脑胶质瘤体素内不相干运动弥散加权成像与T1动态对比增强磁共振成像相关性研究
任 彦,庞浩鹏,狄宁宁,张家文,邱天明,陈 宏,刘含秋,庄冬晓,张 泳,徐田勇,吴劲松,姚振威,冯晓源
1. 复旦大学附属华山医院放射科,上海 200040;
2. 复旦大学附属华山医院神经外科,上海 200040;
3. 复旦大学附属华山医院病理科,上海 200040;
4. 通用电气公司MR科研部,上海 201203;
5. 通用电气MR公司培训部,上海 201203
脑胶质瘤体素内不相干运动弥散加权成像与T1动态对比增强磁共振成像相关性研究
任 彦1,庞浩鹏1,狄宁宁1,张家文1,邱天明2,陈 宏3,刘含秋1,庄冬晓2,张 泳4,徐田勇5,吴劲松2,姚振威1,冯晓源1
1. 复旦大学附属华山医院放射科,上海 200040;
2. 复旦大学附属华山医院神经外科,上海 200040;
3. 复旦大学附属华山医院病理科,上海 200040;
4. 通用电气公司MR科研部,上海 201203;
5. 通用电气MR公司培训部,上海 201203
目的:研究磁共振22 b值eDWI(b值范围0~5 000 s/mm2)体素内不相干运动(intravoxel incoherent motion,IVIM)成像与T1动态对比增强磁共振成像(dynamic contrast enhanced MRI,DCE-MRI)评价脑胶质瘤微循环灌注的相关性。方法:92例手术病理证实的成人胶质瘤患者术前进行MRI、eDWI和T1 DCE-MRI。所有胶质瘤分为低级别组(low-grade glioma,LGG)(Ⅱ级)、高级别组(high-grade gliomas,HGG)(Ⅲ+Ⅳ级);胶质母细胞瘤组(glioblastoma multiforme,GBM)(Ⅳ级)、其他级别组(other-grade gliomas,OGG) (Ⅱ+Ⅲ级)。工作站进行eDWI和DCE-MRI后处理,抽取IVIM双指数模型灌注相关的Dfast和PF两个参数,以及DCE-MRI拟合的血管与血管外细胞外间隙(extravascular extracellular space,EES)转运常数(volume transfer constant,Ktrans)、容积分数(extravascular extracellular space volume fraction,Ve)、EES反向容积转运常数 (Kep)和血浆容积分数(fraction of plasma volume,Vp)4个参数,选取肿瘤最大层面实性区域勾画感兴趣区,测定以上参数的平均值。Dfast、PF分别与Ktrans、Ve、Vp和Kep进行Spearman相关性分析,并比较LGG与HGG、GBM与OGG组之间的差异。受试者工作操作特性(receiver operating characteristic,ROC)曲线分析不同参数对胶质瘤分级的能力。结果:Dfast与Ve、Vp呈中等相关(ρ=0.460和0.412,P<0.01),与Ktrans相关性稍弱(ρ=0.396,P<0.01),与Kep没有相关性;PF与Vp、Ktrans和Ve呈弱负相关(ρ=-0.345、-0.323和-0.249,P均<0.05),与Kep不相关。除Kep外,其余参数在LGG与HGG、GBM与OGG之间均有显著性差异(P均<0.01)。ROC曲线分析各参数鉴别HGG与LGG的能力(AUC,95%CI):Ktrans的AUC值最高,为0.808(0.717,0.899)。ROC曲线分析各参数鉴别GBM与OGG的能力(AUC,95%CI):Dfast最高,为0.802(0.703,0.902)。结论:胶质瘤IVIM灌注相关参数与DCE-MRI密切相关,Dfast是无需外源性造影剂示踪的活体评价脑胶质瘤灌注的潜在标记物。
体素内不相干运动;双指数模型;磁共振动态对比增强成像;胶质瘤
