多巴胺对多壁碳纳米管侧壁的生物功能化及其聚(酰胺-酰亚胺)基复合材料的制备
Shadpour Mallakpour, Amin Zadehnazari
(1.Organic Polymer Chemistry Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan84156-83111, I. R. Iran;2.Nanotechnology and Advanced Materials Institute, Isfahan University of Technology, Isfahan84156-83111, I. R. Iran;3.Center of Excellence in Sensors and Green Chemistry, Department of Chemistry, Isfahan University of Technology, Isfahan84156-83111, I. R. Iran)
多巴胺对多壁碳纳米管侧壁的生物功能化及其聚(酰胺-酰亚胺)基复合材料的制备
Shadpour Mallakpour1,2,3,Amin Zadehnazari1
(1.OrganicPolymerChemistryResearchLaboratory,DepartmentofChemistry,IsfahanUniversityofTechnology,Isfahan84156-83111,I.R.Iran;2.NanotechnologyandAdvancedMaterialsInstitute,IsfahanUniversityofTechnology,Isfahan84156-83111,I.R.Iran;3.CenterofExcellenceinSensorsandGreenChemistry,DepartmentofChemistry,IsfahanUniversityofTechnology,Isfahan84156-83111,I.R.Iran)
采用微波辐射法,以多巴胺(Dop)为改性剂,多壁碳纳米(MWCNTs)管与多巴胺间形成共价键合的功能化。通过FT-IR、XRD、TGA、SEM和TEM对多巴胺功能化的MWCNT- Dop进行表征。MWCNTs得到高度功能化,可作为一种MWCNTs酚基化的有效方法。采用溶液共混工艺制备出MWCNT-Dop增强的聚(酰胺-酰亚胺)基复合材料。通过高能超声,MWCNT-Dop (0~15 wt%)分散于聚合物基体中。TGA结果表明,该复合材料具有良好的热稳定性。拉伸力学测试表明,随着MWCNT-Dop含量的增加,该复合材料的弹性模量增加。
多壁碳纳米管; 共价键功能化; 热重分析; 形貌; 力学性能
(1.OrganicPolymerChemistryResearchLaboratory,DepartmentofChemistry,IsfahanUniversityofTechnology,Isfahan, 84156-83111,I.R.Iran;2.NanotechnologyandAdvancedMaterialsInstitute,IsfahanUniversityofTechnology,Isfahan, 84156-83111,I.R.Iran;3.CenterofExcellenceinSensorsandGreenChemistry,DepartmentofChemistry,IsfahanUniversityofTechnology,Isfahan, 84156-83111,I.R.Iran)
1 Introduction
Carbon nanotubes (CNTs) have attracted tremendous attention owing to their unique and exceptional mechanical, thermal, and electrical properties[1]. CNTs exhibit a high surface area to volume ratio and hence very high tensile strength, elastic modulus, and compressive strength per weight due to the covalent bonding between sp2carbon atoms similar to graphite[2]. They are extremely flexible since they are capable to reversibly absorb large stress by deforming through twisting and buckling motions without fracturing. One of the important applications of CNTs is to act as fillers in composites, especially the polymer matrix composite. A large part of the CNT/polymer composites exploit CNTs as conductive fillers[3]. And the CNT/polymer composites are promising in applications such as electromagnetic interference (EMI) shielding[4], photovoltaic devices[5], gas sensors[6], thermal management[7], corrosion protection[8], and transparent conductive coatings[9]. The aims are to develop cheap, light and easy-to-process “conductive plastics”, for future applications, where metals and semiconductors are still preferred. The key issues for producing technically interesting CNT/polymer composites are to disperse the individual CNT into the polymer matrix completely and efficiently, and to control the properties of the filler-matrix interface. A critical issue in the nanocomposite fabrication and processing is to uniformly distribute and disperse nanofillers in polymer matrices[10]. The dispersion of CNTs in polymer matrix is especially problematic since CNTs are often held together in bundles by very strong van der Waals interactions. Several methods were developed over the last years to circumvent this issue. These methods can be broadly classified into three general categories, which are direct mixing, chemical surface modification, and third component-assisted dispersion[11]. In the “direct mixing”method, the CNTs are dispersed in the polymer by mechanical force generated via shear-intensive stirring or ultrasonication[12-15]. This is also one of the most convenient methods to achieve CNT dispersion. In the chemical surface modification method, the walls of CNTs were chemically modified to improve the interactions at the polymer-filler interface, such as carboxylation[16], hydroxylation[17], fluorination[18], bromination[19]and amidation[20]. In the third component-assisted dispersion approach, a third component is added to assist the dispersion of CNTs in solvents and polymer matrices. The third component could be surfactants[21], polyelectrolytes[22], block copolymers[23], or proteins and DNAs[24,25]. These chemicals adsorb onto the walls of CNTs during sonication and the dispersion is realized by repulsive electrostatic interactions between the surfactants adsorbed on CNTs. Though this technique is effective, the use of a surfactant can impede the tube-to-tube contact, thereby decreasing the final conductivity of the composite.
Dopamine hydrochloride is a well-known bioactive molecule containing catecholamine moiety, acting as a neurotransmitter in the brain. It has been received considerable attention because of its known or suspected role in a variety of neuropsychiatric disorders such as Parkinson’s disease and schizophrenia[26]. The main effect of dopamine is to increase systemic vascular resistance, thereby raising the newborn’s blood pressure. In certain areas of the brain where dopamine is released it gives one the feeling of pleasure or satisfaction[27]. These feelings of satisfaction become desired, and the person will grow a desire for the satisfaction. To satisfy that desire the person will repeat behaviors that cause the release of dopamine[28]. However, it is the role that dopamine plays in pleasure and motivation that attracts the most neurobiologists attention.
Dopamine-functionalized multi-walled CNTs (MWCNT-Dop) were prepared by microwave irradiation method, which were used as fillers in poly(amide-imide) containing dopamine side chain (PAI) to prepare MWCNT-Dop/PAI composite by ultrasonication-assisted solution blending method. The distribution of MWCNT-Dop in the PAI, their interfacial interactions, and the thermal and mechanical characteristics of the composite were investigated.
2 Experimental
2.1Materials
Carboxylated MWCNTs manufactured by a thermal chemical vapor deposition via an acid oxidation method were purchased from Neutrino Co. (Iran), which have outer diameters of 8-15 nm, inner diameters of 3-5 nm, an average length of 50 μm, a carboxyl content of 2.56 wt% and a purity of > 95 wt%. Other chemicals used in this study were obtained commercially from Fluka Chemical Co. (Switzerland), Aldrich Chemical Co. (Milwaukee, WI) and Merck Chemical Co. (Germany). Dopamine hydrochloride, 3,5-dinitrobenzoylchloride, trimellitic anhydride (TMA), L-isoleucine (Ile) amino acid, glacial acetic acid, propylene oxide, tetrabutylammonium bromide (TBAB), and triphenyl phosphite (TPP) were used as received without further purification. Propylene oxide was used as an acid scavenger. Hydrazine monohydrate and 10 wt% palladium on activated carbon were used as received. N,N′-dimethylformamide (DMF) and N,N′-dimethylacetamide (DMAc) as solvents were distilled over barium oxide under reduced pressure before use. All other reagents were used as received from commercial sources.
