基于硫酸根自由基的活化过硫酸盐新型高级氧化技术研究新进展
袁 蓁,隋铭皓,袁博杰,王菁宇,秦 捷,许光益
(同济大学环境科学与工程学院,上海 200092)
基于硫酸根自由基的活化过硫酸盐新型高级氧化技术研究新进展
袁蓁,隋铭皓,袁博杰,王菁宇,秦捷,许光益
(同济大学环境科学与工程学院,上海200092)
基于硫酸根自由基的活化过硫酸盐高级氧化技术近年来受到了广泛的关注,硫酸根自由基有较强的氧化能力并且对难降解有机物具有优异的处理效果。主要对现有过硫酸盐活化技术如热活化、UV活化、金属活化的研究进展进行了阐述,并介绍了新型活化技术如电化学活化、超声活化和碳纳米管活化的最新研究成果。目前,活化过硫酸盐技术仍存在活化效率不高、目标污染物矿化程度较低等问题,同时,由于实际水体的背景有机物较为复杂,氧化过程有可能会生成有害的消毒副产物。
过硫酸盐;高级氧化技术;硫酸根自由基;消毒副产物
1 引 言
随着检测技术的提高,经常在水体中检测出难降解、毒性高的污染物,而传统水处理工艺无法有效去除此类难降解有机物[1]。针对此问题,高级氧化技术作为一种高效、无选择性的处理工艺得到了广泛的关注和应用[2]。其中,基于羟基自由基的芬顿法、光—芬顿法、TiO2光催化法、UV/H2O2法和O3/H2O2法等高级氧化技术对去除水中的难降解有机物表现出很高的效率[3-4]。
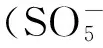
本文将对过硫酸盐活化技术的最新研究进展进行详细的阐述,同时概述多种因素对过硫酸盐新型高级氧化技术处理效果的影响,并提出活化过硫酸盐高级氧化技术应用的现状及问题,以期为相关的研究工作者提供最新的研究热点和方向。
2 过硫酸盐活化方法研究
单过硫酸盐(PMS)的氧化还原电位为1.82 V,过硫酸盐(PS)的氧化电位达到了2.01 V,其本身也具有氧化性[12]。活化技术能激发过硫酸盐产生氧化还原电位更高的硫酸根自由基及其他自由基,从而显著提高氧化能力[14]。
2.1热活化法
通过加热可以使过硫酸盐中的过氧键断裂,1.0 mol的过硫酸盐可以生成2.0mol的硫酸根自由基(如公式(1)所示)[15]。在酸性、中性和碱性条件下的活化能分别为100~116 kJ/mol,119~129 kJ/mol和134~139 kJ/mol[16]。
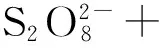
(1)
对于热活化反应,升高温度可以提高活化效率,增加反应速度,有利于目标有机物降解反应的进行。Ghauch和Tuqan发现用热活化过硫酸盐降解β受体阻滞药比索洛尔时,在40 ℃~70 ℃的范围内,降解效率随温度升高而升高[17]。但是,研究发现温度越高也并非一定有利于有机物降解反应的进行。Huang等利用热活化过硫酸盐技术降解59种挥发性有机物,发现其中22种目标物在20 ℃时相较于30 ℃和40 ℃时降解率更高[18]。分析认为,温度的升高可能会加快硫酸根自由基与其他自由基清除剂的反应,从而降低目标污染物的降解效率[19]。
微波加热活化近年来受到了广泛的关注,其相对于传统的热活化方法反应时间更短,并能提高硫酸根自由基的产量,从而提高降解效率,同时微波加热相对于传统加热方法能大量节约能量[20]。Qi等分别运用传统加热方式和微波活化方式活化过硫酸盐降解磺胺甲恶唑。实验结果表明微波活化在90 ℃和130 ℃时,将磺胺甲恶唑全部降解分别只需要4min和16min。而采用传统加热方式,在60 ℃和90 ℃时,在60min的时间内,目标产物仅降解了29.6%和81.4%[21]。由此可见微波活化相对于传统加热活化方法的优势。
2.2UV活化
在UV的照射下,过硫酸盐能发生光解,相对于其他波长,254nm的UV照射由于反应时间较短而得到了最为广泛的应用,其产生硫酸根自由基的原理如反应(2)所示。
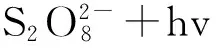
(2)
UV法对过硫酸盐进行活化时,波长和UV剂量是两个关键的影响因素[23]。Liu等在使用UV活化过硫酸盐降解土霉素过程中使用UV254对过硫酸盐进行活化,其发现,在中性条件下,相对于羟基自由基而言,硫酸根自由基占主导地位,并对土霉素的降解贡献最大。同时,其根据反应产物的结构提出了四种不同的土霉素降解机理,分别为羟基化,去甲基化,脱羰和脱水[23]。