体素内不相干运动(intravoxel incoherent motion,IVIM) MRI可评价肿瘤微循环灌注[1-3]。肿瘤血管增殖是恶性胶质瘤的重要组织学特征。动态对比增强磁共振成像(dynamic contrast enhanced magnetic resonance imaging,DCE-MRI)的血管与血管外细胞外间隙(extravascular extracellular space,EES)容积转运常数(volume transfer constant,Ktrans)可评价肿瘤增殖血管的完整性[4-5],用于监测肿瘤血管靶向药物治疗反应[6-7]和预测预后[6],但DCE-MRI需引入外源性造影剂。由于对外源性造影剂在体内沉积的担心和潜在的肾功能损害使其临床应用受限[8]。而基于内源性氢质子示踪的IVIM-MRI,无需外源性造影剂,是真正无创的微血管灌注评价技术[9]。但IVIM-MRI能否评价肿瘤血管增殖和肿瘤血管完整性及其与DCE-MRI之间的关系尚不清楚,IVIM灌注相关参数与Ktrans相关性如何,能否成为评价肿瘤血管微循环灌注的替代标记,有待更多的研究证实。现有的胶质瘤IVIM-MRI研究样本普遍较小,不同研究eDWI使用的b值数量和范围也不相同[2,10-12],甚至产生了相互矛盾的结果[10-11]。本研究将重点探讨优化后的高宽b值(0、10、20、30、50、100、150、200、300、400、500、600、800、1 000、1 500、2 000、2 500、3 500、4 000、4 500和5 000 s/mm2) IVIM-MRI与DCE-MRI的相关性,旨在研究IVIM-MRI评价肿瘤增殖血管的可行性和临床意义。
1 资料和方法
1.1临床资料
将92例经手术病理确诊的幕上胶质瘤纳入本组研究。其中男性63例、女性29例;平均年龄44岁,中位年龄43岁,最小18岁,最大69岁。病例纳入标准:① 年龄:18岁以上成年人;② 术前拟诊胶质瘤患者;③ 初诊胶质瘤患者,未进行过针对胶质瘤的治疗;④ 体内无影响MRI检查的植入物,如起搏器、动脉瘤夹和颅面部植入物等;⑤ 无幽闭恐惧症;⑥ 无造影剂过敏史;⑦术前检查无肾功能不全。病例排除标准:① 经手术病理证实非胶质瘤患者;② 幕下和脑干胶质瘤患者;③ 患者明显运动不能进行图像后处理;④ 胶质瘤实性部分面积<10 mm2;⑤ 肿瘤以囊变为主,囊壁厚度<5 mm。
肿瘤部位:单侧额叶30例(左16、右14),额颞叶7例(左5、右2),颞叶22例(左13、右9),额顶叶6例(左4、右2),颞枕叶1例(左),基底节10例(左5、右5),顶叶5例(左4、右1),枕叶1例(左),顶枕叶1例(左),侧脑室1例(左),跨胼胝体8例。所有病例均进行iMRI导航下的手术切除和(或)立体穿刺活检,其中穿刺24例、手术切除60例、联合穿刺和手术切除8例。
1.2 MRI
1.2.1常规MRI
所有入组胶质瘤患者均在3.0T MRI设备(Discovery MR750,GE Medical Systems,Milwaukee,WI,USA)进行扫描,采用8通道线圈。患者仰卧位,头先进,头部置于线圈中央,头颅和身体正中矢状面与检查床台面中线重合。按预先设定扫描序列:首先进行三平面(横断面、矢状面和冠状面)定位像扫描,然后冠状面和横断面定位像定位,扫描线平行于中线,进行矢状面T1WI扫描,扫描范围覆盖至双侧颞部头皮,层厚4 mm,层间距0 mm。冠状面上将扫描线与大脑中线垂直,矢状面上扫描线与胼胝体膝部和压部下缘连线平行,进行横断面MRI[包括T1WI、T2WI、T2液体衰减反转恢复(fluid attenuated inversion recovery,FLAIR)、DWI]扫描,扫描范围覆盖顶部头皮至枕骨大孔,层数28~32,详细参数信息见表1。
