不同产圈模式对母猪繁殖性能及应激水平的影响
张校军,王占彬,鲍伟光,,高乾坤,万熙卿,顾宪红*,郝 月,崔艳军
(1.河南科技大学动物科技学院,洛阳 471003; 2.中国农业科学院北京畜牧兽医研究所,动物营养学国家重点实验室,北京 100193;3.北京清泉湾养猪有限公司,北京 102104)
不同产圈模式对母猪繁殖性能及应激水平的影响
张校军1,王占彬1,鲍伟光1,2,高乾坤1,万熙卿3,顾宪红1*,郝月2,崔艳军2
(1.河南科技大学动物科技学院,洛阳 471003; 2.中国农业科学院北京畜牧兽医研究所,动物营养学国家重点实验室,北京 100193;3.北京清泉湾养猪有限公司,北京 102104)
旨在研究不同产圈对母猪繁殖性能及应激水平的影响,以期探究一种能在实际生产中推广应用的福利友好型哺乳母猪饲养模式。选取胎次相同,妊娠期、体况相近的24头二元杂交母猪(大白×长白),随机分为3个处理组,即限位栏产圈+高床组(Farrowing crate+high bed group,FCB,n=8)、自由产圈+高床组(Freedom farrowing pen+high bed group,FFPB,n=8)、自由产圈+部分发酵床地面组(Freedom farrowing pen+partially fermented bed surface group,FFPF,n=8)。在母猪预产期的前7 d将其转入不同的产圈,分娩后第21天仔猪断奶。结果显示:1)FFPB、FFPF母猪分娩时长显著低于FCB(P<0.05),FFPB母猪分娩间隔显著低于FCB(P<0.05);FFPB母猪血液催产素(Oxytocin,OT)及催乳素(Prolactin,PRL)含量有升高的趋势(P<0.10)。2)在母猪转入产房后第2天,FFPB和FFPF母猪腰部体表温度显著低于FCB(P<0.05)。在分娩后第7天,FFPB与FFPF母猪唾液α-淀粉酶(α-amylase,AMY)含量显著低于FCB(P<0.05)。在分娩后第14天,FFPF母猪唾液AMY含量显著低于FCB(P<0.05),FCB母猪唾液皮质醇(Cortisol,COR)有明显升高的趋势。结果表明:在分娩当天,自由产圈母猪血液繁殖激素含量升高,产程及分娩间隔时间减少;分娩后的1~2周内,自由产圈母猪应激水平明显降低,更接近福利友好型哺乳母猪饲养模式。
产圈;母猪;繁殖性能;应激
随着中国畜牧业的快速发展,规模化、集约化程度越来越高,在集约化生产过程中,猪场管理者过分的追求效益,使得各个阶段的猪只饲养空间不足、生存环境贫瘠恶劣,造成猪只体质下降、免疫力降低,从而导致发病率和死亡率增加[1]。妊娠母猪分娩环境对母猪和仔猪的生产性能及应激都会产生不同程度的影响[2-4]。目前,母猪产圈一般分为两种模式,一种为限位栏式产圈,另一种为自由式产圈,规模化猪场产房多采用限位栏饲养模式[5],由于限位栏空间小,母猪在其中运动量不足,造成母猪抵抗力下降;还可能会导致母猪分娩时间变长,母性变差,仔猪死胎数增加[6-9]。与分娩限位栏相比,自由式分娩猪栏为母猪提供了充足的运动空间,母性行为得到充分显示,可能会表现出良好的繁殖性能。但也有其他研究发现,限位栏模式的产圈与其他模式产圈对母猪繁殖性能的影响没有显著差异[10-11]。此外,关于不同产圈模式对哺乳仔猪压死率的影响也存在争议。顾招兵等[12]研究发现,自由式产圈较限位栏产圈的仔猪压死率更低。J.Hales等[13]得出相反的结论。在母猪转入产房后,由于环境的改变,其机体会发生一系列内分泌的变化。此外,由内分泌引起的应激水平的变化可能会影响母猪的分娩进程。处于应激状态的母猪对仔猪的压死率更高[14]。有研究表明,与自由产圈相比,限位栏产圈中的母猪做窝等正常行为得不到满足,皮质醇(Cortisol,COR)含量升高,产生更强烈的生理应激[15-16]。
本研究以妊娠后期二元杂交母猪为试验动物,研究不同产圈对母猪繁殖性能、应激水平的影响,以期探究一种能在实际生产中推广应用的福利友好型哺乳母猪饲养模式。
1 材料与方法
1.1试验动物饲养管理
本试验于2015年8月-10月在北京清泉湾养猪有限公司有机猪养殖基地完成。采用单因子试验设计,选取胎次、妊娠期相同、体况相近的二元杂交母猪(大白×长白)24头,随机分配到3个处理组,每个处理设置8个重复。设置3个产圈处理组,即限位栏产圈+高床组(Farrowing crate+high bed group,FCB)、自由产圈+高床组(Freedom farrowing pen+high bed group,FFPB)、自由产圈+部分发酵床地面组(Freedom farrowing pen+partially fermented bed surface group,FFPF)。母猪在妊娠期前7 d转入不同的产圈。在分娩前7 d内每天给母猪定量供应3.5 kg饲料,分娩后第1周根据母猪产后恢复情况供应饲料0.0~4.5 kg,从第2周开始每5~7 d增加0.5 kg饲料。母猪自由饮水。分娩后第21天下午18:00仔猪断奶。免疫程序参照北京清泉湾养猪有限公司的哺乳母猪养殖制度。
1.2产圈设计
FCB:产圈长×宽×高=2.35 m×1.75 m×0.9 m,限位栏长×宽×高=2.3 m×0.6 m×1.1 m,床高0.4 m。FFPB:产圈长×宽×高=2.35 m×1.75 m×0.9 m,产圈内壁两侧安置有仔猪防压架,防压架距围栏侧壁0.2 m、距地面高0.3 m,床高0.4 m。FFPF:产圈(除发酵床以外的地面)长×宽×高=1.75 m × 1.75 m × 0.9 m,发酵床长×宽=0.6 m × 1.75 m,产圈内壁两侧安置有仔猪防压架,防压架距围栏侧壁0.2 m、距地面高0.3 m,母猪分娩当天~分娩后的第5天,在发酵床上方横置一根钢管,在此期间,不让母猪在发酵床上躺卧。
1.3繁殖性能的测定
1.3.1繁殖性能在转入当天、分娩当天及分娩后第7、21天用背膘仪测量母猪最后一根肋骨处距背中线6.5 cm处的背膘厚度,即P2点背膘厚度。分娩时用秒表记录分娩总时长、每头仔猪娩出时间间隔;记录产仔猪数、产活仔猪数、死胎数及整个哺乳期仔猪压死率;每天下午18:00称料,记录母猪日采食量。
