人胰腺癌细胞培养上清对树突状细胞TIM-3表达及其功能的影响*
夏媛媛, 蔡长青, 卢桠楠, 甘慧泉, 周泉波
(1广东医学院,广东 湛江 524023; 2广东省第二人民医院肿瘤二科,广东 广州 510317; 3中山大学孙逸仙纪念医院麻醉科,广东 广州 510120; 4广东省人民医院检验科,广东 广州 510080;5中山大学孙逸仙纪念医院肝胆胰外科,广东 广州 510120)
人胰腺癌细胞培养上清对树突状细胞TIM-3表达及其功能的影响*
夏媛媛1,2,蔡长青2△,卢桠楠3,甘慧泉4,周泉波5△
(1广东医学院,广东 湛江 524023;2广东省第二人民医院肿瘤二科,广东 广州 510317;3中山大学孙逸仙纪念医院麻醉科,广东 广州 510120;4广东省人民医院检验科,广东 广州 510080;5中山大学孙逸仙纪念医院肝胆胰外科,广东 广州 510120)
目的: 研究人胰腺癌微环境对树突状细胞(DCs)T细胞免疫球蛋白及黏蛋白-3(TIM-3)表达及其功能的影响,初步探讨肿瘤微环境调节DCs上TIM-3表达的可能机制。方法:流式细胞术检测肿瘤浸润树突状细胞(TIDC)以及癌旁组织、胰腺癌患者和健康人外周血诱导DCs上TIM-3的表达;观察人胰腺癌细胞培养液上清对健康人外周血单个核细胞经rhGM-CSF和rhIL-4诱导扩增制备DCs上 TIM-3表达的影响;酶联免疫吸附法(ELISA)检测TIM-3+DCs组和对照组的DCs分别与凋亡胰腺癌细胞Capan-2共培养上清中细胞因子IFN-β和IL-12水平。结果:胰腺癌组织中TIDC上TIM-3的表达明显高于癌旁组织及患者和健康人外周血的DCs(P<0.01)。人胰腺癌细胞株Canpan-2、SW1990和Panc-1的上清液较人皮肤成纤维细胞Hs27显著上调DCs上TIM-3表达(P<0.05),联合阻断VEGF、IL-10和PGE2可明显降低Canpan-2细胞上清对DCs上TIM-3的上调作用(P<0.05)。TIM-3高表达DCs组较低表达组分泌的IL-12和IFN-β水平低,而阻断TIM-3后,IFN-β和IL-12水平均升高(P<0.01)。而这种升高趋势可在加入DNase和RNase后消失。结论:人胰腺癌TIDC上TIM-3表达升高导致其固有免疫功能受损;肿瘤细胞分泌的VEGF、IL-10和PGE2可能参与TIM-3的表达调控。
胰腺癌; 树突状细胞; 肿瘤微环境; T细胞免疫球蛋白及黏蛋白-3
胰腺癌是常见的消化系肿瘤之一,由于手术及放化疗对治疗效果的局限性[1],肿瘤免疫治疗已成为新的研究热点,肿瘤微环境对肿瘤的发生、发展以及免疫逃逸的影响更是引起人们的广泛关注。目前认为,树突状细胞(dendritic cells,DCs)是功能最强大的专职抗原提呈细胞(antigen-presenting cells,APC)。肿瘤及其营造的微环境能够通过各种机制导致DCs的分化和成熟障碍,抑制DCs抗原提呈和激活T细胞的能力[2-3]。研究表明[4-5]:T细胞免疫球蛋白及黏蛋白-3(T-cell immunoglobulin and mucin-3, TIM-3)对DCs参与的肿瘤免疫起负性调节作用。