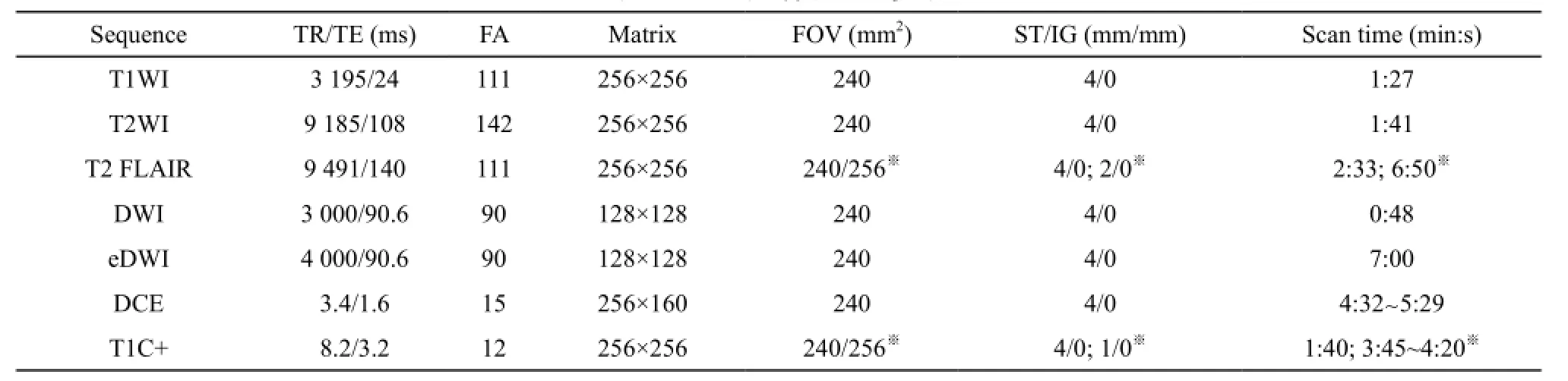
表1 MRI扫描序列及参数
1.2.2IVIM成像
采用多b值eDWI序列[b值:0、10、20、30、50、100、150、200、300、400、500、600、800、1 000、1 500、2 000、2 500、3 500、4 000、4 500和5 000 s/mm2;激励次数(number of excitation,NEX)值随着b值增加,设定为从1到4),层厚4 mm,层间距0 mm;横断面扫描范围与T2WI、T2 FLIAR、T1WI、DWI相同。详细参数信息见表1。
1.2.3T1灌注DCE-MRI
采用3D LAVA序列,范围覆盖整个瘤区。扫描层厚和层间距同其他横断面扫描序列,肿瘤信号范围参考T2 FLIAR序列。记录肿瘤中间层的位置信息,向上及向下各10~12层。记录最上层和最下层空间位置信息。T1-DCE序列(包括T1-Mapping)设置中手动输入刚才记录的位置信息,层厚4 mm,层间距0 mm,总层数20~24层。动态增强扫描前先进行4个翻转角(5°、8°、12°和15°) T1 Mapping扫描,动态增强扫描开始至第4期图像重建结束后,启动预先设置的高压注射器(Opistar LE,Liebel-Flarsheim Company,Cincinnati,USA)按钮,造影剂为钆喷酸葡胺(gadolinium-diethylenetriamine pentaacetic acid,Gd-DTPA;北京北陆药业股份有限公司),剂量为0.01 mmol/kg体重,通过手背或肘静脉预先留置的静脉针快速团注,速率4~5 mL/s,生理盐水15~20 mL冲管。共扫描60期,每次采集时间4 min 32 s~5 min 28 s。详细参数信息见表1。
1.2.4T1增强MRI (T1C+,contrast-enhanced T1WI)
采用3D-BRAVO序列,横断面扫描,扫描野和扫描范围同T2 FLIAR、T2WI,层厚4 mm或1 mm (术中穿刺导航序列),NEX=1,详细参数信息见表1。
1.3数学模型及后处理
1.3.1IVIM双指数模型
所有数据采用GE AW 4.6工作站提供的Functool 9.4.05中MADC程序进行后处理。IVIM双指数函数用于eDWI数据后处理,计算公式如下:
S(b)/S0=[(1-PF)×exp (-bDslow)] + [PF×exp(-bDfast)]。
双指数函数基于IVIM理论[9],其基本思想是假设组织内自由扩散水分子分为快和慢两种成分。S(b)和S0分别代表不同b值的信号强度,S0代表b值为0 s /mm2时的信号强度;Dslow 和Dfast代表慢和快成分的弥散系数,后者又称假弥散系数,与微循环灌注相关;PF代表快成分所占的分数;所有b值被用于双指数函数的拟合,b值200 s /mm2作为快慢成分界值,采用分段法,先用高b值部分计算Dslow,然后移去高b值部分,用剩下的低b值部分计算Dfast,最后计算PF,同时产生功能伪彩图Dslow、Dfast和PF,并以Processed Dicom格式保存。
1.3.2T1灌注DCE-MRI
采用GE AW 4.6工作站提供的GenIQ (Brain DCE)软件进行后处理,采用经典的Tofts-Kermode两室模型[13]。首先进行运动校正和T1校正,为减少后处理时间,选择包含肿瘤的容积范围进行后处理;动脉输入函数(arterial input function,AIF)采用自动选择模式,绘制肿瘤区域感兴趣区(region of interest,ROI)动态增强曲线(图2G、2H)和(图3G、3H),分别计算Ktrans、血管外细胞外间隙容积分数(extravascular extracellular space volume fraction,Ve)、EES反向容积转运系数(Kep)和血浆容积分数(fraction of plasma volume,Vp),并以Processed Dicom格式保存。
