18F-脱氧葡萄糖PET/CT与弥散加权成像在原发性中枢神经系统淋巴瘤中的相关性研究
周维燕,文剑波,华逢春,孔艳艳,张政伟,陆秀宏,管一晖
1. 复旦大学附属华山医院PET中心,上海 200235;
2. 复旦大学附属华山医院放射科,上海 200040
18F-脱氧葡萄糖PET/CT与弥散加权成像在原发性中枢神经系统淋巴瘤中的相关性研究
周维燕1,文剑波2,华逢春1,孔艳艳1,张政伟1,陆秀宏1,管一晖1
1. 复旦大学附属华山医院PET中心,上海 200235;
2. 复旦大学附属华山医院放射科,上海 200040
目的:本研究回顾性分析原发性中枢系统淋巴瘤(primary central nervous system lymphoma,PCNSL)病灶18F-脱氧葡萄糖(18F-fluorodeoxyglucose,18F-FDG)半定量摄取与表观扩散系数(apparent diffusion coefficient,ADC)之间的关系。方法:纳入14例在复旦大学附属华山医院同时行FDG PET/CT及弥散加权成像(diffusion weighted imaging,DWI)检查的初诊PCNSL患者。手动在经滤波反投影(filtered back projection,FBP)重建后的脑PET图勾画PCNSL病灶感兴趣区(region of interest,ROI),测算肿瘤FDG摄取半定量参数平均标准摄取值(mean standardized uptake value,SUVmean)及最大标准摄取值(maximum standardzed uptake value,SUVmax)。在ADC图上肿瘤实质、对侧相应正常脑白质区各自勾画ROI并分别测定ADC值,病灶ADC值取最小值(ADCmin),对侧正常脑白质ADC值取平均值(ADCmean),rADC为病灶ADCmin与对侧正常脑白质ADCmean的比值。采用Pearson分析对SUV与ADC半定量参数之间的相关性进行分析。结果:14例PCNSL患者共计纳入18个病灶,SUVmax与rADC(r=-0.584,P=0.011)、SUVmean与rADC(r=-0.559,P=0.016)均呈负相关。结论:基于病灶分析的治疗前SUV与ADC值存在负相关,PCNSL肿瘤细胞的代谢信息与其致密程度存在相关性,为DWI作为FDG PET诊断PCNSL和进行疗效监测的替代技术提供了一定的理论依据。
18F-脱氧葡萄糖;正电子放射断层成像;弥散加权成像;原发性中枢系统淋巴瘤

周维燕 ,复旦大学附属华山医院PET中心,博士研究生在读。
主要研究方向:神经退行性疾病新型PET探针合成及临床前评价,肿瘤乏氧显像等,目前已发表论文(含SCI收录)、综述多篇。出席SNMMI会议1次。
原发性中枢神经系统淋巴瘤(primary central nervous system lymphoma,PCNSL)约占原发性脑肿瘤的5%。中枢神经系统内并无内源性淋巴组织,所以PCNSL病因尚未完全明确,与EB病毒及巨细胞病毒感染有关[1]。90%的PCNSL病理类型是弥漫大B细胞型,通常为高级别,较少见的病理类型还有Burkitt淋巴瘤和T细胞淋巴瘤。与系统性淋巴瘤不同,PCNSL首诊常表现为神经系统症状,如颅内压升高、局部神经功能缺损、癫痫发作、眼科或精神症状等[2]。该病与获得性免疫缺陷综合征(acquired immunodeficiency syndrome,AIDS)相关,但近年来免疫正常人群PCNSL发病率呈上升趋势[3]。PCNSL的治疗方法及预后不同于其他脑恶性肿瘤,因此寻找可靠的非创伤性影像学方法来准确评估PCNSL十分必要。