那么,在胰腺癌中DCs是否表达TIM-3? TIM-3是否参与了胰腺癌DCs介导的固有免疫应答?肿瘤微环境通过何种途径调节其表达?本课题通过观察人胰腺癌组织中肿瘤浸润树突状细胞(tumor-infiltrating dendritic cells,TIDC)上TIM-3的表达情况,分析胰腺癌细胞通过肿瘤微环境影响TIDC表型的可能因素,进一步探讨TIM-3对TIDC 固有免疫的影响。为研究胰腺癌细胞免疫逃逸机制及寻找新的胰腺癌的免疫治疗靶点打下基础。
材 料 和 方 法
1材料
1.1主要试剂与仪器重组人粒-巨噬细胞集落刺激因子(rhGM-CSF)、重组人白细胞介素4(rhIL-4)、RPMI-1640和DMEM培养基(HyClone);胎牛血清(FBS)、0.25%胰蛋白酶(Gibco);淋巴细胞分离液(TBD);抗人CD1α单克隆抗体(monoclonal antibo-dy, mAb)、抗人S-100 mAb和IL-10 mAb(Abcam);免疫组化试剂盒(博士德生物公司);抗人CD11c-FITC抗体及同型对照抗体(BD);抗人TIM-3-PECy7抗体(eBioscience);TIM-3 阻断抗体(BioLegend);VEGFR2 mAb(R&D);PGE2受体EP2封闭肽(AH6809)(Cayman);人IL-12和IFN-β ELISA试剂盒(Elabscience);CCK-8(Dojindo)。顺铂(cis-diaminodichloroplatin,DDP)冻干粉(齐鲁制药);AnnexinⅤ/PI凋亡检测试剂盒(凯基公司);DNase和RNase(TaKaRa);流式细胞仪(FACSCalibur,BD);低速离心机和低温高速离心机(Beckman);细胞培养箱(Forma Series Ⅱ, Thermo);超净台(VS-840K-U, 苏净);倒置普通光学显微镜和荧光显微镜(Nikon);酶标仪(Denley Dragon Wellscan MK2)。
1.2标本来源人胰腺癌患者手术标本及外周血4例均来源中山大学孙逸仙纪念医院肝胆外科,瘤体重量4.20~7.82 g,平均6.04 g。患者年龄48~70岁,平均60.25岁,男7例、女2例,均符合肿瘤手术切除指征,未发现远处转移,术后病理诊断为胰腺癌;癌旁组织取自距肿瘤切缘2~3 cm的正常胰腺组织。健康人外周血浓缩白细胞来源于广州市中心血站。
本研究符合医学伦理学标准,经医院伦理委员会批准,得到患者家属的知情同意。
1.3细胞株来源人胰腺癌细胞株SW1990、Capan-2、Panc-1及人皮肤成纤维细胞(human skin fibroblast) Hs27由中山大学孙逸仙纪念医院肝胆外科实验室提供。
2方法