理论上,紫外线强度越强,生成硫酸根自由基的速率也就越快[24],Shu等在使用UV活化过硫酸盐处理酸性蓝113时发现当采用高强度的UV时,酸性蓝113的降解效率明显高于使用低强度UV时的效率[24]。
值得注意的是,尽管小试实验结果表明UV/PS氧化效率高,但在使用UV/PS法工程应用中,由于背景有机物种类复杂,普遍对UV有吸收,使得UV难以有效地穿透并活化过硫酸盐,从而降低污染物降解效率[21]。
2.3金属活化
在使用金属对过硫酸盐活化时,金属离子可以为过硫酸盐提供电子,从而激发硫酸根自由基的产生[25],Fe2+、Fe3+、Mn2+、Ni2+、Co2+、Ag+和Cu2+等金属对过硫酸盐的活化能力均见报道[26-27]。Anipsitakis和Dionysiou指出,Co2+对单过硫酸盐活化效果高于其他过渡金属,其活化原理如公式(3)所示:
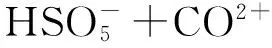
(3)
Fe2+由于价格便宜、毒性较低且催化效率较高而广泛应用于活化过硫酸盐,但值得注意的是,铁离子本身可以作为硫酸根自由基的捕获剂(反应如公式(4)所示)[28-29],因此常采用缓释Fe2+的方法来活化过硫酸盐。

(4)
纳米零价铁因为其活化效率高、价格便宜、对环境污染较小并且能缓释Fe2+的特点得到了广泛的关注和应用[30]。其活化效率较Fe2+高,对降解污染物有更好的促进作用[31]。Song等使用纳米零价铁活化过硫酸盐提升厌氧消化污泥的脱水性能,结果表明,污泥毛细管抽吸时间减少90%,大大提高了脱水效率、降低了经济成本[32]。Diao等使用膨土岩负载纳米零价铁催化过硫酸盐同时去除水中的苯酚和Cr6+,研究发现,在酸性条件下,过硫酸盐更容易分解,苯酚和Cr6+的去除率分别达到了72.3%和99.8%[33]。单一的金属活化容易产生金属的积累和聚集,对环境造成污染,与其他活化方法结合可以减少金属的使用,同时可以有效地提高活化效率和降解效果,因此,将金属活化方法和其他技术相结合逐渐受到重视。
2.4电化学(EC)活化
由于电化学方法具有可循环利用性,环境友好性,并且能在耗费较少投入和运行成本的情况下较高效地降解水中污染物,近年来电化学法活化过硫酸盐作为一种新型的活化技术受到普遍的关注[12]。
以常用的Fe2+为例,传统的Fe2+活化过程中,由于Fe2+快速转化为Fe3+,反应进程很快,导致过硫酸盐利用率较低[34]。同时Fe3+也很难再转化为Fe2+,从而产生大量的金属污泥[29]。使用电化学的方法较好地解决了这一技术难题,其中,Fe2+活化过硫酸盐的反应如公式(5)所示[35]:
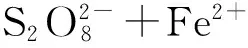
(5)
而通过阴极还原,Fe3+又能转化为Fe2+(如公式(6)所示):
Fe3++e-→Fe2+
(6)
Lin等对比了PMS单独氧化、Fe3+活化PMS和电化学法降解降固醇酸效果,研究发现用PMS单独氧化、Fe3+活化PMS几乎不能降解降固醇酸(2.3%),单独使用电化学法时可以降固醇酸降解率为36.2%;当使用金属-电化学复合法活化过硫酸盐时,降固醇酸降解率达到82.0%[29],并且,增加PMS、Fe3+投加量和加大电流密度能提高降解效果[29]。均相系统中金属的回收需要额外的分离和处理,因此Lin等使用Fe3O4作为催化剂开发了EC/Fe3O4/过二硫酸盐(PDS)非均相系统降解酸性橙7,结果表明,该多相金属氧化物催化复合电化学活化PDS体系实现高效降解有机物的同时也解决了金属回收的问题。60min内酸性橙7完全被降解(酸性橙7初始浓度:25 mg/L;PDS投加浓度:10 mM;Fe3O4投加量:0.8 g/L;电流密度:8.4 mA/cm2,初始pH: 6)[36]。
2.5其他新型活化方法

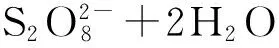
(7)
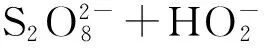
(8)
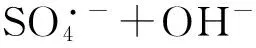
(9)
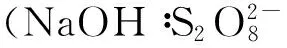
超声活化也是近年兴起的新型过硫酸盐活化技术,通过超声活化,过硫酸盐和水产生硫酸根自由基和羟基自由基(如公式(10)和公式(11)所示),自由基和氧化物进而降解污染物[40-41]。
