Effect of Glycyrrhiza uralensis Fisch polysaccharide on growth performance and immunologic function in mice in Ural City, Xinjiang
Jie Chen, Xiao-Qing Zhu, Li Yang, Yan Luo, Meng-Yuan Wang, Xiao-Ting Liu, Ke-Xun Liang, Xin-Li Gu
College of Animal Science and Technology, Shihezi University, Shihezi 830002, Xinjiang, China
Effect of Glycyrrhiza uralensis Fisch polysaccharide on growth performance and immunologic function in mice in Ural City, Xinjiang
Jie Chen, Xiao-Qing Zhu, Li Yang, Yan Luo, Meng-Yuan Wang, Xiao-Ting Liu, Ke-Xun Liang, Xin-Li Gu✉
College of Animal Science and Technology, Shihezi University, Shihezi 830002, Xinjiang, China
ARTICLE INFO
Article history:
Accepted 10 August 2016
Available online 20 November 2016
Glycyrrhiza uralensis Fisch polysaccharide
Immune organ index
Growth performance
Immunologic function
Objective: To discuss the effect of Glycyrrhiza uralensis (G. uralensis) Fisch polysaccharide on growth performance and immunologic function in mice in Ural City, Xinjiang and to provide important data supporting the application of Glycyrrhiza polysaccharide. Methods: A total of 100 Kunming mice aged 3 weeks old were randomly divided into 5 groups with 20 mice in each group (10 were females and 10 were males). About 0.5 mL normal saline was given to the mice of control group every day and 0.5 mL G. uralensis Fisch polysaccharide was given to the mice of other groups at the concentration of 1, 20, 50 and 100 mg/mL respectively. The growth performance (average body weight, average daily feed intake and feed efficiency), immune organ indexes (spleen index and thymus index) and immunologic function (serum IL-2, CD4+/ CD8+and the activity of NK cells) of mice in each group were detected continuously. Results: The average body weight, feed efficiency, serum IL-2, CD4+/CD8+and the activity of NK cells of mice were increased with the increase of administrated time after administrating G. uralensis Fisch polysaccharide and were reached up the largest level on Day 28. At the same time, each index was proportional to the given dose and was significantly higher than those of control group and reached up the largest level at the administrated dose of 100 mg/mL. After administrating G. uralensis Fisch polysaccharide, the spleen index and thymus index of mice were increased with the increase of administrated dose and the spleen index and thymus index of mice administrated with the dose of 100 mg/mL were maximum which was more than 1.51 times and 1.43 times of that in control group respectively and the comparative differences showed statistical significance (P<0.05). The average daily feed intake of mice in each group was increased with the passage of time and at the same time, the comparison of average daily feed intake of mice in each group was not significantly different (P>0.05). Conclusions: G. uralensis Fisch polysaccharide can significantly improve the growth performance and immunologic function of mice and laid a research basis for the clinical application of G. uralensis Fisch polysaccharide.
1. Introduction
Polysaccharide is a biopolymer connected by ketose or aldose through glucosidic bond which widely exists in various kinds of plant tissue and possesses significant and important biological activity and pharmacological effects[1]. In recent years, a large number of studies have shown that polysaccharide can widely regulate various biological phenomena and influence signaltransduction and feelings among cells and also has an important regulating role on immune function and physical function[2,3]. G. uralensis Fisch (G. uralensis) is a characteristic Chinese medicine in Xinjiang region which possesses some efficacies like fortifying the spleen and supplementing Qi, and clearing heat and removing toxicity and is widely used in various Chinese medicine formulas[4]. Glycyrrhiza polysaccharide is one of the main active ingredients of licorice and its biological activity is closely connected with the spatial structure and spatial structure given priority to with alpha-D-pyran polysaccharide[5]. Glycyrrhiza polysaccharide possesses strong immune activity and antioxidant activity[6], meanwhile it has some functions such as enhancing body function, reduce the production of cancer cells and antivirus which is widely used in some aspects of anti-tumor, immunoregulation and anti-aging etc. and suitable for people of all ages and it is been recognized[7,8]. However, it is still lack of relevant data about the effect of G. uralensis Fisch polysaccharide on growth performance and immunologic function so far which seriously impact the application of G. uralensis Fisch polysaccharide[9].