2.1人胰腺癌组织免疫组化检测将人胰腺癌组织及癌旁胰腺组织手术标本进行常规固定、包埋、切片,分别用鼠抗CD1α单抗及鼠抗S-100单抗作为Ⅰ抗,生物素化的羊抗鼠IgG作为Ⅱ抗,参照SABC免疫组化试剂盒书说明书对胰腺癌组织及癌旁组织切片进行免疫组织化学染色,DAB显色后在光学显微镜下观察染色结果。
2.2人胰腺癌组织及癌旁组织中浸润性DCs的制备手术新鲜肿瘤组织经0.25%胰蛋白酶和0.04%Ⅳ型胶原酶37 ℃充分消化,组织碎块研磨,200目孔径钢网滤过收集单细胞悬液,洗涤,调整细胞密度1×1010/L,淋巴细胞分离液分离细胞后调整细胞密度为1×109/L,37 ℃、5% CO2细胞培养箱中培养过夜,次日收集悬浮细胞,洗涤,用含2%FBS的PBS重悬细胞。
2.3外周血DCs的诱导及体外培养胰腺癌患者及健康人外周血经淋巴细胞分离液分离单个核细胞(peripheral blood mononuclear cells,PBMC),用含10% FBS的RPMI-1640培养液重悬细胞,调整为(2~3)×109/L,加入6孔板(每孔2 mL),37 ℃、5% CO2细胞培养箱培养4 h,洗去悬浮的淋巴细胞,取贴壁单个核细胞加入含10% FBS的RPMI-1640培养液,补充终浓度为100 μg/L的 rhGM-CSF和50 μg/L 的rhIL-4,每3 d换液并补充相应浓度的rhGM-CSF、rhIL-4,第6天收获未成熟的树突状细胞(immature dendritic cells, imDC),备用。台盼兰染色,检测活细胞率。
2.4流式细胞术检测DCs表面TIM-3的表达各组DCs用流式洗液(PBS加入2% FBS)洗2次后,重悬并分别加入抗人TIM-3-PECy7抗体和抗人CD11c-FITC抗体,4 ℃避光孵育30 min, 洗涤2次后上机检测。
2.5流式细胞术分选CD11c+DCs将外周血诱导培养获得的imDC 用CD11c-FITC抗体标记,4 ℃避光孵育30 min, PBS再洗2次后上流式细胞仪进行CD11c+DCs的无菌分选,鉴定纯度。分选获得CD11c+DCs离心浓缩(1 000 r/min,5 min),用含10% FBS的RPMI-1640培养液重悬,备用。分选流式细胞仪严格执行无菌维护流程[6]。
2.6细胞培养及胰腺癌细胞培养上清的制备人胰腺癌细胞株(Capan-2、SW1990、Panc-1)与人皮肤成纤维细胞株(HS27)在含10% FBS的RPMI-1640培养液中,37 ℃、5% CO2细胞培养箱培养,常规传代。将胰腺癌细胞株与Hs27细胞经胰酶消化后,以5×108/L密度接种于10 cm培养皿中(10 mL),37 ℃、5% CO2培养48 h后收集培养上清,离心(1 500 r/min, 5 min)去除细胞碎片,微孔滤膜(0.22 μm)过滤后分装,-80 ℃保存备用。
2.7胰腺癌细胞培养液上清对DCs表达TIM-3的影响健康人外周血单核细胞体外诱导培养至第6天生成的imDC,分成2组,第1组分别加入含有25%的3种胰腺癌细胞上清的培养液,第2组加入含25% HS27细胞上清的培养液,培养48 h, 收集各组DCs,流式细胞术检测TIM-3的表达情况。