1.3.2母猪血浆催产素及催乳素水平在母猪分娩当天用耳缘静脉采血方法采集母猪血液。采集方法:利用母猪保定器保定,用酒精棉球消毒耳部皮肤后,左手压住耳朵,使耳缘静脉怒张,右手取5 mL负压采血管,针头沿血管平行方向刺入血管采血,取血后拇指压迫止血(采血时间较短,最大程度的减少对猪的应激)。采血后离心机离心10 min(3 000 r·min-1),然后将分离出的血浆-20 ℃冷冻保存,待用酶联免疫吸附法(ELISA)测定催产素(Oxytocin,OT)(上海鑫乐生物科技有限公司试剂盒)、催乳素(Prolactin,PRL)(上海鑫乐生物科技有限公司试剂盒)含量。每个处理采集8个样品。
1.4应激水平的测定
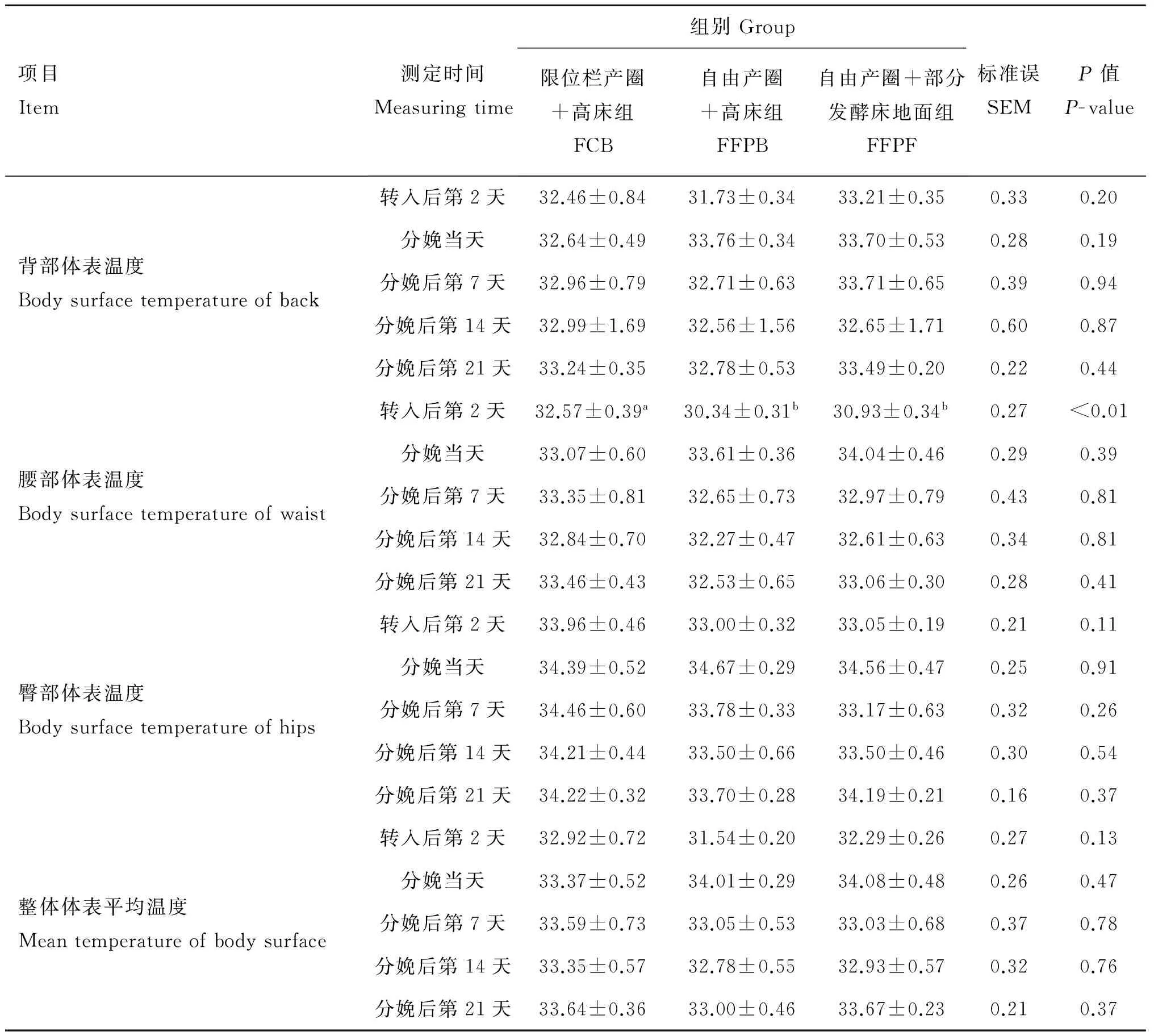
1.4.1体表温度在转入产圈后第2天、分娩当天及分娩后7、14、21 d的上午09:00及下午03:00用红外测温仪对母猪的背部(肩胛处)、腹部(脊椎胸腰段结合部,距背中线20 cm左右处)、臀部(尾根左侧20~25 cm处)进行体表温度测定,同一部位3次重复(使用平均值)。仪器距母猪体表的测定距离为15~20 cm。
1.4.2母猪唾液应激激素和应激蛋白水平母猪在转入产圈后第2天、分娩当天及分娩后7、14、21 d的上午05:30-08:30采用唾液采集装置(用铁丝绑上纱布条)无抓捕采集母猪唾液。采样时,将唾液采集器悬挂于圈栏上方,供母猪(仔猪)自由咀嚼,当纱布湿后剪下,放于20 mL注射器中挤出唾液,置于离心管,离心5 min(4 000 r·min-1)取上清液,-20 ℃冷冻保存,待用放射免疫法测定COR(北京华英生物技术研究所试剂盒)、C-反应蛋白(C-reactive protein,CRP)(北京华英生物技术研究所试剂盒)含量,碘—淀粉比色法测定α-淀粉酶(α-amylase,AMY)(南京建成生物工程研究所试剂盒)含量。每个处理采集8个样品。
1.5数据分析
所有数据均采用Excel进行整理,采用SPSS 17.0统计软件进行统计处理单因素方差分析,邓肯法进行组间差异显著性检验,P<0.05表示差异显著,0.05 2.1不同产圈对母猪繁殖性能的影响 不同产圈对母猪繁殖性能的影响结果列于表1。由表1可知,母猪的产仔猪数、产活仔猪数、死胎率、仔猪出生窝重及仔猪在整个哺乳期的压死率差异不显著(P>0.05)。FFPB和FFPF母猪的分娩时长显著低于FCB(P<0.05),FFPB母猪分娩间隔显著低于FCB和FFPF(P<0.05)。在转入产房至分娩后第21天,各个时期母猪的掉膘量差异不显著(P>0.05)。 不同产圈对母猪繁殖激素的影响结果列于表2。由表2可知,在分娩当天,各组间母猪血液OT及PRL的含量差异不显著(P>0.05),但FFPB母猪血液OT及PRL的含量有升高的趋势(P<0.10)。 2.2不同产圈对母猪体表温度的影响 不同产圈对母猪体表温度的影响列于表3。由表3可知,在母猪转入产房后第2天,FFPB和FFPF母猪腰部体表温度显著低于FCB(P<0.05),其他部位及整体体表平均温度差异不显著(P>0.05)。其他时期各组间母猪体表平均温度差异不显著(P>0.05)。 2.3不同产圈对母猪应激激素及蛋白的影响 不同产圈对母猪唾液COR、CRP、AMY含量的影响列于表4。由表4可知,在转入产圈后第2天,各组间母猪唾液COR、CRP、AMY含量差异不显著(P>0.05)。在分娩后第2天,母猪唾液COR、CRP、AMY含量差异不显著(P>0.05)。在分娩后第7天,FFPB与FFPF母猪唾液AMY含量显著低于FCB(P<0.05),唾液COR、CRP含量差异不显著(P>0.05)。在分娩后第14天,FFPF母猪唾液AMY含量显著低于FCB(P<0.05),各组间COR、CRP含量差异不显著(P>0.05),但FCB母猪唾液COR有升高的趋势(P<0.10)。在分娩后第21天,母猪唾液COR、CRP、AMY含量差异不显著(P>0.05)。 表1产圈对母猪繁殖性能的影响 Table1Effect of farrowing pens on reproductive performance of sows 项目Item限位栏产圈+高床组FCB自由产圈+高床组FFPB自由产圈+部分发酵床地面组FFPF标准误SEMP值P-value产仔猪数Littersize10.50±0.9411.43±0.729.57±0.650.470.30产活仔猪数Livelittersize9.75±0.8010.29±0.788.71±0.680.440.37死胎率/%Stillbirthrate6.77±2.7110.08±4.049.42±1.860.020.70出生窝重/kgBirthweightoflitter14.96±0.8416.06±1.2713.34±0.940.610.20仔猪压死率/%Crushingmortality5.73±3.077.93±3.3111.07±5.672.300.65分娩时长/minDurationoffarrowing200.75±9.11a160.29±9.47b174.29±6.19b5.96<0.01分娩间隔/minAverageinterval19.71±1.11a14.42±1.37b18.68±1.27a0.840.02母猪平均日采食量/(g·d-1)Averagefeedintakeofsows674.11±30.514596.94±56.984556.12±121.7043.270.54转入产房当天~分娩当天母猪掉膘量/mmThedayoftransferredtofarrowingroomstothedayoffarrowing,loseweightofsows0.88±0.481.29±0.491.14±0.400.220.75转入产房当天~分娩后第7天母猪掉膘量/mmThedayoftransferredtofarrowingroomsto7daysafterfarrowing,loseweightofsows3.00±0.423.29±0.472.29±0.810.340.50转入产房当天~分娩后第21天母猪掉膘量/mmThedayoftransferredtofarrowingroomstotheweaningday,loseweightofsows4.00±0.635.00±0.654.29±1.430.550.77 同行数据后所标字母相异表示差异显著(P<0.05),所标字母相同表示差异不显著(P>0.05)。