1.4图像后处理及ROI定义
1.4.1图像对齐和配准
图像序列内对齐:92例胶质瘤eDWI原始数据在导入工作站内置软件MADC 程序(多b值双指数拟合软件)进行计算前,进行运动校正。DCE-MRI序列运动校正由AW 4.6工作站内置软件GenIQ软件执行。
图像序列间的配准:Processed Dicom格式处理的伪彩图Dfast、PF、Ktrans、Ve、Vp、Kep及常规序列T2 FLAIR和T1C+同时选中导入工作站内置IR软件,自动配准后进行ROI放置。图像校正及配准流程见图1。
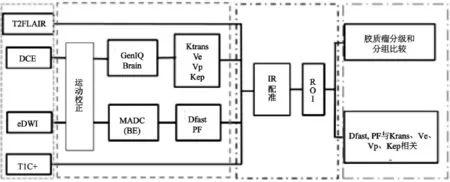
图1 IVIM与DCE-MRI灌注图像后处理及分析流程
1.4.2ROI定义和放置
由两名有经验的神经放射科医师(均不知病理诊断结果)共同决定ROI放置[1,14]。在GE AW4.6工作站内置IR软件中进行ROI勾画。ROI放置标准:不强化肿瘤:以T2 FLAIR作为参考,选择肿瘤最大面积层面进行ROI放置[10]。绘制肿瘤实质区域ROI,手动勾画T2 FLAIR高信号区域,结合DWI、T1WI及T1C+序列,避开囊变、出血及大血管区域(图2A~F、2I~L);强化肿瘤:以T1C+作为参考,选择肿瘤强化最大面积层面进行ROI放置,手动勾画T1C+强化实质区域,结合T2 FLAIR、DWI、T1WI,避开囊变、出血及大血管区域(图3A~F、2I~L);所有肿瘤ROI分别勾画两次,取平均值。
1.5组织病理学检查
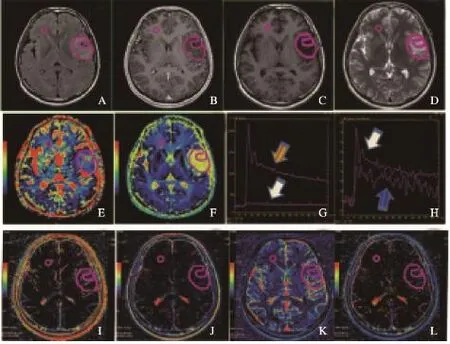
图2 胶质瘤WHOⅡ级
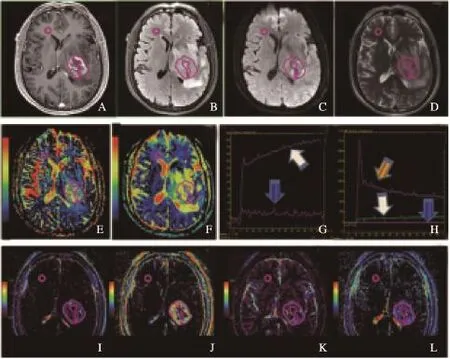
图3 胶质瘤WHO Ⅳ级
所有手术病理标本在复旦大学附属华山医院神经病理数据库资料中收集,肿瘤切片常规进行苏木精-伊红 (hematoxylin eosin,HE) 染色和Ki-67、P53、GFAP、Olig2、IDH1等免疫组织化学染色(immunohistological chemical staining, IHC),由两名神经病理科医师根据HE及IHC结果共同诊断。根据2007年世界卫生组织(World Health Organization,WHO)中枢神经系统肿瘤分类和分级标准进行诊断[15],分为WHO Ⅱ、Ⅲ和Ⅳ级;然后将所有胶质瘤分为低级别组(Ⅱ级;low-grade glioma,LGG)、高级别组(Ⅲ+Ⅳ级;high-grade glioma,HGG);胶质母细胞瘤组(Ⅳ级;glioblastoma multiforme,GBM)、其他肿瘤组(Ⅱ+Ⅲ级;other grade glioma,OGG)。
1.6统计学处理
相关性分析采用Spearman检验,根据相关系数ρ的大小,将结果分为相关性差或一般(<0.4)、相关性中等(0.4~0.7)、相关性良好(>0.7)[16]。两组胶质瘤比较采用t检验(正态分布)或Mann-Whitney U检验(非正态分布),Shapiro-Wilk检验用于数据正态性检验。ROC曲线分析不同参数分级胶质瘤的能力,并计算曲线下面积(area under curve,AUC)(<0.5:完全没有诊断能力;0.5~0.7:差;0.7~0.8:中等;0.8~0.9:良好;>0.9:优)。
2 结 果
按2007 WHO中枢神经系统肿瘤分类标准[15],92例胶质瘤中,WHOⅡ级48例、Ⅲ级17例、Ⅳ级27例。肿瘤区域Dfast (×10-3mm2/s)和PF分别为Ⅱ级:2.32±0.0.379、0.58±0.138;Ⅲ级:2.43±0.324、0.43±0.104;Ⅳ级:3.15±1.005、0.45±0.103。LGG组与HGG组、GBM组与OGG组之间Dfast和PF差异有统计学意义(P<0.01)(表2)。