2.8阻断胰腺癌细胞培养液上清中VEGF、IL-10和PGE2对DCs表达TIM-3的影响收集体外诱导培养至第6天生成的imDC,分为4组,第1、2、3组在含25%Capan-2细胞上清的培养液中分别加入anti-VEGFR2抗体、anti-IL-10抗体和PGE2受体EP2封闭肽,第4组在含25%Capan-2细胞上清的培养液中联合加入anti-VEGF-R2抗体、anti-IL-10抗体和PGE2受体EP2封闭肽,培养48 h,流式细胞术检测TIM-3的表达情况。
2.9CCK-8法测药物细胞毒性试验取对数生长期的胰腺癌细胞,胰酶消化后1 000 r/min离心5 min后弃上清,用10% FBS的RPMI-1640重悬细胞,调整细胞密度为5×107/L,每孔取100 μL种96孔板,放置于37 ℃、5% CO2细胞培养箱培养24 h,待细胞贴壁后,加入10 μL含不同浓度药物的培养基,每种浓度设5个复孔,培养24 h后吸弃含药物的培养基,每孔加入100 μL新鲜含10% FBS的RPMI-1640培养液及10 μL CCK-8,孵育1 h后观察显色程度,酶标仪测定在450 nm处的吸光度(A)值。GraphPad Prism 5软件计算药物不同浓度抑制率,得到IC50。
2.10DCs与凋亡的Capan-2细胞共培养的分组取对数生长期的Capan-2细胞,分别按不同浓度加入DDP,作用时间分别为12、24、36、48 h,胰酶消化收集细胞,按AnnexinⅤ/PI凋亡检测试剂盒说明书操作,流式细胞术测细胞凋亡率,筛选出DDP作用Capan-2细胞的最佳作用浓度和时间。将健康人外周血单核细胞经体外诱导培养、流式分选的CD11c+DCs分成4组,第1组加入含有25%Capan-2细胞上清的培养液培养48 h, 第2组加入含25%成纤维细胞上清的培养液培养48 h,第3组在第1组基础上再加入TIM-3单克隆抗体。将前3组DCs与用顺铂处理后凋亡的Capan-2细胞按5∶1比例混匀共培养 24 h,第4组在第3组处理的DCs基础上与DNase和RNase处理的凋亡Capan-2细胞共培养,收集各组培养液,4 ℃、1 500 r/min离心10 min,取上清液,待测。
2.11ELISA检测各组DCs分泌的细胞因子IFN-β和IL-12的水平在酶标包被板上设立标准孔,每组2个复孔,具体步骤按试剂说明书进行,运用酶标仪自动分析检测结果,读出具体检测值。实验重复3次,取平均值作为最终结果。
3统计学处理
采用SPSS 13.0软件,对所有数据均进行正态性检验, 正态分布的计量资料以均数±标准差(mean±SD) 表示,组间比较采用单因素方差分析(one-way ANOVA),方差齐组间多重比较采用LSD分析,方差不齐组间多重比较采用Dunnett’s T3分析。以P<0.05为差异有统计学意义。
结 果
1胰腺癌组织及癌旁组织中DCs的观察