In this study, we extract the polysaccharide from G. uralensis Fisch and study the effect of G. uralensis Fisch polysaccharide on growth performance (average body weight, average daily feed intake and feed efficiency), immune organ indexes (spleen index and thymus index) and immunologic function (serum IL-2, CD4+/CD8+and the activity of NK cells) in Kunming mice and also discuss the effect of glycyrrhiza polysaccharide on growth performance and immunologic function in mice, Xinjiang and provide important data supportting the application of G. uralensis.
2. Materials and methods
2.1. Research methods
About 5 kg medicinal powder of G. uralensis Fisch was extracted and soaked at 90% ethyl alcohol for 48 h to remove impurities and lipophilic small molecule compounds. Hot reflux extraction was performed 3 times and each extracting time was 3 h, 2 h, 1.5 h in order. The material was centrifuged at 5 000 g for 10 min and combined with supernatant extract and then was concentrated. The right amount of 90% ethyl alcohol was added into the solution to make the concentration of ethyl alcohol to be 85% and was precipitated at 4 ℃ for 24 h. The sediment was extracted after centrifuging at 5 000 g for 10 min and freezed and dried. The dried sample and crude polysaccharide were obtained and distilled water was dissolved the configured into 1% crude polysaccharide solution. The protein was removed by using Sevag method, namely, Sevag reagent (chloroform: N-butyl alcohol=4:1) and polysaccharide samples (1:1) were shaked well for 20 min and kept still for 30 min and the denatured layer of lower protein was removed by centrifuging. The samples were concentrated and operation was conducted repeatedly for several times to removed the proteins. The samples which were concentrated to remove the proteins were washed with 95% ethyl alcohol and were concentrated again and freeze-dried to obtain crude polysaccharide samples.
2.2. Anthrone-sulfuric acid method detecting the content of polysaccharide
The right amount of glucose standard sample was made into 0.1 mg/mL glucose standard solution after precise weighing and adding water. About 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 mL glucose standard solutions were obtained respectively and placed into 10 mL test tube with cork. The water was added up to 2.0 mL and anthrone-sulfuric acid was added precisely up to 6.0 mL and then all were mixed well and heated in water bath for 15 min and put into ice bath immediately for 15 min. The absorbance was detected at the wavelength of 625 nm of ultraviolet spectrophotometer and the absorbance was regarded as ordinate and concentration was regarded as ordinate to draw a standard curve. The content of polysaccharide was measured.
2.3. Mice groups
SPF Kunming mice aged 3 weeks old were purchased from Xinjiang Medical University (half male and half female) and the body weight was between 20-25 g [Certificate of Animal Quality No.: SCXK (Xin) 2013-0001]. Breeding mice and related experiments were carried out strictly in accordance with the related requirements of the Laboratory Animal Administration Rules. The experimental operations were all accomplished in Animal Nutrition and Feed Science Laboratory and Chinese Veterinarian Laboratory of College of Animal Science and Technology, Shihezi University. All experiments related with animals were approved by the Ethics Committee of Shihezi University. All Kunming mice were randomly divided into 5 groups with 20 mice in each group (10 were females and 10 were males), namely, 1 mg/mL group, 20 mg/mL group, 50 mg/mL group, 100 mg/mL group and control group. About 0.5 mL normal saline was given into the mice of control group every day and 0.5 mL G. uralensis Fisch polysaccharide was given into the mice of other groups at the concentration of 1, 20, 50 and 100 mg/mL respectively. Experimental period was persisted for 28 days.
2.4. Detection of growth performance in mice
Each mouse in every experimental group was marked before experimental treatment. The body weight and daily feed intake were detected continuously using electronic scales under the situation of empty stomach before and after feeding to calculate averagebody weight, average daily feed intake and feed efficiency, feed efficiency= (average body weight of mice - initial body weight of mice)/ average daily feed intake.
2.5. Detection of immune function in mice
2.5.1. Detection of immune organ index in mice
The body weight of mice with empty stomach in each group was weighted and sterile spleen and thymus were weighted and recorded after death with cervical dislocation. Spleen index= spleen mass of mice/empty body weight of mice; thymus index= thymus mass of mice/empty body weight of mice.