2.2Characterization
The equipment used for the functionalization of MWCNTs and the synthesis of the polymer was a Samsung microwave oven (2450 MHz, 900 W) (Seoul, South Korea). Melting points of the monomers were measured on a melting-point equipment (Gallenhamp, Cambridge, United Kingdom) without correction.1H nuclear magnetic resonance (NMR) spectrum was recorded on a Bruker (Rheinstetten, Germany) Avance 500 instrument at room temperature in dimethylsulphoxide-d6(DMSO-d6). Multiplicities of proton resonance were designated as singlet (s), doublet (d), triplet (t) and multiplet (m). Fourier transform infrared (FT-IR) spectra of the samples were recorded with a Jasco-680 (Tokyo, Japan) spectrometer at a resolution of 4 cm-1. They were scanned at a wavenumber range of 400-4 000 cm-1. Band intensities were assigned as weak (w), medium (m), strong (s), and broad (br). Vibration bands were reported as wavenumber (cm-1). Elemental analysis was performed in an Elementar Analysensysteme GmbH (Hanau, Germany). Inherent viscosity was measured using a Cannon Fenske Routine Viscometer (Mainz, Germany) at a concentration of 0.5 g/L in DMF and at 25 ℃. Optical specific rotation was measured at the concentration of 0.5 g/L in DMF at 25 ℃ using a quartz cell (1.0 cm) with a Jasco Polarimeter (JASCO Co., Ltd., Tokyo, Japan). Thermal stability of the MWCNTs, PAI and MWCNT-Dop/PAI composites was evaluated by thermogravimetric analysis (TGA) (STA503 TGA, Bahr-Thermoanalyse GmbH, Hüllhorst, Germany) in nitrogen flow rate of 60 cm3/min. A heating rate of 10 ℃/min and a sample amount of 10±2 mg were used in each experiment. The X-ray diffraction (XRD) was used to characterize the crystalline structure of the samples. XRD patterns were collected using a Bruker, D8ADVANCE (Rheinstetten, Germany) diffractometer with a copper target at the wavelength ofλCuKα= 0.154 nm, a tube voltage of 40 kV, and a tube current of 35 mA. The samples were scanned at a 2θrate of 0.05 °/min from 10° to 80°. For XRD studies, rectangular pellets prepared by compression molding were used. The morphology of the MWCNTs and dispersion morphology of the MWCNT-Dop on the PAI matrix were observed using field emission scanning electron microscopy (FE-SEM). The images were taken at 15 kV using a HITACHI S-4160 instrument (Tokyo, Japan). Transmission electron microscopic (TEM) images were obtained using a Philips CM 120 microscope (Germany) with an accelerating voltage of 100 kV. For TEM studies, ultra-thin sections (30-80 nm) of MWCNT-Dop and the composites were prepared using a Leica Ultramicrotome. Tensile testing was performed at room temperature on a Testometric Universal Testing Machine M350/500 (Mainz, Germany), according to ASTM D 882 (standards). Tests were carried out with a cross-head speed of 12.5 mm/min until reaching a deformation of 20% and then, at a speed of 50 mm/min until break. The dimensions of the test specimens were 35×2×0.04 mm3. Measured values reported here represented an average of the results for tests run on at least five specimens. Tensile strength, tensile modulus, and strain were obtained from these measurements. Preparation of MWCNT-Dop/PAI composites was carried out on a MISONIX ultrasonic XL-2000 SERIES (Raleigh, North Carolina, USA). Ultrasonic irradiation was performed with the probe of the ultrasonic horn being immersed directly into the mixture solution with a frequency of 2.25×104Hz and a power of 100 W.
2.3Synthesis of monomers
N-trimellitylimido-L-isoleucine (3) was prepared according to our previous work[29]. 3,5-diamino-N-(3,4-dihydroxyphenethyl)benzamide (7) as a diamine monomer was also prepared according to the our reported procedure (Scheme 1)[30].
2.4Preparation of MWCNT-Dop
We have previously reported the versatility of electrophilic substitution reaction of S-valine amino acid with the carboxylated MWCNTs[20]. Accordingly, dopamine was selected for the functionalization of the carboxylated MWCNTs. Dopamine hydrochloride (0.2 g, 1.05×10-3mol) and propylene oxide (0.2 mL) was mixed with DMAc (20 mL) for 2 h at 60 ℃ to obtain a solution. The pristine carboxylated-MWCNTs (0.1 g) and NaNO2(0.1 g, 1.45×10-3mol) were sonicated with the 20 mL DMAc solution for 2 h until a homogeneous suspension was obtained. The MWCNT suspension was then poured into a porcelain dish (50 mL) and placed in a domestic microwave chamber. The above mentioned MWCNT suspension was heated in the microwave up to 120 ℃ for 15 min with an output power of 700 W. Then the resulting suspension was cooled to room temperature and poured into a 200 mL HCl solution (5%v/v). The mixture was stirred for 30 min at room temperature and then decanted to remove the solvent. When the amount of solvent became about 20 mL, the mixture was subjected to irradiation with a high-intensity ultrasound for 1 h. The obtained homogeneous suspension was placed in an 80 ℃ oven overnight to evaporate most of the solvent. The final products were washed more than 7 times with DMAc and distilled water for the removal of any unreacted residue dopamines, and then dried for 48 h at 50 ℃ to obtain the dopamine-functionalized MWCNTs, named as MWCNT-Dop.
2.5Synthesis of PAI from monomers by polymerization
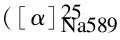

1H NMR (400 MHz, DMSO-d6, ppm): 0.91 (t, 3H, CH3, distorted ), 1.29 (d, 3H, CH3,J= 7.20 Hz), 1.50 (m, 2H, CH2), 2.59 (m, 1H, CH), 2.87 (t, 2H, CH2, distorted), 3.15 (t, 2H, CH2, distorted), 4.63-4.65 (d, 1H, CH,J= 8.40 Hz), 6.45-6.47 (d, 1H, Ar-H,J= 8.40 Hz), 6.61-6.63 (d, 1H, Ar-H, distorted), 7.66 (s, 1H, Ar—H), 7.71 (s, 1H, Ar-H), 7.94 (s, 1H, Ar-H), 8.00 (s, 1H, Ar-H), 8.10 (d, 1H, Ar-H, distorted), 8.46 (s, 1H, Ar—H), 8.51-8.53 (d, 1H, Ar—H, distorted), 8.76 (s, 1H, NH), 10.15 (s, 1H, OH), 10.23 (s, 1H, OH), 10.78 (s, 1H, NH), 10.85 (s, 1H, NH).
These results show a PAI formula of (C30H28N4O7)n. Therefore, the theoretical elemental composition is 64.74% for C, 5.07% for H and 10.07% for N. Elemental analysis result show 64.35% for C, 4.96% for H and 10.29% for N.
2.6Preparation of the MWCNT-Dop/polymer composite film
Two stock solutions were prepared. PAI and MWCNT-Dop were separately dispersed in DMAc with stirring for 1 day at 30-40 ℃. Then, two stock solutions were mixed to achieve the desired weight percentages of MWCNT-Dop from 5 to 15 wt%. The MWCNT-Dop/PAI mixture was stirred for 1 day at 30-40 ℃ and ultrasonicated in a water bath for 1 h. To remove the DMAc solvent, MWCNT-Dop/PAI mixture was poured into uncovered preheated glass Petri dishes and uniformly heated at 60 ℃ for 1 day. The semidried film was further dried under vacuum at 160 ℃ for 8 h to remove the residue solvent to obtain a solid film, MWCNT-Dop/PAI composite film. The curly films, formed after the evaporation of DMAc, could be easily lifted from the glass Petri dishes. The freestanding polymer films with a thickness of 40 μm were then peeled from the glass plate and subjected to different tests. Because MWCNTs are black in color, the films containing more MWCNTs looked darker. The film colors varied from tan for 0 MWCNT-Dop to light gray for 5% MWCNT-Dop and dark black for 10 and 15% MWCNT-Dop. The uniform color found for the films indicates a good distribution of MWCNT-Dop in PAI polymer matrix.