转入当天~分娩前1 d,各试验组n=8;分娩当天~分娩后第21天,FCB(n=8)、FFPB(n=7)、FFPF(n=7)。下同 Different letters within the same row means significant difference (P<0.05),same letters within the same row means not significant difference (P>0.05).The day of transferred to farrowing rooms to 1 day before farrowing,all the groups:n=8;the day of farrowing to the weaning day,FCB(n=8),FFPB(n=7),FFPF(n=7).The same as below 表2产圈对母猪繁殖激素的影响 Table 2Effect of farrowing pens on reproductive hormones of sows 项目Item测定时间Measuringtime组别Group限位栏产圈+高床组FCB自由产圈+高床组FFPB自由产圈+部分发酵床地面组FFPF标准误SEMP值P-value催产素/(ng·mL-1)OT分娩当天158.85±8.15201.44±17.10194.59±12.848.180.06催乳素/(ng·mL-1)PRL分娩当天95.90±4.70126.37±17.7391.61±7.826.920.08 表3产圈对母猪不同部位体表平均温度的影响 Table 3Effect of farrowing pens on reproductive hormones of sows ℃ 3.1产圈对母猪繁殖性能的影响 本研究发现,产圈对母猪产仔数、产活仔数、死胎率、仔猪出生窝重、断奶前仔猪压死率无显著性差异。关于不同产圈对仔猪压死率的影响,报道不一致。M.Melisova等[17]研究表明,在限位栏中母猪由于不能自由活动,能显著降低仔猪的压死率。K.L.Chidgey等[4]也得出相同的结论。但Z.B.Gu等[18]研究发现,自由分娩栏仔猪压死率与限位分娩栏仔猪压死率无显著性差异。A.L.Kilbride等[19]也报道称,不同产圈系统对仔猪的压死率无显著性差异,与本试验结果一致。通过上述报道发现,仔猪的压死率和产圈内合理的防压设施的设计有关联。 不同产圈对母猪分娩时长和分娩间隔都有影响。有研究表明,母猪的平均分娩时长为156~262 min,母猪的平均分娩间隔为15.2~22.4 min[20-22]。与本试验结果类似:FFPB与FFPF母猪分娩时长显著降低,FFPB母猪分娩间隔显著降低。自由产圈改善了母猪的繁殖性能,有助于提高母猪的福利。 表4产圈对母猪唾液应激激素及蛋白的影响 Table 4Effect of farrowing pens on saliva stress hormones and proteins of sows 项目Item测定时间Measuringtime组别Group限位栏产圈+高床组FCB自由产圈+高床组FFPB自由产圈+部分发酵床地面组FFPF标准误SEMP值P-value皮质醇/(ng·mL-1)COR转入后第2天21.38±1.2018.73±3.2314.72±1.261.300.11分娩后第2天29.93±5.4925.36±4.2123.99±3.092.540.62分娩后第7天31.95±8.0218.14±1.8719.45±2.433.250.15分娩后第14天23.99±4.1120.36±2.1814.69±0.951.810.09分娩后第21天17.70±1.6818.45±3.4013.32±1.981.410.30C-反应蛋白/(mg·mL-1)CRP转入后第2天1.47±0.151.50±0.181.52±0.130.090.81分娩后第2天1.59±0.341.76±0.151.72±0.280.160.92分娩后第7天1.48±0.201.02±0.131.58±0.250.120.15分娩后第14天2.30±0.371.57±0.222.00±0.510.220.42分娩后第21天1.59±0.361.75±0.501.69±0.320.230.45α-淀粉酶/(U·dL-1)AMY转入后第2天253.28±49.81215.80±56.91234.92±54.4729.860.89分娩后第2天461.07±55.91365.15±58.19312.39±89.0339.920.31分娩后第7天416.82±82.24a204.56±60.32b202.00±46.23b42.980.04分娩后第14天505.81±63.77a399.16±58.11ab280.91±86.24b43.410.01分娩后第21天420.91±86.91326.49±67.77358.58±69.8943.040.67 同为自由产圈的FFPF母猪分娩间隔显著高于FFPB,可能是由于在发酵床上放置了钢管,母猪在分娩时活动面积变小,正常的行为无法表达,延长了分娩间隔。 母猪背膘厚度对发情间隔及下一胎繁殖性能会产生影响[23-24]。然而本研究发现,在哺乳期,母猪掉膘量差异不显著,就此不同产圈对各组间母猪发情间隔及下一胎的繁殖性能可能不会产生明显影响。在母猪被转入产房~断奶前的各个时期,产圈对母猪的掉膘量及采食量都无显著影响。但在母猪分娩当天~分娩后第7天这一段时间内掉膘量最大,这可能是由于母猪分娩后采食量下降,又需要给仔猪提供乳汁,导致母猪体重急剧下降。 哺乳动物从孕期~分娩前期,需要给胎儿提供营养,生产后哺乳和保护幼儿,体内一系列的激素会发生很大的变化。OT是哺乳动物分娩和泌乳中起神经调节作用的激素,在室旁核(Paraventricular nucleus,PVN)与视上核(Supraoptic nucleus,SON)合成释放[25]。起着调节子宫收缩、催产且缩短产程、促进乳液分泌等的作用[26]。OT的分泌可能和母猪的分娩时长和间隔相关联。C.Oliviero等[27]研究发现,与自由产圈相比,在限位栏产圈中分娩的母猪血液OT含量显著降低,产程及分娩间隔都显著升高。但A.B.Lawrence等[8]报道,在分娩过程的3~4 h,限位栏产圈中母猪血液OT含量显著降低,但产程无显著性差异。