2.5.2. Determination of immune related factors in mice
The peripheral blood was collected before and after feeding and kept at -80 ℃ and then was used to detect subsequently. IL-2 and lactic dehydrogenase were detected by ELISA Kit (Enzymelinked biological technology co., LTD., Shanghai, Wuhan Hua Mei Biological Engineering co., LTD) and related operation was performed strictly according to the specification of the kit. CD4+/ CD8+value was detected by flow cytometry (CytoFLEX, USA) and related antibody was provided by Genscript Biotechnology co., LTD and experimental operation was performed strictly according to the specification.
2.6. Statistical methods
Statistical analysis was performed by using statistical software SPSS20.0. The relevant data were expressed by mean±SD. The comparison of two groups was performed by using t-test. The comparison among groups was performed using ANOVA. Pairwise comparison was conducted by SNK-q test. P<0.05 indicated significantly difference.
3. Results
3.1. The effect of G. uralensis Fisch polysaccharide on growth performance in mice
The average body weight, average daily feed intake and feed efficiency of mice in each group were detected and the results showed that the comparison of average body weight and average daily feed intake of mice in each group before feeding was not significantly different (P>0.05). The body weight and feed efficiency of mice were increased with the increase of administrated time after administrating G. uralensis Fisch polysaccharide and were reached up the largest level on Day 28. At the same time, the body weight and feed efficiency of mice was proportional to the given dose and was significantly higher than those of control group and reached up the largest level at the administrated dose of 100 mg/mL. The comparative differences showed statistical significance (P<0.05). The average daily feed intake of mice in each group was increased with the passage of time and at the same time, the comparison of average daily feed intake of mice in each group was not significantly different (P>0.05) (Table 1).
3.2. The effect of G. uralensis Fisch polysaccharide on immune organ index in mice
The mice with empty stomach were weighted and recorded and the spleen index and thymus index of mice were detected after death with cervical dislocation and the results showed that the spleen index and thymus index of mice in control group were minimum, (35.48±3.21) mg/10 g, (17.02±1.85) mg/10 g, respectively. After administrating G. uralensis Fisch polysaccharide, the spleen index and thymus index of mice were increased with the increase of administrated dose and the spleen index and thymus index of mice administrated with the dose of 100 mg/mL were maximum, (53.57±5.01)mg/10 g, (24.35±2.34) mg/10 g, respectively whichwas more than 1.51 times and 1.43 times of that in control group respectively and the comparative differences showed statistical significance (P<0.05) (Table 2).
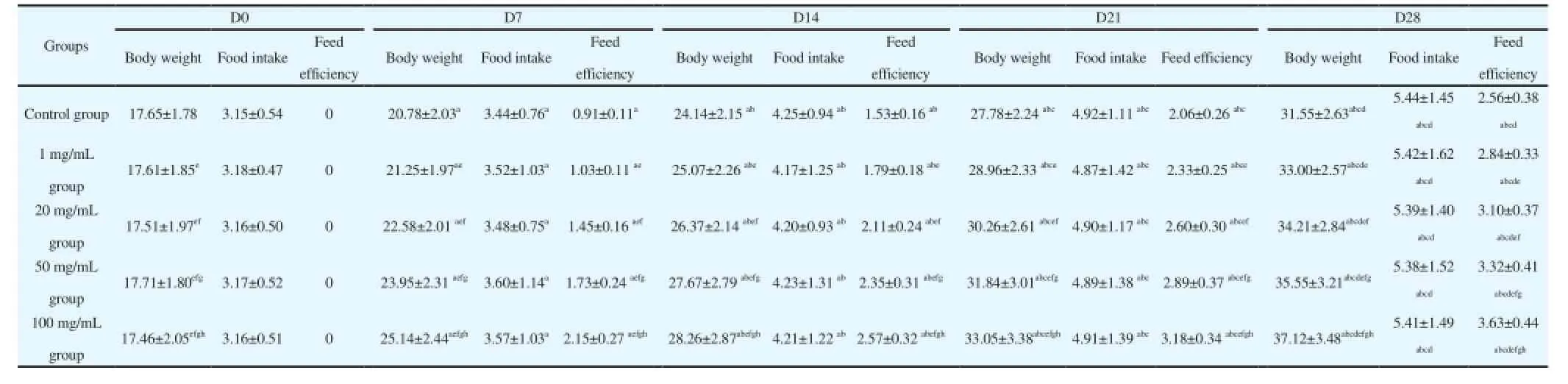
Table 1 The effect of G. uralensis Fisch polysaccharide on growth performance in mice (x±s).