3 Results and discussion
3.1Synthesis and structural characterization of monomers
Diacid monomer 3 was synthesized by the condensation reaction of an equimolar amount of TMA and L-isoleucine in a refluxing acetic acid solution (Scheme 1)[29]. Diamine monomer 7 was synthesized using a two-step process (Scheme 1)[30]. In the first step, aromatic nucleophilic displacement of 3,5-dinitrobenzoylchloride with dopamine hydrochloride in the presence of propylene oxide in DMAc solvent resulted in N-(3,4-dihydroxyphenethyl)-3,5-dinitrobenzamide (6) as a pale yellow solid. In the second step, this dinitro compound was reduced in ethanol in the presence of hydrazine hydrate with a catalytic amount of palladium on the activated carbon at 80 ℃, producing yellow crystals of the diamine 7. The structure of the dinitro 6 and diamine 7 was verified by elemental analysis, FT-IR,1H NMR, and13C NMR spectroscopy.
3.2Synthesis of PAI

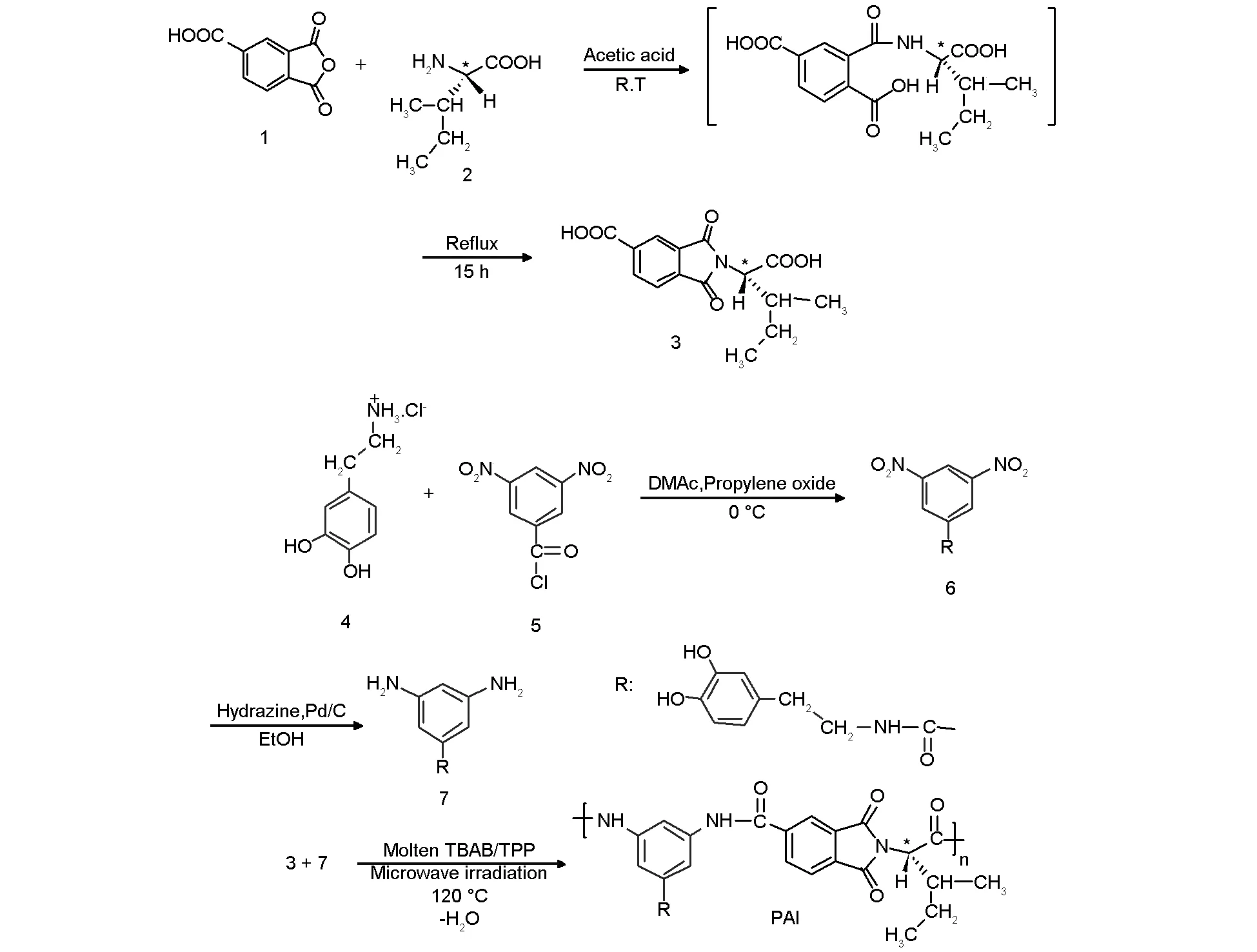
Scheme 1 Synthesis of monomers and PAI.
3.3Preparation of MWCNT-Dop
Microwave-assisted synthesis is a well-known method for a direct covalent linkage of molecules to MWCNTs. In the present work, as we were in need of hydroxyl-substituted arene, we employed dopamine biomolecule for the generation of the dopamine moiety on the side-walls of MWCNTs. The reaction sequence is depicted in Scheme 2. The optimum microwave power level and period of heating were selected according to a previous work[35]. Functionalization was carried out under microwave irradiation. The separation and purification of products, is carried out easily and in a convenient way by simple decanting. The resulting paste was washed with DMAc and water, sonicated and dried. The attachment of dopamine groups on the MWCNTs was confirmed by FT-IR spectroscopy, XRD, TGA, FE-SEM, and TEM.
3.4Preparation of composite films
The dispersion of the MWCNT-Dop in 5%, 10%, and 15% in PAI solutions in DMAc was attained by vigorous stirring with a speed of 15 000 r/min for 1 day, using a homogenizer, which was followed by utrasonication for 1 h to form a series of MWCNT-Dop/polymer composites with different MWCNT contents. The reaction pathway for preparing MWCNT-Dop/PAI composites is shown in Scheme 2. Several researches have recently investigated the properties of the CNT/polymer composites with a CNT content from 1-15 wt% and good results have been obtained and reported. From this point of view, we selected 5%, 10%, and 15% of CNT contents in the composites[36,37]. The functional groups in the polymer chains perform hydrogen interactions with the dopamine-functionalized MWCNTs. These interactions are shown in Fig.1.