本试验发现,FFPB母猪血液OT含量有明显升高的趋势,与之相对应FFPB与FFPF组母猪分娩时长显著降低,FFPB母猪分娩间隔显著降低。OT的分泌常与舒适性事件相关联,已被用作评价动物福利好的指标[28-30]。比如,仔猪的吸吮刺激母猪乳头,引起母猪OT的分泌[31]。S.Chen等[32]研究发现,自然哺乳的小牛的血液OT含量显著地高于人工哺乳组的小牛。M.Kurosawa等[33]报道,抚摸(按摩式)老鼠背部引起血压降低,而大脑注射OT能减缓这一效果。综上所述,自由产圈OT含量明显升高,表明自由产圈更有助于改善母猪的福利水平,更接近友好型哺乳饲养模式。PRL在母性行为,季节性繁殖,分娩以及应激上起着重要的作用[34]。A.A.Valros等[35]研究发现,PRL能激发母猪的母性行为,可能对降低仔猪的压死率起到一定的积极作用。此外,PRL还能刺激乳汁分泌,泌乳能力增强,对其仔猪的生长性能也会产生有益的影响。本研究并没有探索PRL对仔猪生长性能的影响,但FFPB母猪PRL含量较FFPF母猪有升高的趋势,可能是由于在分娩当天,发酵床上放置有铁管,母猪活动面积减少,心理应激水平升高,减低了催乳素的分泌。可以推断FFPB模式促进繁殖激素的分泌,有助于提高生产,更接近友好型哺乳饲养模式。经分析,母猪血浆PRL与分娩后第2天的唾液COR、CRP及AMY无显著相关性,说明母猪应激激素及蛋白对PRL没有产生影响。 3.2产圈对母猪应激水平的影响 3.2.1体表温度动物体温反映其机体的代谢水平,当动物处于恐惧状态时,糖代谢加快,体温升高,摄入的能量用于抵抗外界环境刺激[36]。处于应激状态的动物,其体表温度会升高[37-38]。本试验发现,在转入产房后的第2天,FFPB与FFPF组母猪腰部体表温度显著低于FCB,说明FCB母猪在刚入产房时可能不太适应产圈环境,发生一定程度的应激,而自由产圈更降低了母猪的应激,有助于提高福利水平。除转入产房后第2天外,其他时期母猪各部位及整体体表平均温度无显著性差异,可能是由于母猪逐渐适应产圈环境,产生的应激程度较小,没有引起体表温度的明显变化。而其他部位及整体体表平均温度差异不显著,并且具有显著性差异的体表温度只在腰部部位表现出来,表明腰部体温更适于评估动物的应激水平。 3.2.2应激激素及蛋白K.O’Driscoll等[39]研究发现,下丘脑-垂体-肾上腺(HPA)轴的活跃程度能作为评价动物应激水平的一个方法,而且COR是HPA轴兴奋程度的标记物。P.Katja等[40]报道,动物机体COR的含量可以有效反映动物的应激程度。动物处于应激状态时,COR含量升高[41]。母猪转入产房后,产圈环境会导致母猪产生应激[42]。A.B.Lawrence等[8]研究表明,与自由产圈相比,限位栏产圈的母猪COR含量更高,引起的生理应激强度更大。C.Oliviero等[27]也得出一致的结果。此外,用唾液COR代替血浆COR的检测动物应激的方法已被广泛应用[43]。这种方法采样时对动物的刺激更小。本试验发现,在转入当天~分娩后的一周内,各组间母猪唾液COR含量无显著性差异。在分娩后的第14天,FCB母猪唾液COR含量有明显升高的趋势,可能是由于限位栏中的母猪体况恢复较差,应激程度的降低小于其他组。而在分娩后第21天,各组间母猪唾液COR含量无显著性差异,可能是由于各组母猪已经适应自身所处的环境,环境导致的应激程度没有表现出显著的差异。 H.Murata等[44]报道,动物处于感染、炎症或应激状态时,机体血清CRP含量升高。W.Burger等[45]也研究发现,CRP可以作为评价动物健康、应激水平的参数。本试验发现,在母猪转入产房~分娩后第21天的各个时期,各组间母猪唾液CRP含量差异都不显著。可能是由于母猪应激程度较小,没有能引起CRP含量的明显变化。上述研究用血液CRP评价动物应激水平,而本试验用的是唾液CRP,可能评价效果不明显,但确切原因尚有待进一步研究。 交感-肾上腺髓质(SAM)系统是参与机体产生应激反应的一个重要的神经递质通路系统,AMY作为自主神经交感神经标记物[46],能间接反应SAM轴的活跃程度[47]。有研究表明,心理应激或身体应激都能引起唾液AMY活性的升高[48-49]。本研究发现,在分娩后的第7天,FFPB与FFPF组母猪唾液AMY活性显著低于FCB。在分娩后的第14天,FFPF母猪唾液AMY活性显著低于FCB。其他时期,各组间母猪唾液AMY活性无显著性差异。说明限位栏中的母猪更容易产生应激、情绪状态更差,而有发酵床的自由产圈中的母猪较其他两组表现出了更为积极的情绪状态。可能是发酵床容易满足猪拱、探究等天性行为,使得母猪更愿意呆在有发酵床的自由产圈中。 在分娩当天,自由产圈母猪血液繁殖激素含量升高,产程及分娩间隔时间减少;分娩后的1~2周内,自由产圈母猪唾液COR、AMY含量降低,应激水平明显降低,更接近福利友好型哺乳母猪饲养模式。 [1]刘世荣,刘雁征,李云开.规模化猪场动物福利的环境丰富度调控技术研究进展[J].中国畜牧兽医,2007,34(12):129-131. LIU S R,LIU Y Z,LI Y K.Progress in the study of environmental enrichment regulation technology large-scale pig farm animal welfare[J].ChinaAnimalHusbandry&VeterinaryMedicine,2007,34(12):129-131.(in Chinese) [2]刘洪贵.不同福利措施及品种对母猪的行为、生理、免疫、健康及生产性能的影响[D].哈尔滨:东北农业大学,2013. LIU H G.Effect of the different breeds and methods on behavior,physiological,immunization,Injure and production indicator of pigs[D].Harbin:Northeast Agricultural University,2013.