免疫组化观察9例癌旁胰腺组织和胰腺癌组织中均发现有浸润的CD1α+S-100+DCs,见图1。
2胰腺癌患者TIDC、癌旁组织中DCs、外周血及健康人外周血DCs上TIM-3表达的比较
流式细胞术检测结果显示胰腺癌组织中TIDC上TIM-3表达为(71.79±11.74)%,高于癌旁组织中、胰腺癌患者及健康人外周血DCs上TIM-3表达,数据分别为(11.46±6.68)%、(9.38±3.94)%和(4.10±2.24)%,差异显著(P<0.01)。台盼兰染色检测各组DCs存活率均大于93%,见图2。
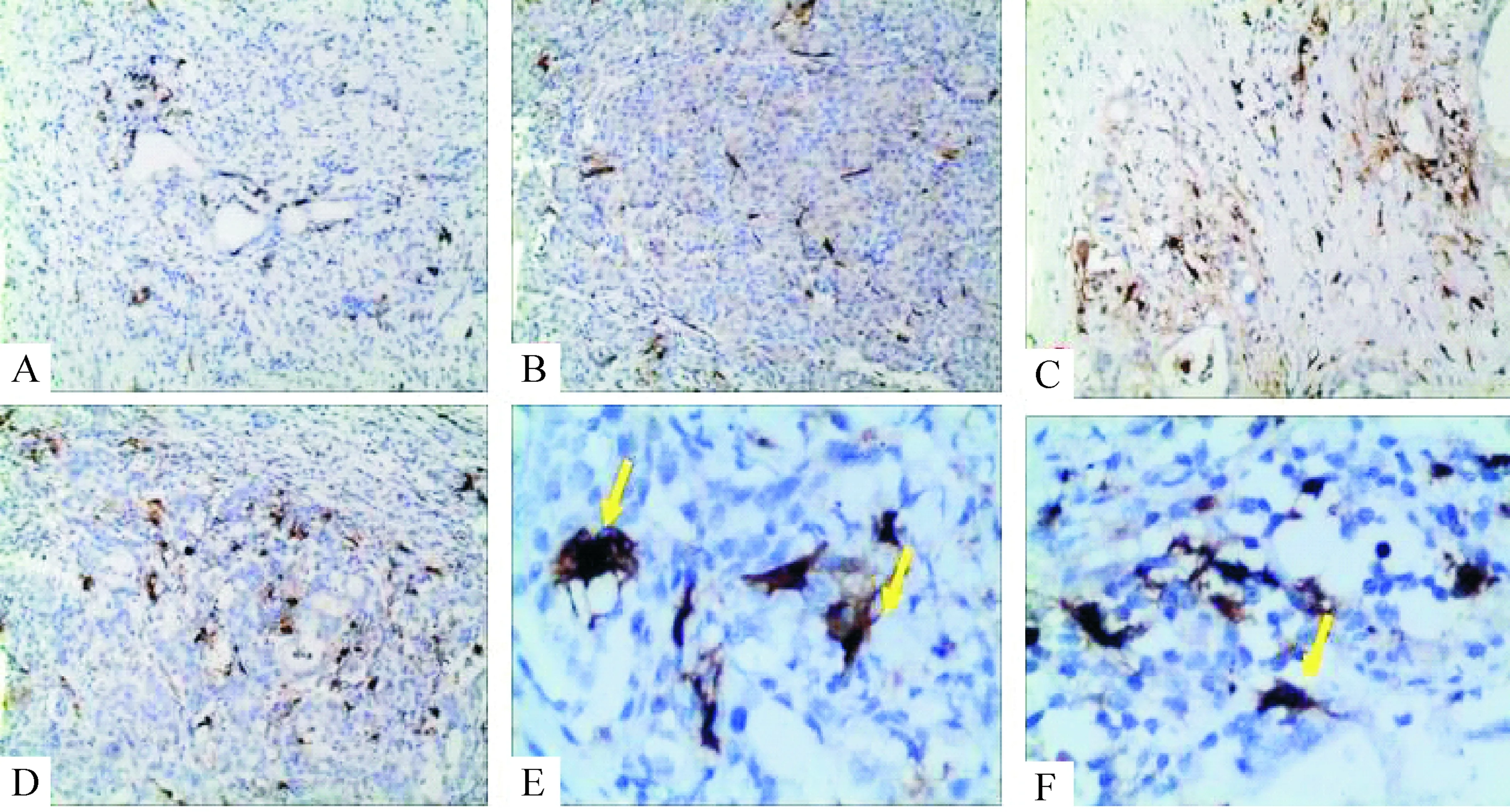
Figure 1.Immunohistochemistry detection of TIDC from pancreatic cancer tissues and adjacent tissues in human. A: CD1α+DCs in para-carcinoma tissue; B: S-100+DCs in para-carcinoma tissue; C: CD1α+DCs in pancreatic cancer tissue; D: S-100+DCs in pancreatic cancer tissue; E: CD1α+DCs in pancreatic cancer tissue; F: S-100+DCs in pancreatic cancer tissue (A~D:×100; E, F:×400).
图1胰腺癌肿瘤组织及癌旁组织中分布的DCs
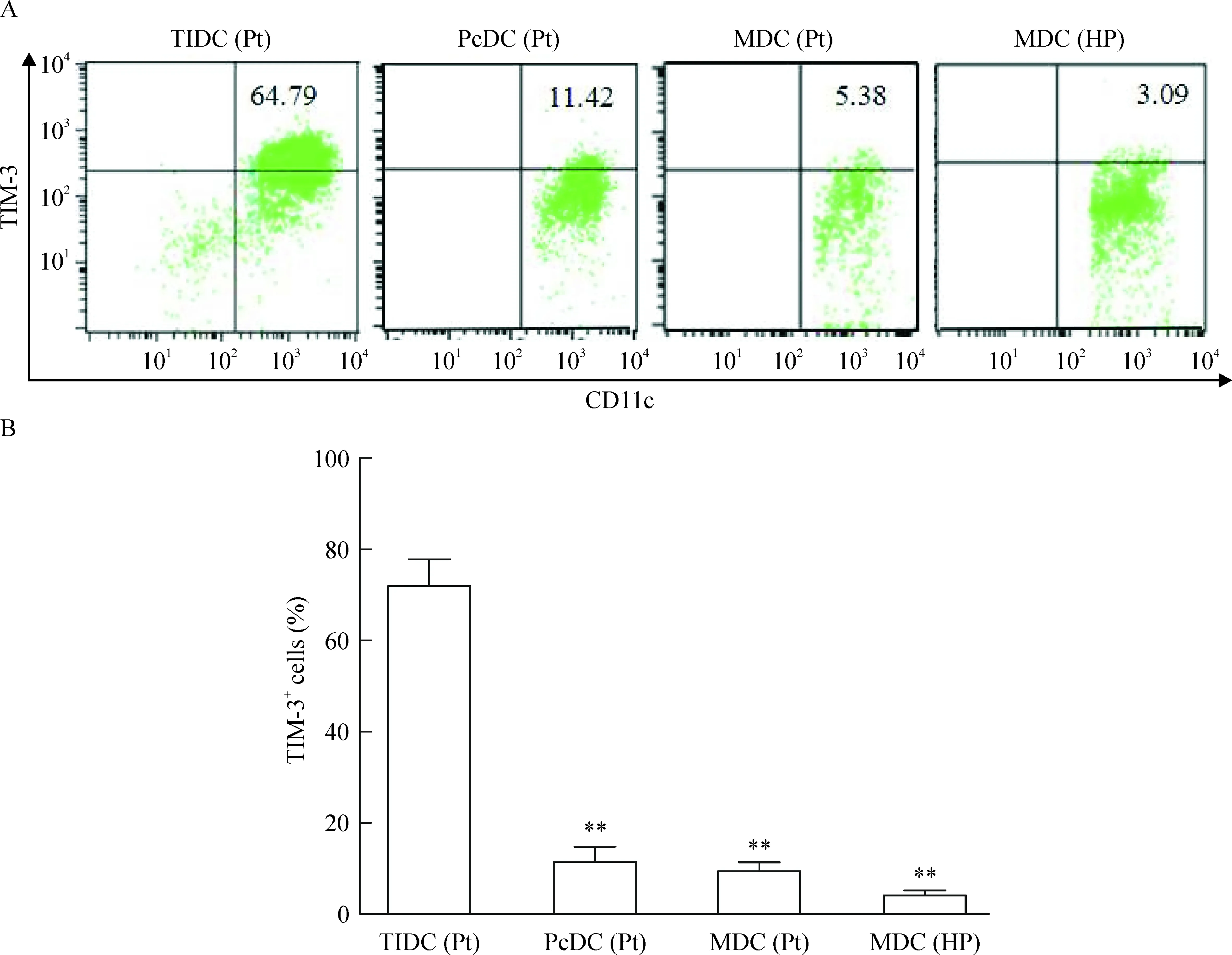
Figure 2.Expression of TIM-3 on surface of TIDC isolated from human pancreatic cancer and of myeloid dendritic cells (MDC) from peripheral blood. A: TIM-3 expression on DCs from pancreatic cancer tissues [TIDC(Pt)], para-carcinoma tissues[PcDC(Pt)] and peripheral blood of the same patients [MDC(Pt)]or healthy population [MDC(HP)], analyzed by flow cytometry. Numbers in top right quadrants indicate percentages of TIM-3+CD11c+cells among CD11c+cells. B: percentages of TIM-3+CD11c+cells among CD11c+cells in the 4 groups. Mean±SD.n=4.**P<0.01vsTIDC(Pt).