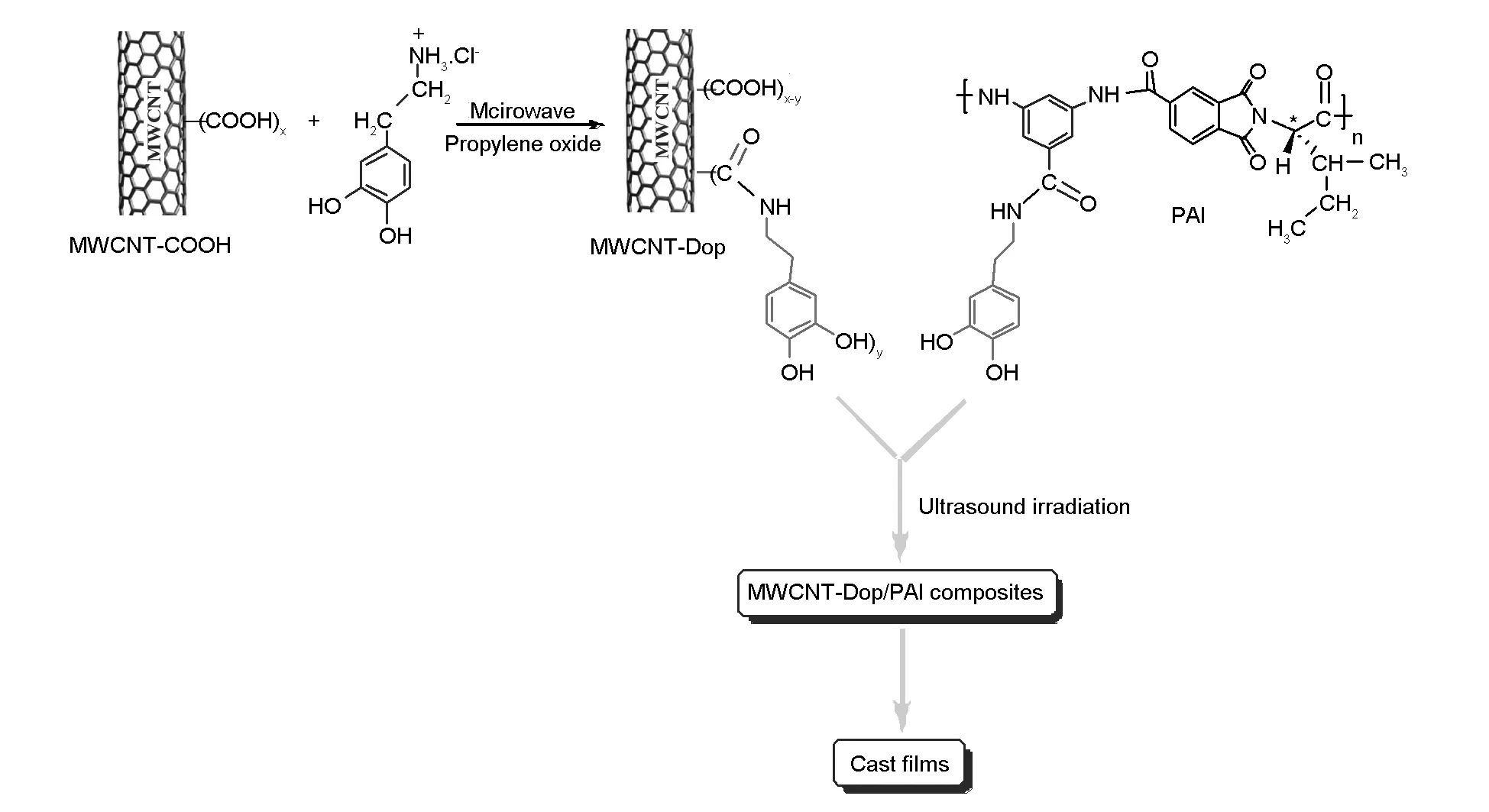
Scheme 2 Fabrication of the MWCNT-Dop and the composites.
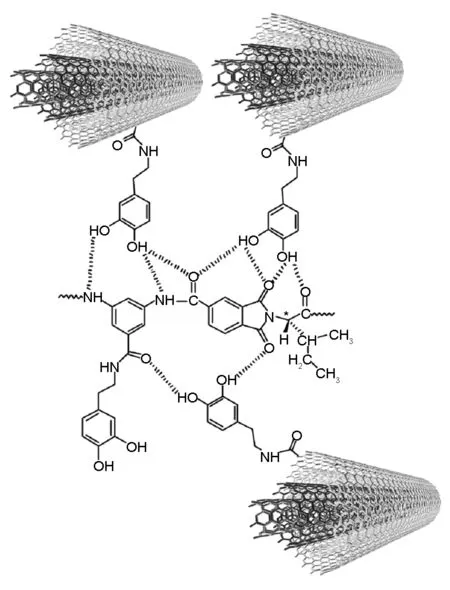
Fig. 1 An illustration of hydrogen bonding between MWCNT-Dop and the PAI.
3.5Characterization of the MWCNT-Dop and the composites
3.5.1FT-IR spectral study


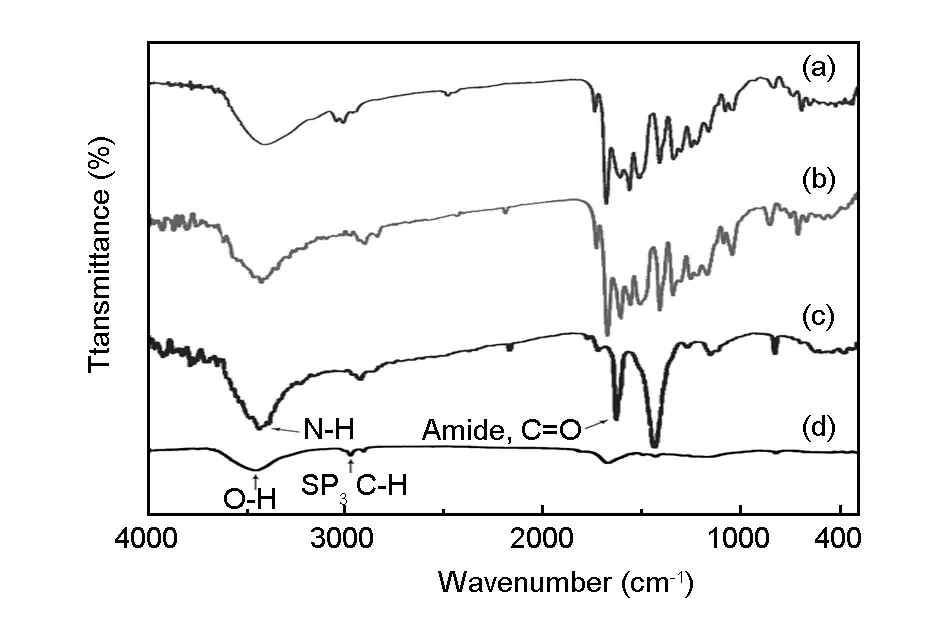
Fig. 2 FT-IR spectra of (a) neat PAI, (b) MWCNT-Dop/PAI 15 wt%(c) MWCNT-Dop, and (d) MWCNT-COOH.
3.5.21H NMR study
The structure of PAI was confirmed by1H NMR spectroscopy as well as elemental analysis. In the1H NMR spectrum of this polymer (Fig.3), the presence of the N—H protons of amide groups at 8.76, 10.78 and 10.85 ppm as three singlet peaks, and O—H groups at 10.15 and 10.23 ppm as two singlet peaks, indicated the presence of amide groups in the polymer side and main chain as well as hydroxyl groups in the polymer side chain. The resonance of aromatic protons appeared in the range of 6.45-8.53 ppm. The proton of the chiral center appeared as doublet at 4.63-4.65 ppm. Elemental analysis of PAI was also in a good agreement with the proposed structure.
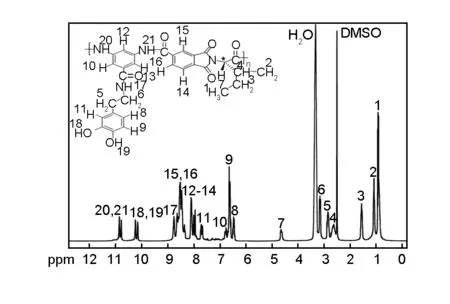
Fig. 3 1H NMR (400 MHz) spectrum of PAI in DMSO-d6 at room temperature.