(in Chinese) [3]BAXTER E M,ADELEYE O O,JACK M C,et al.Achieving optimum performance in a loose-housed farrowing system for sows:The effects of space and temperature[J].ApplAnimBehavSci,2015,169:9-16. [4]CHIDGEY K L,MOREL P C H,STAFFORD K J,et al.Sow and piglet productivity and sow reproductive performance in farrowing pens with temporary crating or farrowing crates on a commercial New Zealand Pig Farm[J].LivestSci,2015,173:87-94. [5]施正香.健康养猪的空间环境构建与养殖技术模式研究[D].北京:中国农业大学,2014. SHI Z X.Study on spatial environmental building and healthy raising technologies in pig loose housing system[D].Beijing:China Agricultural University,2014.(in Chinese) [6]BONDE M,ROUSING T,BADSBERG J H,et al.Associations between lying-down behaviour problems and body condition,limb disorders and skin lesions of lactating sows housed in farrowing crates in commercial sow herds[J].LivestProdSci,2004,87(2-3):179-187. [7]SILVA B A N,OLIVEIRA R F M,DONZELE J L,et al.Effect of floor cooling on performance of lactating sows during summer[J].LivestSci,2006,105(1-3):176-184. [8]LAWRENCE A B,P ETHERICK J C,MCLEAN K A,et al.The effect of environment on behaviour,plasma cortisol and prolactin in parturient sows[J].ApplAnimBehavSci,1994,39(94):313-330. [9]OLIVIERO C,HEINONEN M,VALROS A,et al.Environmental and sow-related factors affecting the duration of farrowing[J].AnimReprodSci,2010,119(1-2):85-91. [10]BRACKE M B M,SPRUIJT B M,METZ J H M,et al.Decision support system for overall welfare assessment in pregnant sows A:Model structure and weighting procedure[J].JAnimSci,2002a,80(7):1819-1834. [11]BRACKE M B M,SPRUIJT B M,METZ J H M,et al.Decision support system for overall welfare assessment in pregnant sows A:Model structure and weighting procedure[J].JAnimSci,2002b,80(7):1835-1845. [12]顾招兵,李明丽,高娅俊,等.自由式分娩猪栏设计及应用效果[J].农业工程学报,2011,27(2):40-44. GU Z B,LI M L,GAO Y J,et al.Design of freedom farrowing pen and application effects[J].TransactionsoftheChineseSocietyofAgriculturalEngineering,2011,27(2):40-44.(in Chinese) [13]HALES J,MOUSTSEN V A,DEVREESE A M,et al.Comparable farrowing progress in confined and loose housed hyper-prolific sows[J].LivestSci,2015,171:64-72. [14]SILVA B A N,OLIVEIRA R F M,DONZELE J L,et al.Effect of floor cooling on performance of lactating sows during summer[J].LivestSci,2006,105(1):176-184. [15]CRONIN G M,SIMPSON G,HEMSWORTH P H.The effects of the gestation and farrowing environments on sow and piglet behaviour and piglet survival and growth in early lactation[J].ApplAnimBehavSci,1996,46(3-4):175-192. [16]JARVIS S,REED B T,LAWRENCE A B,et al.Pituitary-adrenal activation in pre-parturient pigs (Susscrofa) is associated with behavioural restriction due to lack of space rather than nesting substrate[J].AnimWelfare,2002,11(4):371-384. [17]MELISOVA M,VASDAL G,BROOM D M,et al.Increasing the piglets’ use of the creep area—A battle against biology?