图2分离自胰腺癌组织的TIDC及体外诱导的MDC表面TIM-3的表达
3胰腺癌细胞培养液上清对DCs表面TIM-3表达的影响
流式细胞术检测结果显示:含25%Capan-2、SW1990和Panc-1胰腺癌细胞培养液上清的3组DCs上TIM-3的相对表达量分别为(44.52±8.62)%、(52.05±6.48)%和(47.90±10.38)%,均高于Hs27细胞组TIM-3相对表达量[(12.67±4.27)%](P<0.05)。而含胰腺癌细胞上清的3组数值比较无显著差异,见图3。
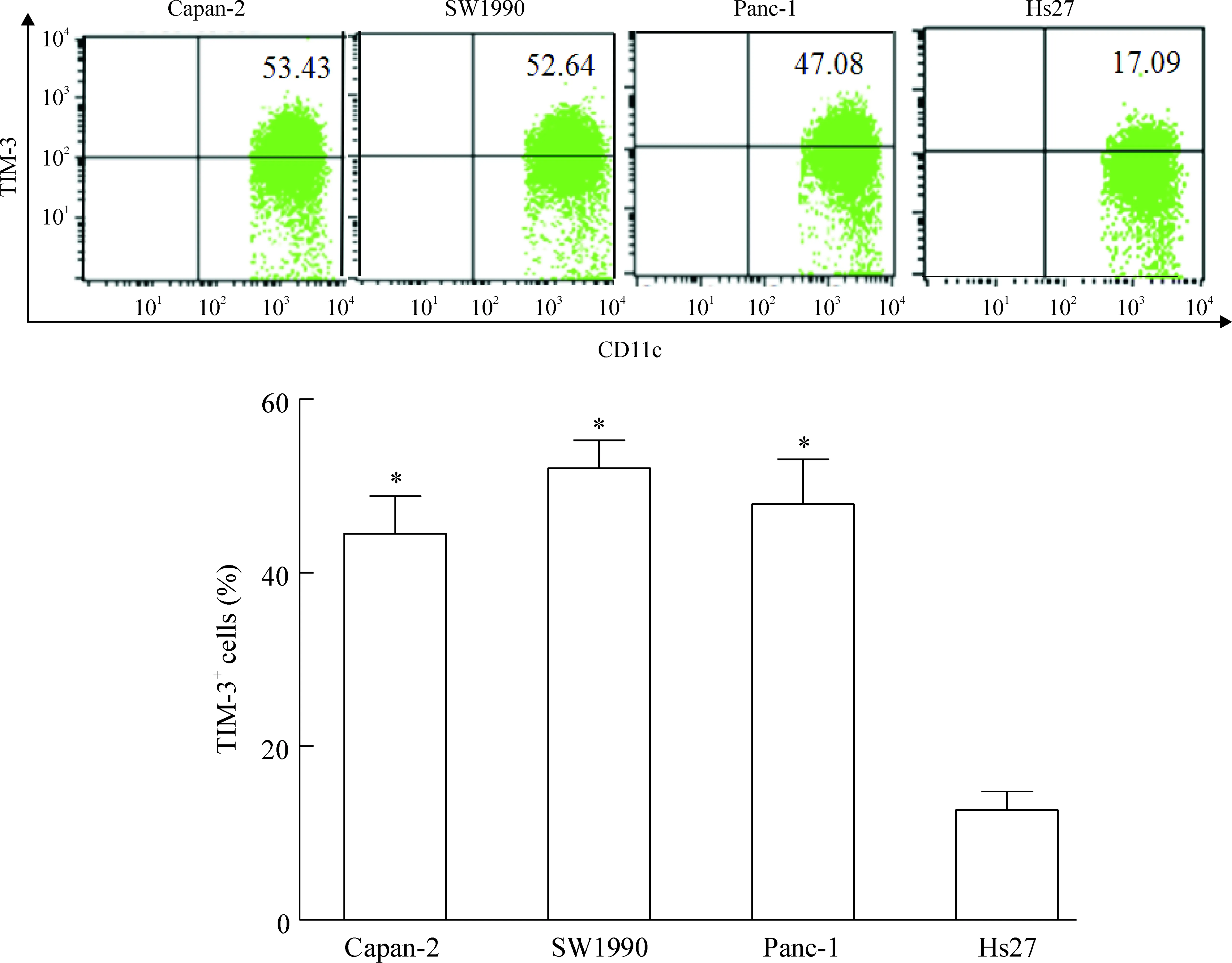
Figure 3.Expression of TIM-3 on surface of DCs treated for 48 h with supernatants of Capan-2, SW1990 and Panc-1 tumor cells or Hs27 cells (25% in total medium). Mean±SD.n=4.*P<0.05vsHs27.
图3胰腺癌细胞及Hs27细胞上清对DCs上TIM-3表达的影响
4阻断胰腺癌上清中VEGF、IL-10和PGE2对DCs表达TIM-3的影响
分别在含25% Capan-2细胞上清的培养液中加入anti-VEGF-R2抗体、anti-IL-10抗体和PGE2受体EP2封闭肽,培养imDC 48 h后,流式细胞术检测DCs上 TIM-3相对表达量,3组表达率分别为(38.09±5.87)%、(43.12±5.08)%和(39.53±4.60)%,与未加抗体组[(44.52±8.62)%]比较,有降低趋势,但差异无统计学意义;联合阻断3种细胞因子作用后,TIM-3的表达水平明显下调[(27.44±5.55)%](P<0.05),但并未完全抑制其表达,见图4。
5DCs与凋亡胰腺癌细胞Capan-2共培养上清中细胞因子IFN-β、IL-12水平
CCK-8法测得DDP对Capan-2胰腺癌细胞24 h的IC50为13.6 mg/L。2倍IC50(27.2 mg/L)的DDP,作用于Capan-2细胞,12 h开始凋亡增加,36 h凋亡逐渐达到高峰值,48 h后以晚期凋亡和坏死为主(图5),于是选择27.2 mg/L作用36 h为DDP致Capan-2细胞凋亡的浓度和时间。凋亡Capan-2细胞与各组CD11c+DCs(流式分选CD11c+DCs纯度>98%,图6)共培养,TIM-3高表达DCs组(Capan-2上清组)、TIM-3低表达DCs组(Hs27上清组)、TIM-3高表达DCs+TIM-3 mAb组和TIM-3高表达DCs+DNase+RNase组上清中细胞因子IL-12和IFN-β水平比较结果见图7。
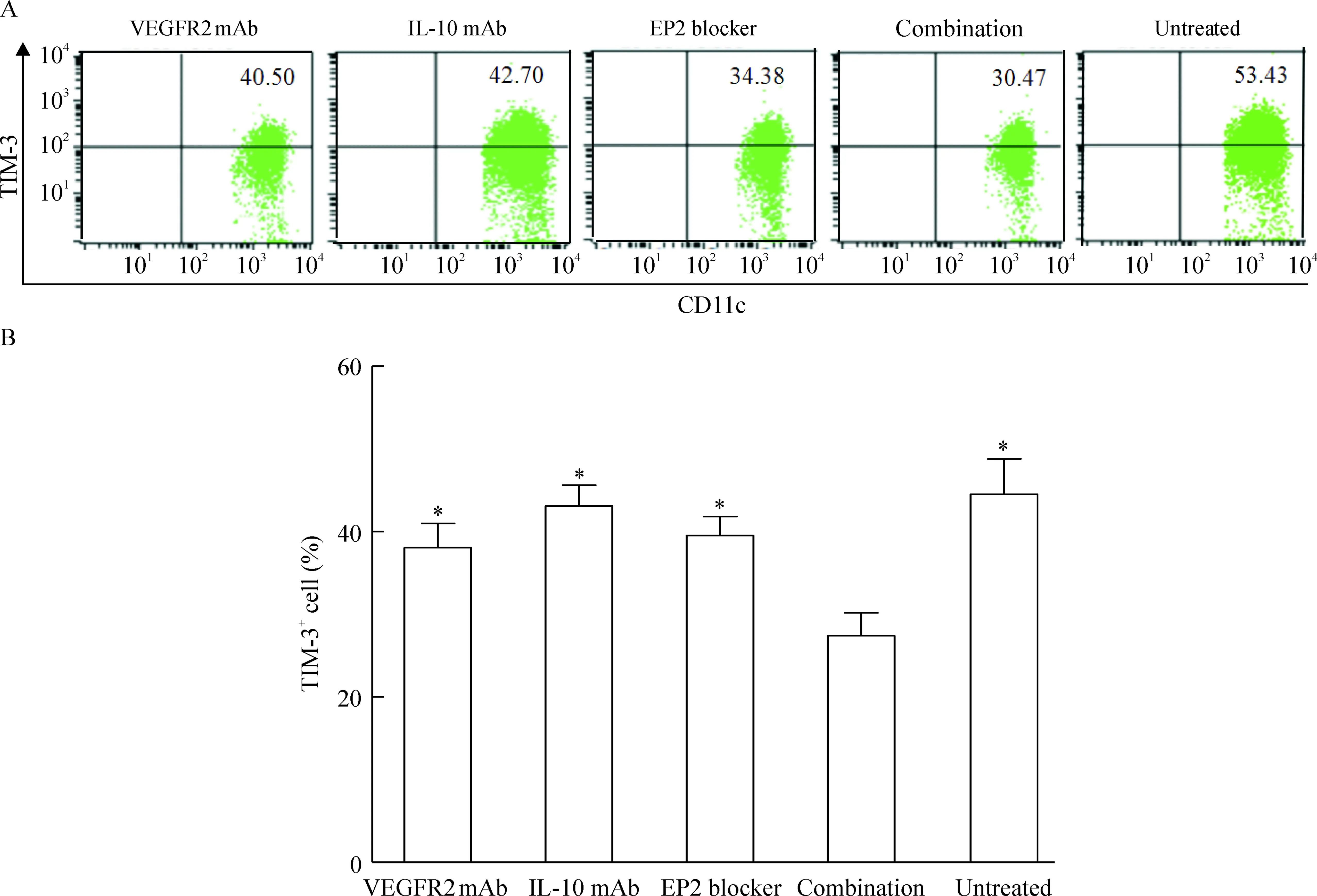