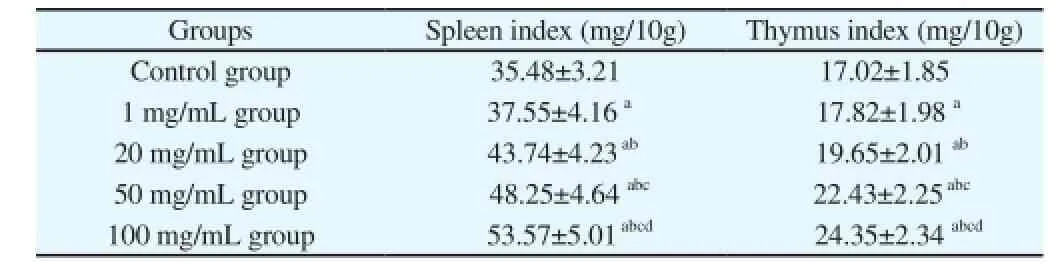
Table 2 The effect of G. uralensis Fisch polysaccharide on immune organ index in mice (x±s).
3.3. The effect of G. uralensis Fisch polysaccharide on immunologic function in mice
The serum IL-2, CD4+/CD8+and the activity of NK cells of mice in each group were detected and the results showed that the comparison of serum IL-2, CD4+/CD8+and the activity of NK cells of mice in each group before feeding was not significantly different (P>0.05). The serum IL-2, CD4+/CD8+and the activity of NK cells of mice were increased with the increase of administrated time after administrating G. uralensis Fisch polysaccharide and were reached up the largest level on Day 28. At the same time, the serum IL-2, CD4+/ CD8+and the activity of NK cells of mice was proportional to the given dose and was significantly higher than those of control group and reached up the largest level at the administrated dose of 100 mg/ mL. The comparative differences showed statistical significance (P<0.05) (Table 3).
4. Discussion
Except protein and nucleic acid, polysaccharide is an important substance in life movement, which is abundant among organisms in nature and widely participates in various biological functions such as structure support, energy storage and immune defense, and so on. More importantly, polysaccharide is a bridge to transfer energy and substance within cells and biotic environment leading to a mutual effect on biomacromolecule and cells[10,11]. In recent years, various polysaccharides have been extracted from different kind of plants whose biological function has also been widely studied, which plays an important role in many aspects such as antineoplastic, decreased hyperglycemic, cruor and immune function. Researches on polysaccharide have also become more and more active. Biological activity of polysaccharide is closely related to its structure, chemical group, formation and purity. Therefore, the focus and emphasis of the present biological research has still aimed at looking for polysaccharides of high activity[12,13]. Uralensis is a common Chinese herbal medicine. G. uralensis is the characteristic traditional Chinese medicine in Xinjiang region. Its quality has been recognized[14]. Uralensis has the effect of clearing heat and removing toxicity, invigorating spleen and replenishing qi, expelling phlegm to arrest coughing and concocting with multiple drugs, which is widely used in the treatment of cough, weakness of the spleen and the stomach, gastric ulcer and food poisoning[15,16]. In recent years, multiple researches have shown that the main active substances of uralensis are G. uralensis Fisch polysaccharide, triterpenoid saponin and flavonoids compounds[17]. Since glycyrrhiza polysaccharide was extracted from G. uralensis Fisch polysaccharide in 1965, clinical and pharmacologic researches have been found that the immunocompetence, antioxidant activity and antineoplastic activity were importantly related to G. uralensis Fisch polysaccharide[18]. G. uralensis Fisch polysaccharide can significantly improve the mitosis, anticomplement and enhance the ability of immune elimination[19]. Multiple researches have shown that G. uralensis Fisch polysaccharide has an effect of improving specific immunity and non- specific immunity of rats and plays an important role on maintaining a healthy body[20]. G. uralensis Fisch polysaccharide has a significant effect of elimination on hydroxyl radical, DPPHfree radical and superoxide anion free radical and can slow down aging and maintain the normality of cells[21,22]. More importantly, G. uralensis Fisch polysaccharide will affect the expression of gene such as BCL-2 and Bax leading to an effect of antineoplastic[23]. At present, polysaccharide has been widely used in various prescriptions and Chinese patent medicine. G. uralensis Fisch polysaccharide also plays an irreplaceable role in the treatment of many diseases[24]. The widely use of polysaccharide in different age and area has earned the focus from people on its toxic and side effect. Effect of G. uralensis Fisch polysaccharide on growth performance and immune function will severely affect the use of polysaccharide[25,26].