(10)
H2O+)))→OH·+H·
(11)
Wang等用超声活化过硫酸盐时发现,该法降解卡马西平的效率相对于单独超声或者单独用过硫酸盐方法效率提升了1倍[40]。研究表明提高过硫酸盐的投加量、超声能量和温度及酸性条件下有利于有机物的降解[40]。超声与其他活化技术如零价铁[42]、热[41~43]非均相催化[44]复合技术也可有效地提高对PMS的活性效率和有机物的降解能力。
碳纳米管近年来在活化过硫酸盐技术的发展中起到了越来越重要的作用。通过用不同种类的酸、臭氧、热能或者负载金属等对碳纳米管进行前处理强化碳纳米管的电化学活性从而提高碳纳米管电子转移的能力,进而实现高效活化PMS[45]。
3 UV活化过硫酸盐氧化消毒副产物研究
活化过硫酸盐技术在对难降解有机物的去除方面有许多其他技术难以比拟的优势,但目前的研究表明其应用仍存一些问题和挑战。若要将此技术运用到实际水处理中,必须考虑其氧化产物在其他工艺中的变化途径,以确保该技术与其他水处理工艺结合时的可行性。大量文献对于活化过硫酸盐目标有机物的转化进行了研究,而对该过程中,尤其是UV活化过硫酸盐中消毒副产物的报道鲜见分析。本文即对UV活化过硫酸盐中消毒副产物的生成研究进展进行阐述。
Chu等研究了一种常用的抗生素氯霉素在经UV/PS氧化后消毒过程中的转化途径。其发现在对饮用水进行消毒前,使用UV/PS对氯霉素进行氧化会生成许多消毒副产物,如一氯甲烷、二氯甲烷、和三氯甲烷,尤其是使用较低剂量的UV且不进行后加氯时,氯代甲烷的产生量更大,而这种方法恰好在饮用水处理厂中使用广泛[46]。因此极有可能产生有害物质。类似的,Fang等提出了溴化物和硫酸根自由基反应生成致癌物溴酸盐的反应途径(如公式(12)~公式(13)所示):

(12)
Br-+Br·→…→HOBr/OBr-
(13)
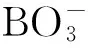
由此可见,活化过硫酸盐方法虽然降解污染物的效果令人满意,但是其过程中可能产生的有毒有害物质仍然不能被忽视,并且更应作为采用该技术时的一个重要因素予以考量。
4 结论与展望
硫酸根自由基由于其极强的氧化性能而对许多难降解有机物表现出非常优异的处理效果,与此同时,活化过硫酸盐技术在过去的十多年中得到了飞速的发展。热能活化相较于微波活化、超声活化耗能较大,设备运行维护成本也较高;金属活化效率虽高,却容易产生金属污泥等污染问题,电化学活化技术由于能重复利用过渡金属而相对较环保、并具备一定的可持续性。显而易见,过硫酸盐活法方法发展趋向于高效性、节能性和绿色性,目标是在能有效活化过硫酸盐的前提下能尽量减少能耗并减少对环境生态的污染。一方面,应当继续探索新的高效活化方法,另一方面应加强现有成熟技术的改进和综合应用。
值得注意的是,研究结果表明活化过硫酸盐技术在处理污染物的同时可能产生毒性更大的副产物,因此,需要将活化过硫酸盐高级氧化技术与前处理技术和后续处理工艺相结合进行综合考量和评估,减少有毒物质前体物的生成,从而抑制有毒有害物质产生。提高活化过硫酸盐高级氧化技术的效率并最大限度地减少有毒副产物生成也将是日后研究的一个重要发展方向。
[1]Ganiyu S O, Van Hullebusch E D, Cretin M, Esposito G, Oturan M A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review [J].Separation and Purification Technology,2015,156, Part 3:891-914.
[2]Rosenfeldt E J,Linden K G, Canonica S, von Gunten U. Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2and UV/H2O2[J].Water Research,2006,40:3695-3704.
[3]Zhang Y,Zhuang Y, Geng J, Ren H, Xu K, Ding L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes [J].Science of The Total Environment,2016,550:184-191.