[J].ApplAnimBehavSci,2010,125(125):96-102. [18]GU Z B,GAO Y J,LIN B Z,et al.Impacts of a freedom farrowing pen design on sow behaviours and performance[J].PrevVetMed,2011,102(4):296-303. [19]KILBRIDE A L,MENDL M,STATHAM P,et al.A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England[J].PrevVetMed,2012,104(3-4):281-291. [20]RANDALL G C.Observations on parturition in the sow.I.Factors associated with the delivery of the piglets and their subsequent behaviour[J].VetRec,1972,90(7):178-182 [21]MADEC F,LEON E.Farrowing disorders in the sow:a field study[J].ZenFürVetReiA,1992,39(6):433-444. [22]DIJK V A J,RENS B T T V,TAVENE M A M,et al.Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated,spontaneous farrowings[J].Theriogenology,2005,64(7):1573-1590. [23]杨菲菲.母猪断奶时背膘厚度对配种间隔和下一胎繁殖性能的影响[J].现代农业科技,2013,23:264-265. YANG F F.Backfat thickness effects on reproductive performance of breeding interval and the next child at the time of weaning[J].ModernAgriculturalScienceandTechnology,2013,23:264-265.(in Chinese) [24]MAESE D G D,JANSSENSG P J,DELPUTTE P,et al.Back fat measurements in sows from three commercial pig herds relationship with reproductive efficiency and correlation with visual body condition scores[J].LivestProdSci,2004,91(1-2):57-67. [25]吴南,苏彦捷.催产素及受体基因与社会适应行为[J].心理科学进展,2012,20(6):863-874. WU N,SU Y J.Oxytocin,oxytocin receptor genotypes and social adaptive behavior[J].AdvancesinPsychologicalScience,2012,20(6):863-874.(in Chinese) [26]INSEL T R.Toward a neurobiology of attachment[J].RevGenPsychol,2000,4(4):176-185. [27]OLIVIERO C,HEINONEN M,VALROS A,et al.Effect of the environment on the physiology of the sow during late pregnancy,farrowing and early lactation[J].AnimReprodSci,2008,105(3-4):365-377. [28]CHEN S,SATO S.Role of oxytocin in improving the welfare of farm animals[J].AsianAustralJAnim, 2016. [29]BROOM D M,FRASER A F.Domestic animal behaviour and welfare[M].London:Cambridge University Press,2007,64-66. [30]UVNAS-MOBERG K.Oxytocin may mediate the benefits of positive social interaction and emotions[J].Psychoneuroendocrinology,1998,23(8):819-835. [31]ALGERS B,ROJANASTHIEN S,UVNAS-MOBERG K.The relationship between teat stimulation,oxytocin release and grunting rate in the sow during nursing[J].ApplAnimBehavSci,1990,26(3):267-276. [32]CHEN S,TANAKA S,OGURA S,et al.Effect of suckling systems on serum oxytocin and cortisol concentrations and behavior to a novel object in beef calves[J].AsianAustralJAnim,2015,28(11):1662-1668. [33]KUROSAWA M,LUNDEBERG T,AGREN G,et al.Massage-like stroking of the abdomen lowers blood pressure in anesthetized rats:influence of oxytocin[J].JAutonomNervSyst,1995,56(1-2):26-30. [34]FAVA M,GUARALDI G P.Prolactin and stress[J].StressMed,2006,3(3):211-216. [35]VALROS A A,RUNDGRENB M,SALONIEMI H,et al.Oxytocin,prolactin and somatostatin in lactating sows:associations with mobilisation of body resources and maternal behaviour[J].LivestProdSci,2003,85(1):3-13. [36]TALLING J C,WARAN N K,WATHES C M,et al.Behavioural and physiological responses of pigs to sound[J].ApplAnimBehavSci,1996,48(3):187-202. [37]韦福鑫.