DCE-MRI灌注参数Ktrans (中位数,25%~75%分位数)(min-1)、Ve (中位数,25%~75%分位数)、Vp (中位数,25%~75%分位数)和Kep (中位数,25%~75%分位数)(min-1)测量值如下。Ⅱ级:0.05 (0.02,0.11),0.15 (0.05,0.27),0.03 (0.02,0.05),0.57(0.25,1.27);Ⅲ级:0.14 (0.06,0.35),0.38(0.12,0.59),0.38 (0.12,0.59),0.06 (0.03,0.12),0.53 (0.32,0.80);Ⅳ级:0.16 (0.12,0.28),0.46 (0.33,0.68),0.11 (0.07,0.21),0.44(0.32,0.66)。除Kep外,Ktrans、Ve和Vp在LGG与HGG、GBM与OGG之间差异有显著统计学意义(P均<0.01)(表2)。
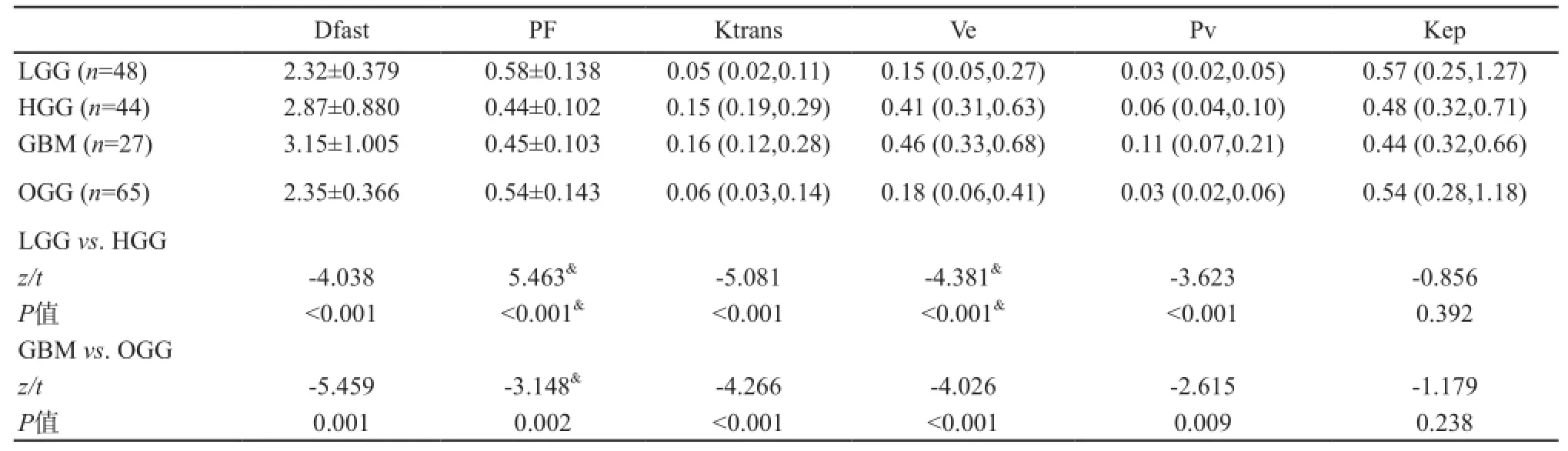
表2 IVIM与DCE-MRI灌注相关参数测量与胶质瘤分级
灌注相关弥散参数与DCE-MRI灌注参数相关性分析显示,Dfast与Ve和Vp中等显著相关(ρ=0.460和0.412,P<0.01),与Ktrans相关性稍弱(ρ=0.396,P<0.01),与Kep没有相关性;PF与Vp和Ktrans呈弱负相关(ρ=-0.345,P<0.01;ρ=-0.323,P<0.01),与Ve呈更弱负相关(ρ=-0.249,P=0.017),与Kep不相关(ρ=-0.006,P=0.955)(图4)。
ROC曲线鉴别LGG与HGG的AUC值分别为Dfast:0.745 (0.643,0.830);PF:0.789 (0.651, 0.848);Ktrans:0.808 (0.717,0.899);Ve:0.765(0.663,0.867);Vp:0.719 (0.613,0.826),P均<0.05。Kep无鉴别诊断价值(P>0.05)。ROC曲线鉴别GBM与OGG的AUC值分别为Dfast:0.802 (0.703,0.902);PF:0.701 (0.588,0.813);Ktrans:0.783 (0.692,0.875);Ve:0.768(0.674,0.861);Vp:0.674 (0.558,0.790),P均<0.05。Kep无鉴别诊断价值(P>0.05)。
3 讨 论

图4 各指标相关性散点图
DCE-MRI可定量评价微血管内造影剂通过不成熟血管壁的程度,与肿瘤新生血管密度显著相关[17]。微血管通透性增加是恶性胶质瘤新生血管的重要组织学特征[18]。根据Tofts-Kermode模型计算的Ktrans、Ve和Vp等参数分别代表不同的肿瘤组织学特征:肿瘤血管通透性、肿瘤间质和肿瘤血管密度,其中Ktrans是定量评价新生肿瘤血管的重要标记,已用于LGG中恶性成分的识别[19]、GBM与不典型淋巴瘤的鉴别[5]、早期预测抗血管药物治疗反应[20]和跟踪靶向药物的疗效[6,21]。DCE-MRI来源的Ktrans、Ve和Vp在胶质瘤分级中的价值在本研究中得到进一步验证。在IVIM理论中,快弥散和慢弥散并不与组织结构直接联系,但慢弥散分数已成为肿瘤细胞密度和细胞增殖的重要标记[22-23]。Dfast及PF与肿瘤血管灌注的关系虽然理论上与血流速度和血容量密切相关,但在实践中不如慢弥散明确,多项研究结果也不一致[14]。尽管IVIM-MRI与DCE-MRI原理不同,但基本理论假设均基于毛细血管水平设计[14,24],因此两者之间参数的相关性可能更准确。