3.5.3 Morphological analysis
The structure of the MWCNT-COOH was examined by XRD. The resultant curves are shown in Fig.4. For the MWCNT-COOH, two peaks appeared at 2θ= 26° and 44°, with the 2θ= 26° corresponding to the (002) diffraction plane of the graphite, and 2θ= 44° toα-Fe (110) and/or Ni (111) diffractions[40]. MWCNT-Dop showed very few changes in the XRD pattern with the MWCNT-COOH. It could be seen that XRD pattern is very similar to the MWCNT-COOH. MWCNT-Dop still had the same cylinder wall structure as raw MWCNTs and interlayer spacing of all samples remained the same. For the PAI, the weak reflection emerged at around 20° was characteristic of the amorphous polymer. XRD patterns of the MWCNT-Dop/PAI composite materials are also presented in Fig.4 and they are in good agreement with the MWCNT-Dop and the pure PAI polymer. Composite samples showed that XRD pattern was similar to the pure PAI polymer when the MWCNT-Dop composition was 5 wt%. For the composites with a MWCNT-Dop content higher than 5 wt%, the MWCNT-Dop/PAI composites exhibited peaks of PAI and MWCNT-Dop, as shown in Fig.4. The reflections at 2θ= 26° and 44° were slightly increased when the MWCNT-Dop content in the composite was 15 wt%.
MCWNT-Dop were imaged with FE-SEM. A representative image of the MWCNT-Dop, along with an image of the MWCNT-COOH, are shown in Fig.5. The FE-SEM image of the MWCNT-COOH surface is approximately smooth. After functionalization with dopamine, the surface of the MWCNT-Dos became a rough and debundled structure, as can be seen in Fig.5. FE-SEM images of neat PAI and the composites were also examined to investigate the morphology and dispersity of the MWCNT-Dop in the composites, as displayed in Fig.6. The pure PAI copolymer showed a corn-like morphology. FE-SEM observation revealed that the PAI particles were self-organized into nanopatterns. The average diameter of polymeric particles was about 43 nm. For the MWCNT-Dop/PAI composites, MWCNT-Dop were well dispersed and embedded in the PAI matrix without a noticeable MWCNT-Dop aggregation. These results strongly showed that due to hydrogen bonding between MWCNT-Dop and several functional groups in the backbone of the PAI, the MWCNT-Dop were mixed well with the PAI matrix, eventually leading to the good dispersion and compatibility of the MWCNT-Dop in the PAI matrix.
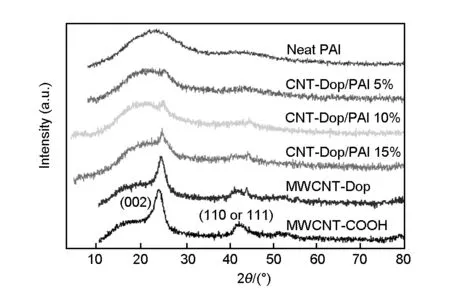
Fig. 4 XRD patterns of MWCNT-COOH, MWCNT-Dop, PAI, and MWCNT-Dop/PAI composites with different MWCNT-Dop contents.
Fig.7 presents TEM images of the MWCNT-Dop (top) and MWCNT-COOH (bottom). As can be seen, they showed a high surface roughness for the MWCNT-Dop. This surface roughness may imply the partial damage of graphitic carbon, which could have resulted from the severe functionalization and/or oxidation processes[41]. Although TEM could not distinguish minute functional groups, it could represent surface deterioration of the CNTs that occurred as a result of the functionalization. As mentioned above, the functionalization reaction disrupted the sp2carbon network of graphitic CNTs, so it may be responsible for the roughness of CNTs’ surfaces. In fact, since the microwave radiation was harsh enough to produce highly disordered carbon (Fig.7), it is likely that it could also damage graphitic MWCNTs[42]. The representative TEM images of the MWCNT-Dop/PAI composites are shown in Fig.8. As shown in this figure, the MWCNT-Dop were well dispersed in the composites. This can be explained by the fact that the MWCNT-Dop stabilizes their dispersion by good interactions with the PAI matrix, resulting from the increased polarity by the functional groups formed on the surfaces of the MWCNTs as well as good interactions of the hydroxyl groups of MWCNT-Dop from dopamine with the carbonyl groups in the PAI matrix. The presence of the functional groups on the surfaces of the MWCNTs resulted in the interfacial interaction between the polymer matrix and the CNTs in the composites. For example, a hydrogen bonding could be formed between the hydroxyl groups at the MWCNT-Dop with the oxygen atoms at the carbonyl groups of the PAI chains as shown in Fig.1.
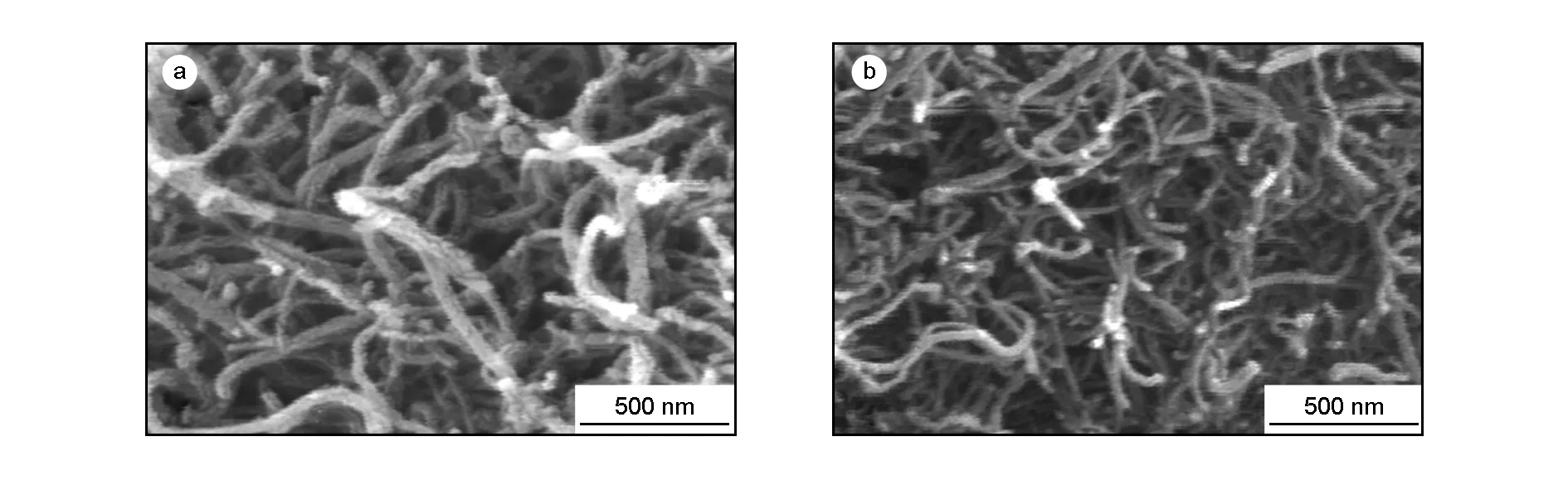
Fig. 5 FE-SEM micrographs of (a) MWCNT-COOH and (b) MWCNT-Dop.