全身18F-脱氧葡萄糖(18F-fluorodeoxyglucose,18F-FDG) PET/CT有助于除外中枢神经系统以外的淋巴瘤病灶,帮助PCNSL诊断的建立。PCNSL在18F-FDG显像中主要表现为高代谢,标准摄取值(standardized uptake value,SUV)有助于PCNSL与具有淋巴瘤相似糖代谢特点的脑肿瘤如高级别胶质瘤鉴别[4]。弥散加权成像(diffusion weighted imaging,DWI)是一项测定组织内自由水分子运动的MR技术,可评估病灶微结构。肿瘤细胞密度是影响DWI信号的重要因素,脑恶性肿瘤细胞密度高,可造成自由水弥散受限,继而DWI呈现高信号[5]。DWI相关参数可帮助PCNSL与高级别胶质瘤鉴别,并进一步用于疗效监测[6]。本研究拟对治疗前同时接受18F-FDG PET/ CT及DWI检查的14例PCNSL病例进行回顾性分析。
1 资料和方法
1.1一般资料
收集2011年3月—2014年11月在复旦大学附属华山医院神经外科接受立体定向活检手术或开颅手术且神经病理证实为PCNSL弥漫大B细胞型的免疫正常14例患者的治疗前完整18F-FDG PET/ CT及完整DWI影像学资料(两次检查间隔时间为0~12 d)。其中男性8例、女性6例;年龄44~78岁,平均(56.2±8.3)岁,中位年龄58.5岁。入组标准:① 中枢神经系统受损表现为首发症状,病变局限于中枢神经系统内(包括眼、脑组织、脊髓、软脑膜);② 胸片、胸部或腹部CT检查、腹部B超、全血常规、骨髓穿刺涂片检查等未发现全身淋巴造血组织和其他部位受累;③ 人类免疫缺陷病毒(human immunodeficiency virus,HIV)感染阴性,近期未使用免疫抑制剂;④ 无糖尿病及合并其他体部肿瘤。所有患者均被详细告知该研究目的和检查流程,并签署知情同意书。
1.2检查方法
1.2.1PET/CT显像
仪器为SIEMENS Biograph 64 PET/CT仪。18F-FDG为本中心应用FDG4模块自动化生产,放化纯度>99%。显像前所有患者禁食6~8 h,血糖控制在<11.1 mmol/L。静脉注射18F-FDG 370~555 MBq后,休息45~60 min开始扫描。采用专门的脑部采集程序进行图像采集,显像前患者头部接受低剂量CT扫描,再以3D模式进行PET采集8 min,经滤波反投影(filtered back projection,FBP)重建。
1.2.2 DWI显像
采用SIEMENS Trio Tim 3.0T磁共振扫描仪,16通道相控阵头线圈。扫描参数:自旋回波(spin echo,SE)序列轴位T1WI (TR=2 000 ms,TE= 9 ms),轴位T2WI (TR=3 000 ms,TE=98 ms),轴位液体衰减反转恢复(fluid attenuated inversion recovery,FLAIR)序列(TR=7 000 ms,TE=93 ms),层厚5.0 mm,层间距1.0 mm。9例患者术前行常规增强扫描,采用经肘静脉注射对比剂Gd-DTPA,剂量为0.1 mmol/kg,分别行轴位(层厚5.0 mm,层间距1.0 mm)、矢状位及冠状位扫描(层厚4.0 mm,层间距1.0 mm)。5例患者在术中导航或穿刺时进行3D增强采用TRA序列(TR=1 900 ms,TE= 293 ms),后在SIEMENS Syngo工作站重建获得多平面成像。DWI采用单次激发平面回波成像(single shot echo planar imaging,SS-EPI)序列(TR=5 100 ms,TE=90 ms),扩散敏感系数b=1 000 s/mm2,层厚5.0 mm,层间距1 mm。
1.3图像处理与分析
1.3.118F-FDG图像分析
采用半定量分析方法评估肿瘤对18F-FDG的摄取:勾画体积为0.04~0.08 mm3的感兴趣区(region of interest,ROI),对放射性摄取明显的肿瘤组织取摄取最明显部位,测定半定量参数SUVmean及SUVmax;对无明显摄取的肿瘤组织,勾勒参照对比MRI T1WI增强图像进行。