Table 3 The effect of G. uralensis Fisch polysaccharide on immunologic function in mice.
The study found that G. uralensis Fisch polysaccharide can significantly improve the growth performance of mice (average weight, average daily feed intake and feed efficiency), immune organ index (spleen and thymus index) and immune function (serum IL-2, CD4+/CD8+and the activity of NK cells). The average daily intake of mice in every group was significantly increased with time, but at the same time, the daily intake of mice was not significantly different. The possible reason was that the growth of mice and the increase of gastric volume and energy consumption in mice would result in the increase of food intake. After Kunming mice were fed with glycyrrhiza polysaccharide, the average weight and the feed efficiency of mice were significantly higher than those of control group, but the food intake did not increased, which indicated that glycyrrhiza polysaccharide can promote the feed utilization rate or reduce energy consumption, thus speeding up the growth in mice. Spleen and thymus are important immune organs of the body. The immune organ index is a manifestation of the function of immune cells and the development of immune organs, which can reflect the immune function of the organism from the side [27]. The spleen index and thymus index of mice were significantly higher after administrating glycyrrhiza polysaccharide which indicates that glycyrrhiza polysaccharide has auxo-action on immune organs of body and can enhance the immune function of body. Immune cells and immune factor are the indispensable part of the body's immune function and the levels of immune cells and immune factor can reflect well the level of body's immune function[28]. Interleukin-2 (IL-2) is an important immune factor secreted by CD4+T lymphocyte which widely participates in anti-virus infection and body's immune response and can improve the activation and proliferation of T cell, B cell and NK cell and secretion of correlation factor. It plays an important role in the treatment of tumor and antivirus[29,30]. G. uralensis Fisch polysaccharide increases the secretion of IL-2 by improving the activation and proliferation of related immunity cells, which can simultaneously increase the secretion of Interleukin-2 dose-dependently. The secretion of IL-2 further improves the activation of NK cells. It has been found by ELISA that LDH level in rats irrigated by G. uralensis Fisch polysaccharide was significantly higher than that of control group, which by analyzing may result from the proliferation and activation of NK cells improved by G. uralensis Fisch polysaccharide. And it further improves the damage on tumour cell and virus-infection cells and the release of LDH from cells. T-lymphocyte subsets is a important cell participating in the function of body's immune defenses, which mainly includes two classes, inhibitory CD8+T lymphocytes and auxiliary CD4+T lymphocytes. CD4+T lymphocytes contain secrete-related factors which improve the production of antibody and regulate the function of immunocompetence. While, CD8+T lymphocytes possess the effects of immunosuppression and cytotoxicity. Therefore, the value of CD4+/CD8+can adequately indicate the situation of immune mechanism[31,32]. Researchers have been found that the G. uralensis Fisch polysaccharide improve the increase of the value of CD4+/ CD8+, which indicates that G. uralensis Fisch polysaccharide can regulate the body immune function.
In conclusion, in this study, we extract the polysaccharide from G. uralensis Fisch and study the effect of G. uralensis Fisch polysaccharide on growth performance (average body weight , average daily feed intake and feed efficiency), immune organ indexes (spleen index and thymus index) and immunologic function (serum IL-2, CD4+/CD8+and the activity of NK cells) in Kunming mice and also discuss the effect of glycyrrhiza polysaccharide on growth performance and immunologic function in mice, Xinjiang and provide important help for the development and application of G. uralensis.
Declare of interest statement
We declare that we have no conflict of interest.
References
[1] Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang Y, Uzayisenga R, et al. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complem Altern Med 2016;16(1):1-8.