双翅目昆虫侵袭应激牛体对牛的护体行为反应、体表温度、心率及免疫机能的影响[D].呼和浩特:内蒙古农业大学,2014. WEI F X.Effects of diptera insect invasion on fly-repelling,skin temperature,heart rate and immune function of the cattle[D].Hohhot:Inner Mongolia Agricultural University,2014.(in Chinese) [38]于世征,郝月,顾宪红.生产性能测定站对生长猪应激水平和采食行为的影响[J].畜牧兽医学报,2013,44(9):1411-1416. YU S Z,HAO Y,GU X H.Effect of electronic feeding stating on stress level an feeding behavior of growing pigs[J].ActaVeterinariaetZootechnicaSinica,2013,44(9):1411-1416.(in Chinese) [39]O’DRISCOLL K,TEIXEIRA D L,O’GORMAN D.The influence of a magnesium rich marine supplementon behaviour,salivary cortisol levels,and skin lesions in growing pigs exposed to acute stressors[J].ApplAnimBehavSci,2013,145(3-4):92-101. [40]KATJA P,GLORIA-BEATRICE W,MARCO S.Blunted salivary and plasma cortisol response in patients with panic disorder under psychosocial stress[J].LivestProdSci,2013,88(1):35-39. [41]DRESCHEL N A,GRANGER D A.Physiological and behavioral reactivity to stress in thunderstorm-phobic dogs and their caregivers[J].ApplAnimBehavSci,2005,95(3-4):153-168. [42]YUN J,SWAN K,OLIVIERO C,et al.Effects of prepartum housing environment on abnormal behaviour,the farrowing process,and interactions with circulating oxytocin in sows[J].ApplAnimBehavSci,2015,162:20-25. [43]HELLHAMMER D H,STEFAN W,BRIGITTE M K.Salivary cortisol as a biomarker in stress research[J].Psychoneuroendocrinology,2009,34(2):163-171. [44]MURATA H,SHIMADA N,YOSHIOKA M.Current research on acute phase proteins in veterinary diagnosis:an overvie[J].VetJ,2004,168(1):28-40. [45]BURGER W,EWALD C,FENNERT E M.Increase in C-reactive protein in the serum of piglets (pCRP) following ACTH or corticosteroid administration[J].ZenFürVetReiA,1998,45(1):1-6. [46]SCHUMACHER S,KIRSCHBAUM C,FYDRICH T,Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?-A review of preliminary findings and the interactions with cortisol[J].Psychoneuroendocrinology,2013,38(6):729-743. [47]NATER UM ROHLEDER N.Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system current state of research[J].Psychoneuroendocrinology,2009,34(4):486-496. [48]KANG Y.Psychological stress-induced changes in salivary alpha-amylase and adrenergic activity[J].NursHealthSci,2010,12(4):477-484. [49]HAMILTON L D,FOGLE E A,MESTON C M.The roles of testosterone and alpha-amylase in exercise-induced sexual arousal in women[J].JSexMed,2008,5(4):845-853. (编辑程金华) Effect of Different Modes of Farrowing Pens on Reproductive Performance and Stress Level of Sows ZHANG Xiao-jun1,WANG Zhan-bin1,BAO Wei-guang1,2,GAO Qian-kun1,WAN Xi-qing3,GU Xian-hong1*,HAO Yue2,CUI Yan-jun2 (1.CollegeofAnimalScienceandTechnology,HenanUniversityofScienceandTechnology,Luoyang471003,China;2.StateKeyLaboratoryofAnimalNutrition,InstituteofAnimalScience,ChineseAcademyofAgriculturalSciences,Beijing100193,China;3.BeijingQingquanwanPigCo.,LTD,Beijing102104,China) This experiment was conducted to study the effect of different patterns of farrowing pens on reproductive performance and stress level of sows,in order to explore a kind of welfare and friendly feeding mode of lactating sows that could be popularized and applied in practical production.Twenty-four hybrid sows (Large White × Landrace) which had same parity,similar pregnancy and body condition were randomly assigned to 3 treatments:farrowing crate+high bed group (FCB,n=8),freedom farrowing pen+high bed group (FFPB,n=8) and freedom farrowing pen+partially fermented bed surface group (FFPF,n=8).The 7thday before farrowing,sows were transferred to different patterns farrowing pens.The 21st day after farrowing,piglets were weaned.The results showed that:1) farrowing time in FFPB and FFPF was significantly lower than in FCB (P<0.05);average farrowing interval in FFPB was significantly lower than in FCB (P<0.05);the levels of blood plasma oxytocin (OT) and prolactin (PRL) in FFPB showed an increasing trend (P<0.10).2) The 2ndday after transferation of sows to farrowing pens,waist surface temperature in FFPB and FFPF was significantly lower than in FCB (P<0.05).The 7thday after farrowing,salivary α-amylase (AMY) level in FFPB and FFPF was significantly lower than in FCB (P<0.05).The 14thday after farrowing,salivary AMY level in FFPF was significantly lower than in FCB (P<0.05),and cortisol (COR) level in FCB showed increasing trend (P<0.10).These results indicated that:at the day of farrowing,reproductive hormones levels in freedom farrowing pens were increased while farrowing duration and average farrowing interval were reduced.Within 1 to 2 weeks after farrowing,stress level in freedom farrowing pens was obvious decreased,indicating freedom farrowing pens were more close to the welfare and friendly feeding mode of feeding sows. farrowing pen;sow;reproductive performance;stress 10.11843/j.issn.0366-6964.2016.10.011 2016-05-04 国家科技支撑计划课题(2012BAD39B02);中国农业科学院科技创新工程(ASTIP-IAS07) 张校军(1990-),男,河南滑县人,硕士生,主要从事畜禽应激与动物福利研究,E-mail:zhangxiaojun14@163.com 顾宪红,研究员,博士生导师, E-mail:guxianhong@vip.sina.com S828.2 A 0366-6964(2016)10-2027-102 结 果


3 讨 论

4 结 论