Figure 4.Expression of TIM-3 on surface of DCs treated with VEGF, IL-10 and PGE2inhibitors.A: expression of TIM-3 on surface of DCs treated for 48 h with supernatants of Capan-2 cells untreated or treated with anti-VEGFR2 (vascular endothelial growth factor receptor 2), anti-IL-10 and EP2 blocker (EP2 receptor bloking peptide) alone, or a combination of all three inhibitors; B: percentages of TIM-3+CD11c+cells among CD11c+cells in the 5 groups. Mean±SD.n=4.*P<0.05vscombination.
图4抑制胰腺癌细胞培养液上清中VEGF、IL-10和PGE2对DCs表达TIM-3的影响
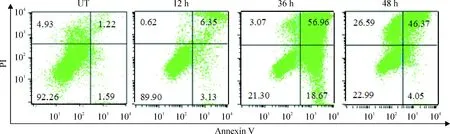
Figure 5.The percentages of apoptotic Capan-2 cells induced by DDP (27.2 mg/L) at different time points. UT: untreated.
图52倍IC50(27.2 mg/L)的DDP作用不同时间后Capan-2细胞的凋亡情况
讨 论
近期有学者研究报道,肺癌及结直肠腺癌荷瘤小鼠肿瘤组织浸润的DCs及晚期肺癌、胃腺癌等患者肿瘤相关性DCs (tumor-associated DCs,TADC) 均高表达TIM-3,而癌旁组织、脾和外周血中DCs表面TIM-3低表达或几乎不表达[5]。TIM-3表达与核酸介导的固有免疫密切相关。
TIM-3是2002 年Monney 等[7]发现并鉴定的Th1细胞表面的含免疫球蛋白和黏蛋白结构域的跨膜蛋白。TIM-3 可通过与其天然配体半乳糖凝集素-9结合,诱导Th1、Th17及Tc细胞凋亡或抑制其分化[8]。研究显示TIM-3还表达于多种固有免疫细胞亚群包括人NK细胞、巨噬细胞、未成熟的DCs[9-10]。而目前人胰腺癌TADC上TIM-3表达情况未见有报道。
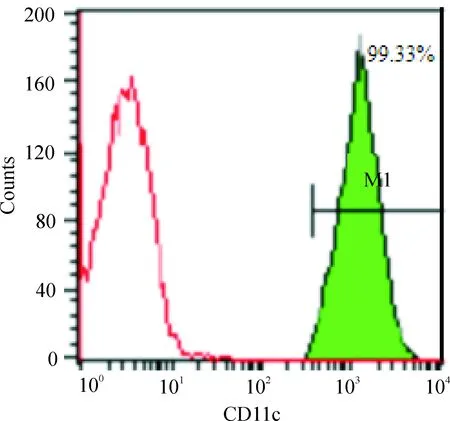
Figure 6.Purity of CD11c+DCs detected by flow cytometry after sorting. The left curve indicates the control antibody-labeled DCs, while the right M1 indicates percentage of CD11c+DCs labeled by fluoresence.