3.5.4Thermogravimetric analysis
The thermal properties of the resulting samples were examined by TGA under nitrogen atmosphere at a heating rate of 10 ℃/min, and the corresponding weight loss temperatures of 5% and 10% (Td5% andTd10%), were all determined from the original TGA curves. Typical TGA curves of representative samples are shown in Fig.9. Furthermore, the results obtained from these analyses are completely tabulated in Tables 1 and 2. A single step degradation was observed in all samples. The curve of pristine the MWCNT-COOH showed a small mass loss in a temperature range of 0-550 ℃. The weight loss of the MWCNT-Dop resulted from the decomposition of the functionalized organic moieties attached to the surface of MWCNTs. TGA data of the pristine and the purified MWCNTs suggested that the MWCNTs were able to withstand oxidation temperatures up to 700 ℃. The initial decomposing temperature of the MWCNT-COOH was 550 ℃, indicating that the acid-treated CNTs were thermally stable up to this temperature, as shown in Table 1. This could be due to the incorporation of COOH groups in the defective sites and tips. When the MWCNT-COOH were functionalized with dopamine, the weight loss at less than 500 ℃ increased from the contribution of dopamine molecules incorporated over MWCNTs. The TGA plot showed that the degradation of specimen MWCNT-Dop occurred in the 245-700 ℃ temperature range. By comparing the MWCNT-Dop with the MWCNT-COOH, it can be concluded that the thermal stability of the MWCNT-COOH was destroyed upon functionalization with dopamine. The thermal behavior data for the PAI and the composites are summarized in Table 2. The onset of decomposition temperature of the composites was slightly higher than that of pure PAI, shifting toward higher temperatures as the amount of MWCNT-Dop was increased. The marginal increase in thermal stability of PAI matrix upon incorporation of the MWCNT-Dop could be related to the high thermal conductivity of CNTs that facilitated heat dissipation within the composites, hence preventing the accumulation of heat at certain points for degradation[43]. The end temperature of decomposition was also retarded with increasing MWCNT-Dop content. The percentage of carbonized residue at 800 ℃ was higher for the composites rather than neat PAI. This indicated that the MWCNT-Dop reduced the degradation of PAI at high temperature as seen in the curves. Therefore, it could be demonstrated that a small amount of MWCNT-Dop acted as effective thermal degradation resistant component in the PAI matrix, increasing the thermal stability of the PAI.

Table 1 Thermal stability of CNT samples obtained from TGAa.
Note:aTemperature at which 5% weight loss was recorded by TGA at a heating rate of 10 ℃/min in nitrogen atmosphere.
Table 2 Thermal properties of PAI and the compositesa.
Note:aTemperature at which 5 and 10% weight loss was recorded by TGA at a heating rate of 10 ℃/min in nitrogen atmosphere.
bPercentage weight of material left undecomposed after TGA analysis at maximum temperature 800 ℃ in nitrogen atmosphere.
cLimiting oxygen index (LOI) evaluating at the char yield at 800 ℃.
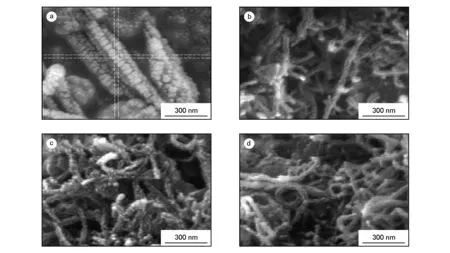
Fig. 6 FE-SEM micrographs of (a) pure PAI and the composites containing (b) 5 wt%, (c) 10 wt% and (d) 15 wt% of MWCNT-Dop.
The limiting oxygen index (LOI) is the minimum concentration of oxygen, expressed as a percentage, which will support combustion of a polymer. It can be used to evaluate the flame retardancy of the polymers. Normal atmospheric air is approximately 21% oxygen, so a material with a LOI of less than 21% would burn easily in air. A material with a LOI of greater than 21% but less than 28% would be considered as “slow burning”. A material with a LOI of greater than 28% would be considered as “self-extinguising”. A self-extinguishing material is one that would stop burning after the removal of the fire or ignition source[44].
Theoretically, two interesting relationships have been found between the LOI and the parameters of the combustion process: char yield or char residue (CR) and heat of combustion.
According to Krevelen[45], there is a linear relationship between LOI and CR only for halogen-free polymers (Eq. 1):
LOI=(17.5+0.4CR)/100
(1)
From this equation, a higher char yield will improve flame retardance. PAI and the composites containing 5%, 10%, and 15%, had LOI values of 35.5, 37.1, 39.1, and 41.5, respectively, as calculated from their char yields. On the basis of the LOI values, such materials can be classified as self-extinguishing materials.
According to Johnson[46], the LOI values of many common materials can be logically well predicted by the expression (Eq. 2):
LOI=80000/ΔHcomb
(2)
whereΔHcombis the specific heat of combustion in J/g. So, in the case of this polymer (PAI) and their composites (5%, 10%, and 15% of MWCNT-Dop),ΔHcombare 22.5, 21.6, 20.4, and 19.3 kJ/g, respectively.

Fig. 7 TEM micrographs of MWCNT-Dop (top) and MWCNT-COOH (bottom) at different magnifications.
3.5.5Mechanical test
Mechanical properties of the MWCNT-Dop/PAI composites were studied. Typical stress-strain curves for pure PAI and the MWCNT-Dop/PAI composites with different MWCNT-Dop contents are displayed in Fig.10. The tensile strength and the strain at tensile strength varied depending on the amount of CNTs added. The results of tensile tests in terms of Young’s modulus, strength and elongation at break of the composite films and respective standard deviations, corresponding to an average of at least five samples tested for each type of the film, are reported in Table 3. The more MWCNT-Dop were added to the composite, the bigger was the reinforcement and the higher was the strain. Furthermore, the incorporation of the dopamine biomolecules on the surface of the MWCNT-COOH and several functional groups into the PAI matrix resulted in a good interfacial adhesion between the MWCNT-Dop and the PAI matrix. Therefore, the improvement of the mechanical properties of the MWCNT-Dop/PAI composites could be related to the good interaction between the MWCNT-Dop and the PAI matrix as well as the good dispersion of the MWCNT-Dop in the PAI matrix.

Fig. 8 TEM micrographs of the MWCNT-Dop/PAI composite containing 10 wt% of MWCNT-Dop at different magnifications.
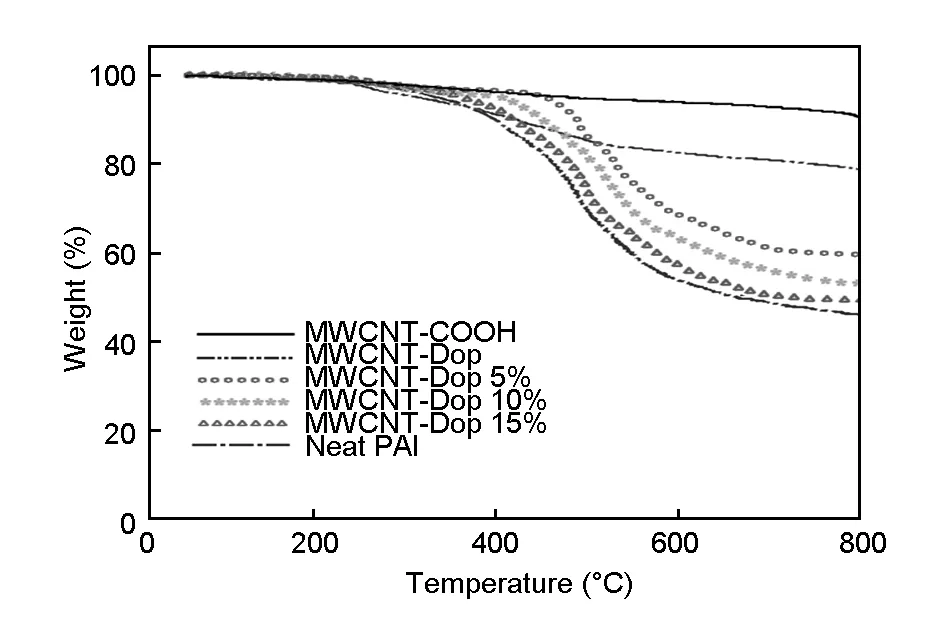
Fig. 9 TGA curves of the composites with different MWCNT-Dop loadings (in nitrogen at a heating rate of 10°C/min).