[2] Span EA, Marletta MA. The framework of polysaccharide monooxygenase structure and chemistry. Curr Opin Struc Biol 2015;35(25):93-99.
[3] Orla McCabe1, Silvia Spinelli, Carine Farenc, Myriam Labbé, Denise Tremblay, Stéphanie Blangy, et al. The targeted recognition of Lactococcus lactis phages to their polysaccharide receptors. Mol Microbiol 2015;96(4):875–886.
[4] Marc J.M. Bonten, Susanne M. Huijts, Marieke Bolkenbaas, Chris Webber, Scott Patterson, Samantha Gault, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New Engl J Med 2015;372(12):1114-1125.
[5] Shen H, Zeng G, Sun B, Cai X, Bi L, Tang G, et al. A polysaccharidefrom Glycyrrhiza inflata Licorice inhibits proliferation of human oral cancer cells by inducing apoptosis via mitochondrial pathway. J Int Soci Oncodevelop Bio Med 2015;36(6):4825-4831.
[6] Jassal PS, Kaur G, Kaur L. Synergistic effect of curcuma longa and glycyrrhiza glabra extracts with copper ions on food spoilage bacteria. Int J Clin Pharm-Net 2015;7(10):371-375.
[7] Hosseinzadeh H, Nassiri-Asl M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: update and review. Phytotherapy Res Ptr 2015;29(12):1868–1886.
[8] Barros L. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. Rsc Adv 2015;5(34):29-39.
[9] Norazah B, Ayotunde OO, Ritchie KJ, Sarker S. Comparative cytotoxicity of Glycyrrhiza glabra roots from different geographical origins against immortal human keratinocyte (HaCaT), lung adenocarcinoma (A549) and liver carcinoma (HepG2) cells. Phytother Res 2015;29(6):944-948.
[10] Crouch LI, Labourel A, Walton PH, Davies GJ, Gilbert HJ. The contribution of non-catalytic carbohydrate binding modules to the activity of lytic polysaccharide monooxygenases. J Biol Chem 2016;291(14):7439-7449.
[11] Wietz M, Wemheuer B, Simon H, Giebel HA, Seibt MA, Daniel R, et al. Bacterial community dynamics during polysaccharide degradation at contrasting sites in the Southern and Atlantic Oceans. Environ Microbiol 2015;17(10): 3822-3831.
[12] Bryant KA, Frenck R, Gurtman A, Rubino J, Treanor J, Thompson A, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 18-49 years of age, naive to 23-valent pneumococcal polysaccharide vaccine. Vaccine 2015;33(43):5854-5860.
[13] Alaa El-Din Shawky Hosny, Mohamed Reda Diab, Rania Abdelmonem Khattab, Heba Osama Awad. Immune response to Vi polysaccharide, heat-killed whole cells, and outer membrane protein of Salmonella typhi. J Infect Dev Countr 2015;9(6):642-649.
[14] Link P, Wetterauer B, Fu Y, Wink M. Extracts of Glycyrrhiza uralensis and isoliquiritigenin counteract amyloid-βtoxicity in Caenorhabditis elegans. Planta Med 2015;81(5):357-362.
[15] Mousavi SA, Li L, Wei G, Lindström K. Evolution and taxonomy of native mesorhizobia nodulating medicinal Glycyrrhiza species in China. Syst Appl Microbiol 2016;39(4):260-265.
[16] Bhargava N, Singh SP, Sharma A, Sharma P, Capalash N. Attenuation of quorum sensing-mediated virulence of Acinetobacter baumannii by Glycyrrhiza glabra flavonoids. Future Microbiol 2015;10(12):1953-1968.
[17] Zeng G, Shen H, Tang G, Cai X, Bi L, Sun B, et al. A polysaccharide from the alkaline extract of Glycyrrhiza inflata induces apoptosis of human oral cancer SCC-25 cells via mitochondrial pathway. Tumor Biol 2015;36(9):6781-6788.
[18] Jing Li, Juan Wang, Jinxin Li, Jianli Li, Shujie Liu, Wenyuan Gao. Salicylic acid induces the change in the adventitious root of Glycyrrhiza uralensis Fisch.: bioactive compounds and antioxidant enzymes. Res Chem Intermediat 2016;42(2): 1503-1519.