[4]Lee C, Yoon J, Von Gunten U. Oxidative degradation of N-nitrosodimethylamine by conventional ozonation and the advanced oxidation process ozone/hydrogen peroxide [J].Water Research,2007,41:581-590.
[5]Yang Y, Jiang J, Lu X, Ma J, Liu Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process [J].Environmental Science & Technology,2015,49:7330-7339.
[6]Yang Y, Guo H, Zhang Y, Deng Q, Zhang J. Degradation of Bisphenol A Using Ozone/Persulfate Process: Kinetics and Mechanism [J].Water, Air, & Soil Pollution,2016,227:1-12.
[7]Neta P, Huie R E, Ross A B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution [J].Journal of Physical and Chemical Reference Data,1988,17:1027-1284.
[9]Cong J,Wen G, Huang T, Deng L, Ma J. Study on enhanced ozonation degradation of para-chlorobenzoic acid by peroxymonosulfate in aqueous solution [J].Chemical Engineering Journal,2015,264:399-403.
[10]Lutze H V, Bircher S, Rapp I, Kerlin N, Bakkour R, Geisler M, von Sonntag C, Schmidt T C. Degradation of Chlorotriazine Pesticides by Sulfate Radicals and the Influence of Organic Matter [J].Environmental Science & Technology,2015,49:1673-1680.
[11]Park S, Lee L S, Medina V F, Zull A, Waisner S. Heat-activated persulfate oxidation of PFOA, 6:2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation [J].Chemosphere,2016,145:376-383.
[12]Govindan K, Raja M, Noel M,James E J. Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide [J].Journal of Hazardous Materials,2014,272:42-51.
[13]Lutze H V, Bakkour R, Kerlin N, von Sonntag C, Schmidt T C. Formation of bromate in sulfate radical based oxidation: Mechanistic aspects and suppression by dissolved organic matter [J].Water Research,2014,53:370-377.
[14]Guan Y H, Ma J,Li X C, Fang J Y,Chen L W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System [J].Environmental Science & Technology,2011,45:9308-9314.