本研究发现Dfast不仅与Vp相关,还与Ve密切相关,且相关趋势更高;其与Ktrans的相关性稍弱,与Kep没有相关性。Dfast与Ve和Ktrans呈显著正相关,提示影响Dfast的因素除血容量和(或)血流量外[25],恶性胶质瘤增高的EES及血管与组织的容积交换也是重要因素。Dfast与血流灌注等多个恶性胶质瘤生物学特征相关,提示其有良好的恶性胶质瘤分级能力。本研究在WHOⅢ级(n=17)与Ⅳ级(n=27)胶质瘤比较中,发现Dfast有显著差异(P=0.006),而其他弥散及灌注参数均无显著统计学差异。
DCE-MRI灌注相关参数与钆剂示踪的微血管密度密切相关[17],而微血管密度预测受示踪剂分子大小的影响。IVIM灌注相关参数与内源性示踪剂水分子弥散关系密切,快速运动的水分子除受微血管密度影响外,血管排列方式和水分子在管腔样结构中的定向流动等也对Dfast有重要影响[26-28]。这反映了GBM微血管复杂的形成机制,即除经典的肿瘤血管生成(angiogenesis)[18],至少还包括血管发生(vasculogenesis)、血管拟态(vascular mimicry)[29]等3种以上其他新生血管形成理论。其中血管拟态过程提示肿瘤细胞可直接形成完全没有血管内皮细胞的功能性管腔样结构[29],这样的结构可能是EES水分子定向流动的重要因素。另外,恶性胶质瘤的新生血管长度往往更短,排列更趋于杂乱,由于缺乏血管壁阻挡,水分子扩散可能更自由、随机。最后,除新生血管的组织形态学差异外,恶性胶质瘤往往还伴有大量坏死及微囊变[15],尽管选取ROI时已尽量避开宏观的坏死和囊变成分,但微观下体素内的微小坏死和囊变无法完全避免,因此这些EES成分成为GBM比WHO Ⅲ级胶质瘤Dfast显著增加的重要原因。
此外,PF与Vp、Ve、Ktrans呈显著负相关,与Bisdas等[14]研究认为PF与Vp、Ve和Ktrans没有相关性不一致。PF的临床意义究竟是什么?显然,与早先Le Bihan等提出的与血管容积分数正相关的理论相矛盾,也与Federau等[1]的研究结果不一致。同时,本研究中PF值 (LGG:0.58±0.138;HGG:0.44±0.102)均明显>30%,不符合生理性脑血流容积分数[1,30]。为进一步阐明PF的意义,本研究对PF与肿瘤增殖指数Ki-67进行相关分析,发现PF与Ki-67呈明显负相关(ρ=-0.595,P<0.001)。理论上讲,肿瘤增殖指数越高,肿瘤细胞生长越趋密集。因此推测,与Le Bihan提出的PF代表脑血流容积分数的初衷不同[25],本研究eDWI设计下(0~5 000 s/mm2)产生的PF主要反映肿瘤间质内水分子弥散所占的容积分数。这与脑胶质瘤的实际表现相符,LGG往往以肿瘤细胞弥漫浸润水肿,T2 FLAIR高信号为主要表现,而HGG肿瘤细胞增殖明显,强化肿瘤实质内肿瘤细胞高度增殖,T2 FLAIR高信号相对降低。本研究结果与Bisdas等[14]认为PF不与任何DCE-MRI和DSC-MRI来源的血流量和血容量参数相关是一致的,但Bisdas没有进一步证明PF的真实意义。以上证据表明,PF与肿瘤间质水容积分数密切相关,与Dfast及Vp等血流灌注相关参数不直接相关,与Le Bihan提出的PF(f)代表血流容积分数的理论也不一致[31],探测微血管血流的不同b值设计可能是主要原因。
IVIM-MRI (b值范围0~5 000 s/mm2)快弥散参数Dfast与DCE-MRI灌注密切相关,是无需外源性对比剂示踪的识别恶性胶质瘤新生血管的重要潜在标记物。
[1] FEDERAU C, MEULI R, O’BRIEN K, et al. Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI [J]. AJNR Am J Neuroradiol,2014, 35(2): 256-262.