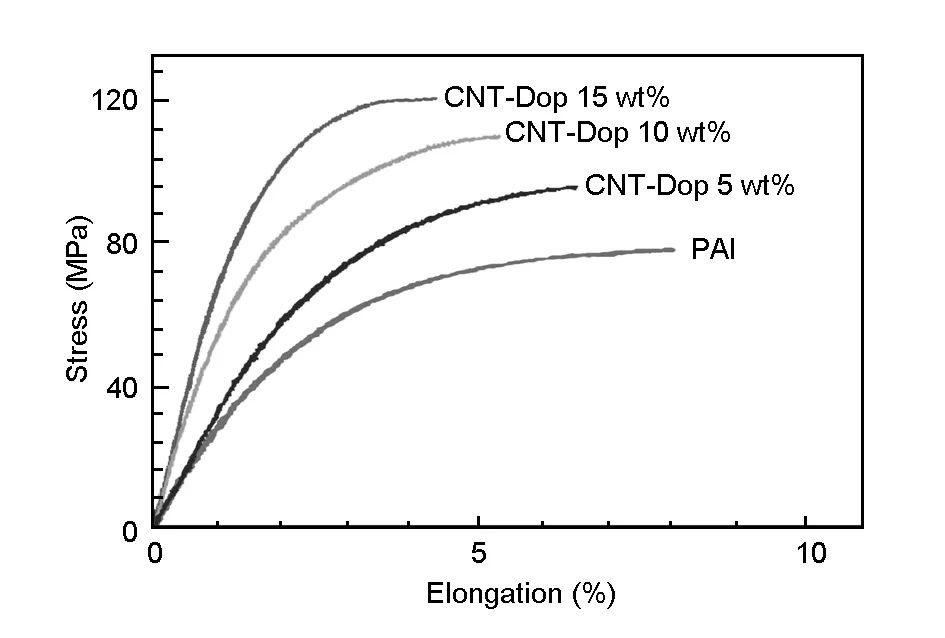
Fig. 10 Stress-strain curves of the composite samples with various MWCNT-Dop loadings.
4 Conclusions
Covalent functionalization of the MWCNT-COOH with dopamine biomolecule was achieved to prepare MWCNT-Dop by microwave irradiation, which were used as fillers to improve fire-retardant and mechanical properties of the PAI polymer. The attached dopamine molecules significantly increased the interaction between the MWCNT-Dop and PAI polymer chains via hydrogen bonding between polar groups in dopamine and PAI, leading to a good dispersion of the MWCNT-Dop in PAI polymer, an increased mechanical strength and an improved fire-retardant performance and thermal stability of PAI polymer. MWCNTs are regarded as a high conducting fillers that help to dissipate heat and thus to prevent heat accumulation and hot-spot formation in PAI polymer.
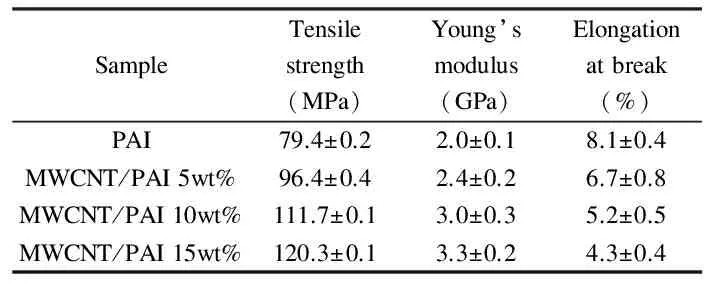
Table 3 Mechanical properties for MWCNT-Dop/PAI composite films.
Acknowledgements
This work was supported by the Research Affairs Division of Isfahan University of Technology (IUT), National Elite Foundation (NEF), and Center of Excellency in Sensors and Green Chemistry Research (IUT).
[1]Thostenson ET, Ren Z, Chou T-W. Advances in the science and technology of carbon nanotubes and their composites: A review[J]. Compos Sci Technol, 2001, 61(13): 1899-1912.
[2]Shokrieh M, Rafiee R. A review of the mechanical properties of isolated carbon nanotubes and carbon nanotube composites[J]. Mech Compos Mater, 2010, 46(2): 155-172.
[3]Min C, Shen X, Shi Z, et al. The electrical properties and conducting mechanisms of carbon nanotube/polymer nanocomposites: A review[J]. Polym Plast Technol Eng, 2010, 49(12): 1172-1181.
[4]Thomassin J M, Huynen I, Jerome R, et al. Functionalized polypropylenes as efficient dispersing agents for carbon nanotubes in a polypropylene matrix; application to electromagnetic interference (EMI) absorber materials[J]. Polymer, 2010, 51(1): 115-121.
[5]Massuyeau F, Zhao Y, El Mel AA, et al. Improved photoconductive properties of composite nanofibers based on aligned conjugated polymer and single-walled carbon nanotubes[J]. Nano Res, 2013, 6(2): 149-158.
[6]Sayago I, Fernandez MJ, Fontecha JL, et al. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites[J]. Sens Actuators B, 2013, 175(1): 67-72.
[7]Cho D-B, Suhr J, Koratkar NA. Carbon nanotube thin film coating for improved thermal management in piezoceramic sheet actuators[J]. J Intell Mater Syst Struct, 2006, 17(3): 209-216.
[8]Richard Prabakar S, Pyo M. Corrosion protection of aluminum in LiPF6by poly(3,4-ethylenedioxythiophene) nanosphere-coated multiwalled carbon nanotube[J]. Corrosion Sci, 2012, 57: 42-48.
[9]Ma R, Menamparambath MM, Nikolaev P, et al. Transparent stretchable single-walled carbon nanotube-polymer composite films with near-infrared fluorescence[J]. Adv Mater, 2013, 25(18): 2548-2553.
[10]Ma P C, Siddiqui N A, Marom G, et al. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review[J]. Composites Part A, 2010, 41(10): 1345-1367.
[11]Moniruzzaman M, Winey K I. Polymer nanocomposites containing carbon nanotubes[J]. Macromolecules, 2006, 39(16): 5194-5205.
[12]Mallakpour S, Zadehnazari A. The production of functionalized multiwall carbon nanotube/amino acid-based poly(amide-imide) composites containing a pendant dopamine moiety[J]. Carbon, 2013, 56(0): 27-37.
[13]Zhang F M, Chang J, Eberhard B. Dissolution of poly(vinyl alcohol)-modified carbon nanotubes in a buffer solution[J]. New Carbon Mater, 2010, 25(4): 241-247.
[14]Liu Q, Tu J, Wang X, et al. Electrical conductivity of carbon nanotube/poly(vinylidene fluoride) composites prepared by high-speed mechanical mixing[J]. Carbon, 2012, 50(1): 339-341.
[15]Ambrogi V, Gentile G, Ducati C, et al. Multiwalled carbon nanotubes functionalized with maleated poly (propylene) by a dry mechano-chemical process[J]. Polymer, 2012, 53(2): 291-299.
[16]Li L X, Li F. The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of carbon nanotubes[J]. New Carbon Mater, 2011, 26(3): 224-228.