1.3.2DWI图像分析
将DWI原始图像输入工作站获得表观扩散系数(apparent diffusion coefficient,ADC)图。参照同一层面的T2WI、T1WI增强图像,依据ADC图于肿瘤实质、对侧相应正常脑白质区各取3个ROI,每个ROI的面积定为0.16 cm2,分别测ADC值,病灶ADC取最小值(ADCmin),对侧正常脑白质取平均值(ADCmean),相对ADC值(relative ADC,rADC)=病灶ADCmin/对侧正常脑白质ADCmean。
1.4统计学处理
2 结 果
2.1病变数目及分布
14例PCNSL患者中,单发病灶8例(占57.14%),多发病例6例(占42.86%),其中2个病灶4例(占28.57%),3个及以上病灶2例(占14.29%)(注:累及多个部位的呈融合状态的病灶记为1个,不重复计数)。其中幕上病灶21个(87.5%),幕下病灶3个(12.5%),病灶累及最常见部位为额颞叶及基底节区。部分病灶水肿异常明显导致ADC图上ROI无法勾画未纳入测量,累计纳入可测量病灶数为18个。另外,病灶增强后小结节状或斑点状病灶(<8 mm)较小,未列入统计;其中1个病灶T1WI增强见强化且FLAIR呈高信号,但FDG摄取未见明显增高,同机融合CT密度亦呈等密度,周围不伴明显水肿,为PET失检病灶。
2.218F-FDG代谢特征及SUV值
18个PCNSL病灶对18F-FDG的摄取均明显高于脑对侧皮质,SUVmean为16.4±9.4,SUVmax为18.2±9.7。
2.3DWI信号特征及ADC值
18个淋巴瘤病灶在DWI (b=1 000 s/mm2)上均表现为高于脑灰质的信号,在ADC图上表现为低或等信号。ADC图上ROI的选择避免周围水肿区及瘤内血管的容积效应。在每一病灶实质部分分别放置3个ROI进行测量,记录病灶ADCmin。然后以同样大小的ROI测量病灶对侧正常白质区ADCmean。病灶ADCmin与对侧正常白质区ADCmean的比值为rADC。病灶ADCmin平均值为(0.542±0.058)×10-3mm2/s,正常白质ADCmean平均值为(0.712±0.052)×10-3mm2/s,rADC平均值为0.764±0.082。
2.4病理结果
均为弥漫大B细胞非霍奇金淋巴瘤,光镜下见瘤组织弥漫密集,呈片状分布,瘤细胞大小较一致,胞质少,核大,肿瘤间质成分相对较少,肿瘤血管内皮增生少见。
2.5 相关性分析
采用软件SPSS 19.0,SUVmean与SUVmax呈显著正相关(Pearson双侧检验:r=0.994,P<0.001),ADCmin与rADC亦呈显著正相关(Pearson双侧检验:r=0.755,P<0.001),SUVmax与rADC(Pearson双侧检验:r=-0.584,P=0.011,图1)及SUVmean与rADC (Pearson双侧检验:r=-0.559,P=0.016)呈负相关,SUV-max与ADCmin之间无显著相关性(r=-0.340,P=0.168)。
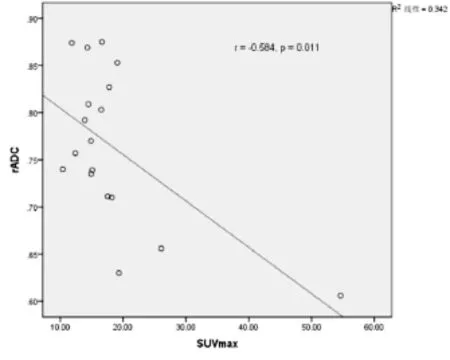
图1 PCNSL患者SUVmax与rADC相关性
3 讨 论
颅内原发性淋巴瘤的起源、病因和发病机制目前尚不完全清楚,但免疫系统缺陷患者的EB病毒病因学说受到较多肯定。本组患者HIV均为阴性,未使用免疫抑制剂,病理证实均为弥漫大B细胞非霍奇金淋巴瘤。PCNSL的常用治疗方案为基于甲氨蝶呤或美罗华的大剂量化疗或全脑放疗,手术并不是一线推荐治疗,患者通常反应良好,并可达到临床缓解[7],所以临床亟需敏感和特异性俱佳的用于诊断及疗效判断的影像学技术。研究表明,FDG PET/CT及DWI技术均可用于淋巴瘤鉴别诊断[8-9](包括侵袭型与惰性淋巴瘤亚型鉴别[10])、疗效[11]及预后评估[12],各自与多种免疫组化指标相关,且存在交叉,那么基线(治疗前)相关参数可能存在某种关联性,已在非小细胞肺癌及其转移淋巴结[13]、乳腺癌[14-15]、腹膜癌[16]、头颈部肿瘤[17]、胰腺腺癌[18]等中证明ADC与SUV存在负相关。