[2] FEDERAU C, MAEDER P, O’BRIEN K, et al. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging [J]. Radiology,2012, 265(3): 874-881.
[3] IIMA M, REYNAUD O, TSURUGIZAWA T, et al. Characterization of glioma microcirculation and tissuefeatures using intravoxel incoherent motion magnetic resonance imaging in a rat brain model [J]. Invest Radiol,2014, 49(7): 485-490.
[4] BERGAMINO M, BONZANO L, LEVRERO F, et al. A review of technical aspects of T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCEMRI) in human brain tumors [J]. Phys Med, 2014, 30(6): 635-643.
[5] KICKINGEREDER P, SAHM F, WIESTLER B, et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation [J]. AJNR Am J Neuroradiol, 2014, 35(8): 1503-1508.
[6] KICKINGEREDER P, WIESTLER B, GRAF M, et al. Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab [J]. J Neurooncol, 2015, 121(2): 373-380.
[7] GAUSTAD J V, SIMONSEN T G, SMISTAD R, et al. Early effects of low dose bevacizumab treatment assessed by magnetic resonance imaging [J]. BMC Cancer, 2015,15(1): 900.
[8] MCDONALD R J, MCDONALD J S, KALLMES D F,et al. Intracranial gadolinium deposition after contrastenhanced MR imaging [J]. Radiology, 2015, 275(3): 772-782.
[9] LE BIHAN D, BRETON E, LALLEMAND D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging [J]. Radiology, 1988,168(2): 497-505.
[10] BISDAS S, KOH T S, RODER C, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: feasibility of the method and initial results [J]. Neuroradiology, 2013, 55(10): 1189-1196.
[11] HU Y C, YAN L F, WU L, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: efficacy in preoperative grading [J]. Sci Rep, 2014, 4: 7208.
[12] BAI Y, LIN Y, TIAN J, et al. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR Imaging [J]. Radiology, 2015,278(2): 496-504.
[13] TOFTS P S, BRIX G, BUCKLEY D L, et al. Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols [J]. J Magn Reson Imaging, 1999, 10(3): 223-232.
[14] BISDAS S, BRAUN C, SKARDELLY M, et al. Correlative assessment of tumor microcirculation using contrast-enhanced perfusion MRI and intravoxel incoherent motion diffusion-weighted MRI: is there a link between them? [J]. NMR Biomed, 2014, 27(10): 1184-1191.
[15] LOUIS D N, OHGAKI H, WIESTLER O D, et al. The 2007 WHO classification of tumours of the central nervous system [J]. Acta Neuropathol, 2007, 114(2): 97-109.
[16] WOO S, LEE J M, YOON J H, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade [J]. Radiology, 2014, 270(3): 758-767.
[17] JIA Z Z, GU H M, ZHOU X J, et al. The assessment of immature microvascular density in brain gliomas with dynamic contrast-enhanced magnetic resonance imaging[J]. Eur J Radiol, 2015, 84(9): 1805-1809.
[18] DAS S, MARSDEN P A. Angiogenesis in glioblastoma [J]. N Engl J Med, 2013, 369(16): 1561-1563.
[19] FALK A, FAHLSTROM M, ROSTRUP E, et al. Discrimination between glioma grades Ⅱ and Ⅲ in suspected low-grade gliomas using dynamic contrastenhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach [J]. Neuroradiology, 2014, 56(12): 1031-1038.
[20] PILUDU F, MARZI S, PACE A, et al. Early biomarkers from dynamic contrast-enhanced magnetic resonance imaging to predict the response to antiangiogenic therapy in high-grade gliomas [J]. Neuroradiology, 2015, 57(12): 1269-1280.
[21] JALALI S, CHUNG C, FOLTZ W, et al. MRI biomarkers identify the differential response of glioblastoma multiforme to anti-angiogenic therapy [J]. Neuro Oncol, 2014, 16(6): 868-879.
[22] LE BIHAN D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure [J]. Radiology, 2013, 268(2): 318-322.
[23] YAN R, HAOPENG P, XIAOYUAN F, et al. Non-Gaussian diffusion MR imaging of glioma: comparisons of multiple diffusion parameters and correlation with histologic grade and MIB-1 (Ki-67 labeling) index [J]. Neuroradiology, 2016, 58(2): 121-132.
[24] GAENS M E, BACKES W H, ROZEL S, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation [J].Radiology, 2013, 266(1): 271-279.