[15]Waldemer R H,Tratnyek P G,Johnson R L,Nurmi J T. Oxidation of Chlorinated Ethenes by Heat-Activated Persulfate:? Kinetics and Products [J].Environmental Science & Technology,2007,41:1010-1015.
[16]MatzekL W,Carter K E. Activated persulfate for organic chemical degradation: A review [J].Chemosphere,2016,151:178-188.
[17]Ghauch A,Tuqan A M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products [J].Chemical Engineering Journal,2012,183:162-171.
[18]HuangK C,Zhao Z,Hoag G E,Dahmani A,Block P A. Degradation of volatile organic compounds with thermally activated persulfate oxidation [J].Chemosphere,2005,61:551-560.
[19]Drzewicz P, Perez-Estrada L, Alpatova A,Martin J W, Gamal El-Din M. Impact of Peroxydisulfate in the Presence of Zero Valent Iron on the Oxidation of Cyclohexanoic Acid and Naphthenic Acids from Oil Sands Process-Affected Water [J].Environmental Science & Technology,2012,46:8984-8991.
[20]Qi C, Liu X, Zhao W, Lin C, Ma J, Shi W, Sun Q, Xiao H. Degradation and dechlorination of pentachlorophenol by microwave-activated persulfate [J].Environmental Science and Pollution Research,2015,22:4670-4679.
[21]QiC, Liu X, Lin C, Zhang X, Ma J, Tan H, Ye W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity [J].Chemical Engineering Journal,2014,249:6-14.
[22]Zhang R, Sun P, T.H. Boyer, L. Zhao, C.-H. Huang, Degradation of Pharmaceuticals and Metabolite in Synthetic Human Urine by UV, UV/H2O2, and UV/PDS [J].Environmental Science & Technology,2015,49:3056-3066.
[23]Liu Y, He X, Fu Y, Dionysiou D D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate [J].Journal of Hazardous Materials,2016,305:229-239.
[24]Shu H Y, Chang M C, Huang S W. UV irradiation catalyzed persulfate advanced oxidation process for decolorization of Acid Blue 113 wastewater [J].Desalination and Water Treatment,2015,54:1013-1021.
[25]Pardo F, Santos A, Romero A. Fate of iron and polycyclic aromatic hydrocarbons during the remediation of a contaminated soil using iron-activated persulfate: A column study [J].Science of The Total Environment,2016,566-567:480-488.
[26]Liang H Y, Zhang Y Q, Huang S B, Hussain I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate [J].Chemical Engineering Journal,2013,218:384-391.
[27]Zhang B T, Zhang Y, Teng Y, Fan M. Sulfate Radical and Its Application in Decontamination Technologies [J].Critical Reviews in Environmental Science and Technology,2015,45:1756-1800.
[28]Romero A, Santos A, Vicente F, González C. Diuron abatement using activated persulphate: Effect of pH, Fe(Ⅱ) and oxidant dosage [J].Chemical Engineering Journal,2010,162:257-265.
[29]Lin H, Wu J, Zhang H. Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process [J].Chemical Engineering Journal,2014,244:514-521.
[30]Rodriguez S, Vasquez L, Costa D, Romero A, Santos A. Oxidation of Orange G by persulfate activated by Fe(Ⅱ), Fe(Ⅲ) and zero valent iron (ZVI) [J].Chemosphere,2014,101:86-92.
[31]Kusic H, Peternel I, Koprivanac N, Loncaric Bozic A. Iron-Activated Persulfate Oxidation of an Azo Dye in Model Wastewater: Influence of Iron Activator Type on Process Optimization [J].Journal of Environmental Engineering,2010,137:454-463.
[32]Song K, Zhou X, Liu Y, Xie G J, et al. Improving dewaterability of anaerobically digested sludge by combination of persulfate and zero valent iron [J].Chemical Engineering Journal,2016,295:436-442.
[33]DiaoZ H, Xu X R, Chen H, et al. Simultaneous removal of Cr(VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism [J].Journal of Hazardous Materials,2016,316:186-193.