颅内原发性淋巴瘤多数位于深部白质,T1WI多呈稍低或等信号,T2WI呈等或稍高信号,瘤周水肿和占位效应较轻,均匀强化,灌注成像被认为可用于鉴别淋巴瘤与胶质瘤。研究显示,FDG PET相关半定量参数可用于鉴别淋巴瘤与其他性质的脑肿瘤[19],但由于颅内病变影像学表现和临床症状可不典型,存在重叠区,常易误诊。PCNSL通常位于幕上,病灶位于脑干及小脑者较为少见,与本组病例一直。PCNSL在化疗前病灶通常并不出现钙化或出血,凭此可与胶质母细胞瘤鉴别。与胶质母细胞瘤相似,PCNSL可跨越胼胝体,同时累及双侧额叶及胼胝体,呈经典蝴蝶状分布,此时病灶通常较大(图2)。
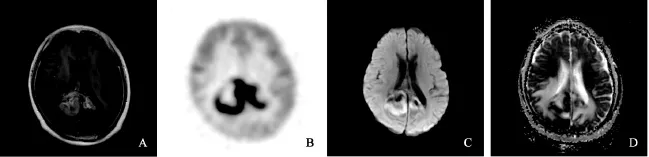
图2 PCNSL患者18F-FDG PET/CT与DWI MRI影像学表现
本研究显示,PCNSL病灶ADCmin平均值为(0.542±0.058)×10-3mm2/s,正常白质ADCmean平均值为(0.712±0.052)×10-3mm2/s。Matsushima等[20]研究证实30例胶质瘤及6例PCNSL患者病灶SUVmax比值(选择对侧额叶作为参考区)与ADCmin存在负相关(r=-0.68,P<0.000 1),其中病灶ADCmin平均为(0.58±0.13)×10-3mm2/s,ADCmean平均值为(0.66±0.16)×10-3mm2/s,SUVmax平均值为25.3±3.99,均高于本研究结果,这可能是由于该研究纳入病例较少而导致差异存在。本研究发现PCNSL多病灶患者额叶代谢常常减低,若选择对侧额叶作为参考计算SUVmax比值将不能真实评估病灶代谢情况,故未计算SUVmax比值。为消除白质纤维弥散各项异性的影响,本研究引入rADC概念,即肿瘤组织ADC值与对侧半球对应正常部位脑白质ADC值的比值,结果显示病灶ADCmin均低于正常白质ADCmean,故rADC均<1。本组14例PCNSL患者18个病灶在DWI上均表现为高于脑灰质的信号,在ADC图上表现为低或等信号,与其相对低弥散特性相一致。
PCNSL病灶18F-FDG半定量摄取值SUVmax通常为14~22,约为脑灰质SUVmean的2.5倍。尽管如此,脑皮质、基底节区及丘脑的正常高代谢本底常掩盖淋巴瘤病灶[21],特别是CT上呈等密度病灶时,易造成假阴性。本研究中漏诊的唯一病灶位于左侧桥壁,FDG代谢未见明显异常增高,但增强MRI显示明显,可能是由于该病灶处于早期,病灶肿瘤细胞尚不够致密所致。因此,在探查早期病灶方面,MRI增强技术可能具有独到优势。
此外,9例单侧脑内病变PCNSL患者中7例对侧小脑出现脑代谢减低的现象,称为交叉性小脑失联络,原因可能是病灶位于大脑中动脉分布脑区,局部脑缺血导致皮质桥小脑束受损,使对侧小脑失去神经支配[22]。
本研究亦发现基于病灶分析的治疗前ADC与SUV存在负相关,表明PCNSL的葡萄糖代谢信息与肿瘤细胞致密程度存在相关性,为DWI作为FDG PET诊断PCNSL和进行疗效监测的替代技术提供了一定的理论依据。PET/MRI一体机技术的引进,可一次扫描获得PET代谢、MRI解剖及其他功能MRI序列信息,有助于反映病灶不同生物学特性,相互提供互补功能影像学信息,且一定时间范围内的异机对照研究为后期同机PET/ MRI相关技术在国内的应用提供了一定的经验与借鉴。
[1] RICARD D, IDBAIH A, DUCRAY F, et al. Primary brain tumours in adults [J]. Lancet, 2012, 379(9830): 1984-1996.