[25] LE BIHAN D, TURNER R. The capillary network: a link between IVIM and classical perfusion [J]. Magn Reson Med, 1992, 27(1):171-178.
[26] AHN C B, LEE S Y, NALCIOGLU O, et al. The effects of random directional distributed flow in nuclear magnetic resonance imaging [J]. Med Phys, 1987, 14(1): 43-48.
[27] HARPEN M D. Comments on “The effects of random directional distributed flow in nuclear magnetic resonance imaging”[J]. Med Phys, 1987, 14(6): 1092-1093.
[28] KWONG K K, MCKINSTRY R C, CHIEN D, et al. CSF-suppressed quantitative single-shot diffusion imaging [J]. Magn Reson Med, 1991, 21(1): 157-163.
[29] YUE W Y, CHEN Z P. Does vasculogenic mimicry exist in astrocytoma? [J]. J Histochem Cytochem, 2005, 53(8): 997-1002.
[30] WIRESTAM R, BORG M, BROCKSTEDT S, et al. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique [J]. Acta Radiol, 2001, 42(2): 123-128.
[31] LE BIHAN D, TURNER R. The capillary network: a link between IVIM and classical perfusion [J]. Magn Reson Med, 1992, 27(1): 171-178.
Correlative assessment of perfusion-related parameters of intravoxel incoherent motion with dynamic contrast enhancement in MR imaging of gliomas
REN Yan1, PANG Haopeng1, DI Ningning1, ZHANG Jiawen1, QIU Tianming2, CHEN Hong3, LIU Hanqiu1, ZHUANG Dongxiao2, ZHANG Yong4, XU Tianyong5, WU Jinsong2, YAO Zhenwei1, FENG Xiaoyuan1(1. Department of Radiology, Huashan Hospital, Fudan University, Shanghai 200040,China; 2. Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai 200040, China; 3. Department of Pathology, Huashan Hospital, Fudan University, Shanghai 200040, China; 4. Department of MR Research, GE Healthcare, Shanghai 201203, China; 5. Department of MR Training, GE Healthcare, Shanghai 201203, China)
Correspondence to: FENG Xiaoyuan E-mail: xyfeng@shmu.edu.cn
Objective: To study the applicable value of 22 b-value (0-5 000 s/mm2) eDWI with biexponential model using the correlative analysis of incoherent intravoxel motion (IVIM) and dynamic contrast enhancement (DCE) in MR imaging of brain gliomas. Methods: For 92 cases with pathologically confirmed gliomas, the combined protocol of conventional MRI, DWI, eDWI,DCE-MRI was performed. The following parameters on tumor regions were measured including eDWI-derived parameters ofDslow, Dfast and PF and DCE-derived parameters of volume transfer constant (Ktrans), extravascular extracellular space volume fraction (Ve), Kep and fraction plasma volume (Vp). According to 2007 central nervous system World Health Organization (WHO)classification, 92 gliomas were classified into groups of high-grade glioma (HGG; grade Ⅲ+Ⅳ) and low-grade glioma (LGG;grade Ⅱ); glioblastoma multiforme (GBM; grade Ⅳ) and other grade glioma (OGG; grade Ⅱ+Ⅲ). Correlative analyses were performed between perfusion-related parameters of Dfast and PF and perfusion parameters of Ktrans, Ve, Vp and Kep. Meanwhile,comparisons of multiple parameters were made for HGG vs. LGG and GBM vs. OGG, as well as receiver operating characteristic(ROC) curve analyses to discriminate HGG from LGG and GBM from OGG. Results: Positive correlations were observed for Dfast with parameters of Ktrans, Ve and Vp with ρ values of 0.396, 0.460 and 0.412 (all P<0.01), respectively, but no correlation between Dfast and Kep. There were negative correlations between PF and parameters of Ktrans, Ve and Vp with ρ values of -0.323 and -0.319,respectively, all P<0.05), and no significant correlation between PF and Kep. Other than Kep, there were significant differences for the remaining parameters between HGG and LGG, between GBM and OGG. ROC analyses showed the maximal area under curve(AUC) values of 0.808 for Ktrans to identify HGG from LGG and 0.802 for Dfast to discriminate GBM from OGG, respectively. Conclusion: There are close correlations between IVIM and DCE in MR imaging of tumor perfusion of brain gliomas. As a potential candidate biomarker, it is hopeful for Dfast to evaluate the characteristics of tumor microvascular perfusion without exogenous contrast tracker.
Intravoxel incoherent motion; Biexponential model; Dynamic contrast enhanced magnetic resonance imaging;Glioma
R445.2
A
1008-617X(2016)03-0223-10
冯晓源 E-mail:xyfeng@shmu.edu.cn
(2016-09-10)