[19] Haiyang Gao, Zhe Sun, Chaoni Xiao, Xiaohui Zhenga, Yajun Zhang. The metabonomic study of Shaoyao-Gancao decoction in a rat model of acute bronchial asthma by 1H NMR. Anal Methods 2016;8(3):570-581.
[20] Tu Y, Zhang GF, Deng KD, Diao Qiyu. Effects of supplementary bee pollen and its polysaccharides on nutrient digestibility and serum biochemical parameters in Holstein calves. Anim Prod Sci 2015;55(10):1318-1323.
[21] Menchicchi B, Hensel A, Goycoolea FM. Polysaccharides as bacterial antiadhesive agents and smart constituents for improved drug delivery systems against Helicobacter pylori infection. Curr Pharm Design 2015;21(33):4888-4906.
[22] Hajirahimkhan A, Simmler C, Dong H, Lantvit DD, Li G, Chen SN, et al. Induction of NAD(P)H:Quinone oxidoreductase 1 (NQO1) by glycyrrhiza species used for women's health: differential effects of the michael acceptors isoliquiritigenin and licochalcone A. Chem Res Toxicol 2015;28(11): 2130-2141.
[23] Kim D S, Hurh B S, Shin K S. Chemical characteristics and immunostimulatory activity of polysaccharides from fermented vinegars manufactured with different raw materials. J Korean Phys Soc 2015;44(2): 191-199.
[24] Jeong SJ, Lim HS, Seo CS, Kim JH, Jin SE, Yoo SR, et al. Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-βB in HaCaT cells. Phytomedicine 2015;22(2):326-332.
[25] Ma C, Wei F, Zhang J, Feng NC. Study of Pb(2+), Cd(2+) and Ni(2+) adsorption onto activated carbons prepared from glycyrrhiza residue by KOH or H3PO4 activation. Water Sci Technol 2015;72(3):451-462.
[26] Dunlap TL, Wang S, Simmler C, Chen SN, Pauli GF, Dietz BM, et al. Differential effects of Glycyrrhiza species on genotoxic estrogen metabolism: Licochalcone A downregulates P450 1B1, whereas isoliquiritigenin stimulates it. Chem Res Toxicol 2015;28(8):1584-1594.
[27] Tang J, Chen Z. The protective effect ofβ-aminobutyric acid on the development of immune function in chickens under heat stress. J Anim Physiol An N 2015;103(5):99-102.
[28] Deretic V, Kimura T, Timmins G, Pope M, Santosh C, Michael M. Immunologic manifestations of autophagy. J Clin Invest 2015;125(1):75-84.
[29] Zhu E, Gai S, Opel C, Kwan BH, Surana R, Mihm MC, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 2015;27(4):489-501.
[30] Weist BM, Kurd N, Boussier J, Chan SW, robev EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 2015;16(6):635-641.
[31] Takeuchi A, Badr ME, Miyauchi K, Ishihara C, Onishi R, Guo Z, et al. CRTAM determines the CD4+cytotoxic T lymphocyte lineage. J Exp Med 2016;213(1): 123-138.
[32] Marshall MR, Varsha P, Mahantappa H, Maier-Peuschel M, Müller ML, Becherer U, et al. VAMP8-dependent fusion of recycling endosomes with the plasma membrane facilitates T lymphocyte cytotoxicity. J Cell Biol 2015;210(1):135-151.
Document heading 10.1016/j.apjtm.2016.08.004
30 June 2016
in revised form 7 July 2016
Chen Jie (1986 -), Female, Doctor, Mainly engaged in animal nutrition and feed science research.
Tel: 18699145825
E-mail: chenjie4984@163.com.
✉ Xin-Li Gu, College of Animal Science and Technology, Shihezi University, Shihezi 830002, Xinjiang, China.
Tel: 0993-2038582
E-mail: xlgu@shzu.edu.cn
Foundation project: This study was supported by Scientific Research Innovation Project of Graduate Education Innovation Fund from Xinjiang (Grant No. XJGRI2014057).
 Asian Pacific Journal of Tropical Medicine2016年11期
Asian Pacific Journal of Tropical Medicine2016年11期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
- Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
- Expression and mechanism of action of miR-196a in epithelial ovarian cancer
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