[34]Hussain I, Zhang Y, Huang S. Degradation of aniline with zero-valent iron as an activator of persulfate in aqueous solution [J].RSC Advances,2014,4:3502-3511.
[35]Cai C,Zhang H,Zhong X, Hou L. Electrochemical enhanced heterogeneous activation of peroxydisulfate by Fe-Co/SBA-15 catalyst for the degradation of Orange II in water [J].Water Research,2014,66:473-485.
[36]Lin H, Zhang H, Hou L. Degradation of C. I. Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process [J].Journal of Hazardous Materials,2014,276:182-191.
[37]Qi C, Liu X, Ma J, Lin C, Li X, Zhang H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants [J].Chemosphere,2016,151:280-288.
[38]FurmanO S, Teel A L, Watts R J. Mechanism of Base Activation of Persulfate [J].Environmental Science & Technology,2010,44:6423-6428.
[39]Marchesi M, Thomson N R, Aravena R, Sra K S,Otero N, Soler A. Carbon isotope fractionation of 1,1,1-trichloroethane during base-catalyzed persulfate treatment [J].Journal of Hazardous Materials,2013,260:61-66.
[40]Wang S, Zhou N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process [J].Ultrasonics Sonochemistry,2016,29:156-162.
[41]Weng C H, Tsai K L. Ultrasound and heat enhanced persulfate oxidation activated with Fe0aggregate for the decolorization of C.I. Direct Red 23 [J].Ultrasonics Sonochemistry,2016,29:11-18.
[42]Wang X,Wang L, Li J, Qiu J, Cai C, Zhang H. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation [J].Separation and Purification Technology,2014,122:41-46.
[43]Deng D, Lin X, Ou J, et al. Efficient chemical oxidation of high levels of soil-sorbed phenanthrene by ultrasound induced, thermally activated persulfate [J].Chemical Engineering Journal,2015,265:176-183.
[44]Cai C, Zhang H, Zhong X, Hou L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange Ⅱ in water [J].Journal of Hazardous Materials,2015,283:70-79.
[45]Cheng X, Guo H, Zhang Y, Liu Y, Liu H, Yang Y. Oxidation of 2,4-dichlorophenol by non-radical mechanism using persulfate activated by Fe/S modified carbon nanotubes [J].Journal of Colloid and Interface Science,2016,469:277-286.
[46]Wenhai C, Tengfei C, Erdeng D, Deng Y, Yingqing G, Naiyun G. Increased formation of halomethanes during chlorination of chloramphenicol in drinking water by UV irradiation, persulfate oxidation, and combined UV/persulfate pre-treatments [J].Ecotoxicology and Environmental Safety,2016,124:147-154.
[47]Fang J Y, Shang C. Bromate Formation from Bromide Oxidation by the UV/Persulfate Process [J].Environmental Science & Technology,2012,46:8976-8983.
Research Progress of Novel Sulfate Radical-Based Advanced Oxidation Process Using Activated Persulfate
YUAN Zhen, SUI Ming-hao, YUAN Bo-jie, WANG Jing-yu, QIN Jie, XU Guang-yi
(CollegeofEnvironmentalScience&Engineering,TongjiUniversity,Shanghai200092,China)
The novel sulfate radical-based advanced oxidation process using activated persulfate has taken extensive attention for its extraordinary efficiency of degradation of recalcitrant organics. This paper introduced current persulfate activation methods like heat activation, UV activation and metal activation, as well as emerging methods including electrochemical activation, ultrasonic activation and carbon nanotube activation. As for the activated persulfate technique, the relatively low activation rate and mineralization efficiency of model compounds remained to be improved at present. Meanwhile, due to the complex background organics, it was likely to generate poisonous disinfection by-products during the persulfate oxidation process.
Persulfate; advanced oxidation process; sulfate radicle; disinfection by-product
2016-06-28
袁蓁(1992-),男,湖南株洲人,同济大学市政工程专业2015级在读硕士,研究方向为饮用水深度处理技术。
X703
A
1001-3644(2016)05-0142-05