[2] MCNAMARA S. Treatment of primary brain tumours in adults [J]. Nurs Stand, 2012, 27(14): 42-47.
[3] OLSON J E, JANNEY C A, RAO R D, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance,epidemiology, and end results analysis [J]. Cancer, 2002,95(7): 1504-1510.
[4] KAWAI N, MIYAKE K, YAMAMOTO Y, et al.18F-FDG PET in the diagnosis and treatment of primary central nervous system lymphoma [J]. Biomed Res Int,2013, 2013: 247152.
[5] ZACHARIA T T, LAW M, NAIDICH T P, et al. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy [J]. J Neuroimaging, 2008, 18(4): 411-417.
[6] MOFFAT B A, CHENEVERT T L, LAWRENCE T S, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response [J]. Proc Natl Acad Sci USA, 2005, 102(15): 5524-5529.
[7] KASENDA B, FERRERI A J, MARTURANO E, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—a systematic review and individual patient data metaanalysis [J]. Ann Oncol, 2015, 26(7): 1305-1313.
[8] DOSKALIYEV A, YAMASAKI F, OHTAKI M, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T [J]. Eur J Radiol, 2012, 81(2): 339-344.
[9] NAKAJIMA S, OKADA T, YAMAMOTO A, et al. Primary central nervous system lymphoma and glioblastoma: differentiation using dynamic susceptibilitycontrast perfusion-weighted imaging, diffusion-weighted imaging, and18F-fluorodeoxyglucose positron emission tomography [J]. Clin Imaging, 2015, 39(3): 390-395.
[10] MOSAVI F, WASSBERG C, SELLING J, et al. Wholebody diffusion-weighted MRI and18F-FDG PET/CT can discriminate between different lymphoma subtypes [J]. Clin Radiol, 2015, 70(11): 1229-1236.
[11] PALMEDO H, URBACH H, BENDER H, et al. FDGPET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up [J]. Eur J Nucl Med Mol Imaging,2006, 33(2): 164-168.
[12] MAYERHOEFER M E, KARANIKAS G, KLETTER K, et al. Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an18F-FDG-PET/ CT-controlled prospective study in 64 patients [J]. Clin Cancer Res, 2015, 21(11): 2506-2513.
[13] SCHAARSCHMIDT B M, BUCHBENDER C,NENSA F, et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value(SUV) in lymph node metastases of non-small cell lung cancer (NSCLC) patients using hybrid18F-FDG PET/ MRI [J]. PLoS One, 2015, 10(1): e0116277.
[14] KITAJIMA K, YAMANO T, FUKUSHIMA K, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma [J]. Eur J Radiol,2016, 85(5): 943-949.
[15] BABA S, ISODA T, MARUOKA Y, et al. Diagnostic and prognostic value of pretreatment SUV in18F-FDG/ PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging [J]. J Nucl Med, 2014, 55(5): 736-742.
[16] SCHWENZER N F, SCHMIDT H, GATIDIS S, et al. Measurement of apparent diffusion coefficient with simultaneous MR/positron emission tomography in patients with peritoneal carcinomatosis: comparison with18F-FDG-PET [J]. J Magn Reson Imaging, 2014, 40(5): 1121-1128.