[17]Bradley R H, Cassity K, Andrews R, et al. Surface studies of hydroxylated multi-wall carbon nanotubes[J]. Appl Sur Sci, 2012, 258(11): 4835-4843.
[18]Wang H Y, Chua D H C. Triple layered core-shell structure with surface fluorinated ZnO-carbon nanotube composites and its electron emission properties[J]. Appl Sur Sci, 2013, 265(0): 66-70.
[19]Chang Y H, Lin P Y, Wu M S, et al. Extraordinary aspects of bromo-functionalized multi-walled carbon nanotubes as initiator for polymerization of ionic liquid monomers[J]. Polymer, 2012, 53(10): 2008-2014.
[20]Mallakpour S, Zadehnazari A. Functionalization of multi-wall carbon nanotubes with amino acid and its influence on the properties of thiadiazol bearing poly(amide-thioester-imide) composites[J]. Synthc Met, 2013, 169(0): 1-11.
[21]Zhong W, Claverie JP. Probing the carbon nanotube-surfactant interaction for the preparation of composites[J]. Carbon, 2013, 51(0): 72-84.
[22]Kong H, Luo P, Gao C, et al. Polyelectrolyte-functionalized multiwalled carbon nanotubes: preparation, characterization and layer-by-layer self-assembly[J]. Polymer, 2005, 46(8): 2472-2485.
[23]Song W, Zheng Z, Tang W, et al. A facile approach to covalently functionalized carbon nanotubes with biocompatible polymer[J]. Polymer, 2007, 48(13): 3658-3663.
[24]Karajanagi Sandeep S, Asuri P, Sellitto E, et al. Protein-carbon nanotube conjugates[J]. Biomolecul Catal, 2008, 986: 100-115.
[25]Awasthi K, Singh D P, Singh S K, et al. Attachment of biomolecules (protein and DNA) to amino-functionalized carbon nanotubes[J]. New Carbon Mater, 2009, 24(4): 301-306.
[26]Wise R A. Dopamine, learning and motivation[J]. Nature Rev Neurosci, 2004, 5(6): 483-494.
[27]McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients[J]. Brain, 1998, 121(6): 1155-1164.
[28]Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction[J]. Eur J Pharmacol, 2000, 393(1-3): 295.
[29]Mallakpour S, Hajipour A R, Shahmohammadi M H. Direct polycondensation of N-trimellitylimido-L-isoleucine with aromatic diamines[J]. J Appl Poly Sci, 2003, 89(1): 116-122.
[30]Mallakpour S, Hatami M, Ensafi AA, et al. Synthesis and characterization of novel dopamine-derivative: Application of modified multi-wall carbon nanotubes paste electrode for electrochemical investigation[J]. Chin Chem Lett, 2011, 22(2): 185-188.
[31]Sugamoto K, Matsushita Y I, Matsui T. Microwave-assisted beckmann rearrangement of aryl ketoximes catalyzed by In(OTf)3in ionic liquid[J]. Synth Commun, 2011, 41(6): 879-884.
[32]Du F Y, Xiao X H, Li G K. Ionic liquid aqueous solvent-based microwave-assisted hydrolysis for the extraction and HPLC determination of myricetin and quercetin from Myrica rubra leaves[J]. Biomed Chromatogr, 2011, 25(4): 472-478.
[33]Mallakpour S, Zadehnazari A. Advances in synthetic optically active condensation polymers: A review[J]. Express Polym Lett, 2011, 5(2): 142-181.
[34]Mallakpour S, Zadehnazari A. Synergic effects of molten ionic liquid and microwave irradiation in preparation of optically active nanostructured poly(amide-imide)s containing amino acid and dopamine moiety[J]. Polym Plast Technol Eng, 2012, 51(11): 1090-1096.
[35]Amiri A, Maghrebi M, Baniadam M, et al. One-pot, efficient functionalization of multi-walled carbon nanotubes with diamines by microwave method[J]. Appl Surf Sci, 2011, 257(23): 10261-10266.
[36]Al-Saleh M H, Sundararaj U. Electromagnetic interference shielding mechanisms of CNT/polymer composites[J]. Carbon, 2009, 47(7): 1738-1746.
[37]Lahelin M, Annala M, Nykänen A, et al. In situ polymerized nanocomposites: Polystyrene/CNT and poly(methyl methacrylate)/CNT composites[J]. Compos Sci Technol, 2011, 71(6): 900-907.
[38]Lee H J, Oh S J, Choi J Y, et al. In situ synthesis of poly(ethylene terephthalate)(PET) in ethylene glycol containing terephthalic acid and functionalized multiwalled carbon nanotubes (MWNTs) as an approach to MWNT/PET nanocomposites[J]. Chem Mater, 2005, 17(20): 5057-5064.
[39]Hsiao S H, Guo W, Lee W F, et al. Synthesis and characterization of electrochromic poly(amide-imide)s bearing methoxy-substituted triphenylamine units[J]. Mater Chem Phys, 2011, 130(3): 1086-1093.
[40]Perez-Cabero M, Rodriguez-Ramos I, Guerrero-Ruiz A. Characterization of carbon nanotubes and carbon nanofibers prepared by catalytic decomposition of acetylene in a fluidized bed reactor[J]. J Catal, 2003, 215(2): 305-316.
[41]Zardini HZ, Amiri A, Shanbedi M, et al. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method[J]. Colloids Surf B, 2012, 92: 196-202.
[43]Huxtable S T, Cahill D G, Shenogin S, et al. Interfacial heat flow in carbon nanotube suspensions[J]. Nature Mater, 2003, 2(11): 731-734.
[44]Troitzsch J, Bönold H J, Rieber M, et al. International plastics flammability handbook: Principles, regulations, testing and approval: Hanser publication, 1983.
[45]Van Krevelen D. Some basic aspects of flame resistance of polymeric materials[J]. Polymer, 1975, 16(8): 615-620.
[46]Johnson P. A general correlation of the flammability of natural and synthetic polymers[J]. J Appl Polym Sci, 1974, 18(2): 491-504.
Preparation of dopamine-functionalized multi-wall carbon nanotube/poly(amide-imide)composites and their thermal and mechanical properties
Shadpour Mallakpour1,2,3,Amin Zadehnazari1
Covalent functionalization of MWCNT-COOH with dopamine was conducted by microwave irradiation and the dopamine-functionalized MWCNTs (MWCNT-Dop) were used as fillers in poly(amide-imide) (PAI) to improve its thermal and mechanical properties. Results indicate that the MWCNT-Dop is dispersed in PAI very well, which is ascribed to a good interfacial interaction between the MWCNT-Dop and PAI by hydrogen bonding. The temperatures at which 10 wt% weight loss occurs, and the limiting oxygen indices increase from 398 to 483 ℃ and 35.5% to 41.5%, respectively, as the MWCNT-Dop content increases from 0 to 15 wt%. The tensile strength and Young’s modulus also increase from 79.4 to 120.3 MPa and 2.0 to 3.3 GPa, respectively, as the MWCNT-Dop content increases from 0 to 15 wt%.
Multi-walled carbon nanotubes; Covalent functionalization; Thermogravimetric analysis; Morphology; Mechanical properties
date: 2015-12-01;Reviseddate: 2016-01-05
Shadpour Mallakpour. E-mail: mallak@cc.iut.ac.ir, mallakpour84@alumni.ufl.edu, mallak777@yahoo.com
1007-8827(2016)01-0018-13
TB332
A
Shadpour Mallakpour. E-mail: mallak@cc.iut.ac.ir, mallakpour84@alumni.ufl.edu, mallak777@yahoo.com
10.1016/S1872-5805(16)60001-X
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).