[17] HAN M, KIM S Y, LEE S J, et al. The correlations between MRI perfusion, diffusion parameters, and18F-FDG PET metabolic parameters in primary headand-neck cancer: a cross-sectional analysis in single institute [J]. Medicine (Baltimore), 2015, 94(47): e2141.
[18] SAKANE M, TATSUMI M, KIM T, et al. Correlation between apparent diffusion coefficients on diffusionweighted MRI and standardized uptake value on FDGPET/CT in pancreatic adenocarcinoma [J]. Acta Radiol,2015, 56(9): 1034-1041.
[19] YAMAGUCHI S, HIRATA K, KOBAYASHI H, et al. The diagnostic role of18F-FDG PET for primary central nervous system lymphoma [J]. Ann Nucl Med, 2014,28(7): 603-609.
[20] MATSUSHIMA N, MAEDA M, UMINO M, et al. Relation between FDG uptake and apparent diffusion coefficients in glioma and malignant lymphoma [J]. Ann Nucl Med, 2012, 26(3): 262-271.
[21] KAWAI N, MIYAKE K, OKADA M, et al. Usefulness and limitation of FDG-PET in the diagnosis of primary central nervous system lymphoma [J]. No Shinkei Geka,2013, 41(2): 117-126.
[22] YOU D L, SHIEH F Y, TZEN K Y, et al. Cerebral perfusion SPECT in transient ischemic attack [J]. Eur J Radiol, 2000, 34(1): 48-51.
Relationship between 18F-fluorodeoxyglucose PET/CT and diffusion weighted imaging in primary central nervous system lymphoma
ZHOU Weiyan1, WEN Jianbo2, HUA Fengchun1, KONG Yanyan1, ZHANG Zhengwei1, LU Xiuhong1, GUAN Yihui1(1. PET Center, Huashan Hospital, Fudan University, Shanghai 200235, China; 2. Department of Nuclear Medicine, Huashan Hospital, Fudan University, Shanghai 200235, China)
Correspondence to: GUAN Yihui E-mail: guanyihui@hotmail.com
Objective: To retrospectively evaluate the relationship between18F-fluorodeoxyglucose (18F-FDG) uptake using PET/CT and apparent diffusion coefficient (ADC) in patients with primary central nervous system lymphoma (PCNSL). Methods: A total of 14 PCNSL cases with 18 lesions underwent both FDG PET/CT scan and diffusion weighted imaging at onset. The meanstandardized uptake value (SUVmean) and maximum standardized uptake value (SUVmax) were calculated to assess the tumor FDG uptake on brain PET/CT images with filtered back projection (FBP) reconstruction. On ADC map, ADCmin of the lesions and ADCmean of the contralateral normal white matters were calculated. The relative ADC (rADC) was obtained by the ratio of ADCmin of the tumor to ADCmean of the contralateral normal white matter. Pearson’s correlation analysis was used to assess the relationship between FDG uptake and ADC-derived parameters. Results: Negative correlations between SUVmax and rADC, between SUVmean and rADC were found for PCNSL cases (r=-0.584, P=0.011; r=-0.559, P=0.016, respectively). Conclusion: There is negative correlation between SUV and rADC before treatment. Both FDG PET/CT and DWI are useful methods in diagnosing PCNSL by evaluating tumor metabolic activity and cellular density. They are closely correlated. DWI could be an alternative imaging technique for FDG PET/CT in the diagnosis and therapy monitoring of PCNSL.
18F-fluorodeoxyglucose; Positron emission tomography; Diffusion weighted imaging; Primary central nervous system lymphoma
R445.5
A
1008-617X(2016)03-0257-06
国家自然科学基金(No:81271516、81571345);上海市科委项目(No:14DZ1930402);复旦大学老年医学专项支持计划青年学者创新研究项目(No:IDF151006);上海市卫生局资助项目(No:20134313)
管一晖 E-mail:guanyihui@hotmail.com
(2016-09-03)