图6流式细胞术分选CD11c+DC的纯度检测
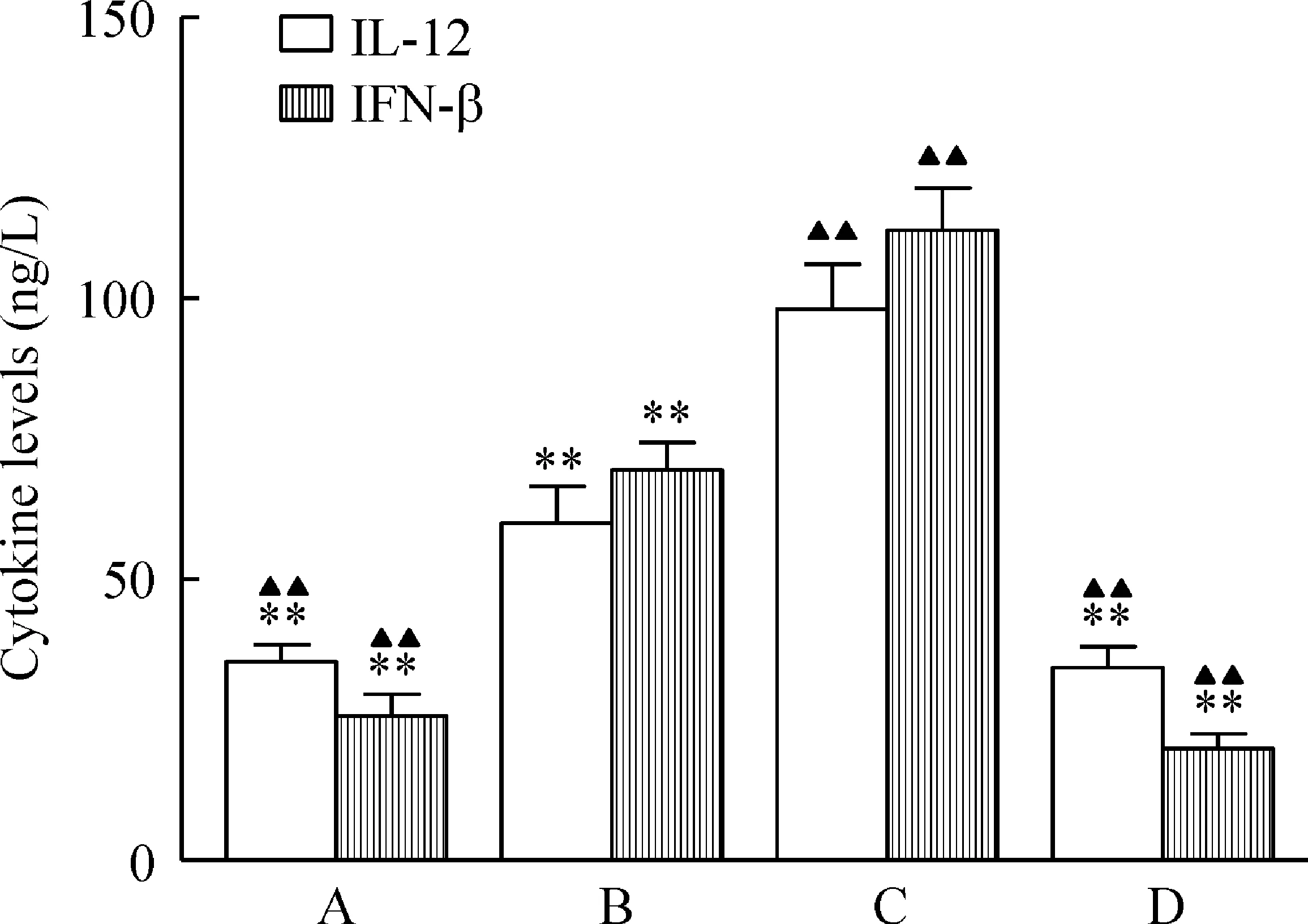
Figure 7.TIM-3 suppressed IL-12 and IFN-β production in DCs. The levels of IL-12 and IFN-β produced by four groups of dendritic cells after co-culture with apopto-tic Capan-2 cells at the ratio of 5∶1 for 24 h, detected by ELISA. A: Capan-2 Sup+DCs; B: Hs27 Sup+DCs; C: Capan-2 Sup+DCs+TIM-3 mAb; D: Capan-2 Sup+DCs+TIM-3 mAb+DNase+RNase. Mean±SD.n=4.**P<0.01vsC;▲▲P<0.01vsB.
图7DCs上TIM-3表达对IL-12和IFN-β分泌的影响
本研究通过免疫组化发现胰腺癌患者肿瘤组织中存在大量浸润性CD1α+S-100+DCs。我们进一步研究发现:胰腺癌组织中TIDC上TIM-3的表达明显高于癌旁组织中DCs及患者和健康人外周血DCs,提示胰腺癌局部微环境可能与DCs上TIM-3 表达增高有关。研究肿瘤微环境中TIDC表型及功能状态变化的原因,有助于深入认识肿瘤免疫逃逸机制,找到促进机体抗肿瘤免疫应答的治疗方法。
许多研究表明TIDC的分化和成熟发生障碍与肿瘤复杂的微环境关系密切[2-3]。在胰腺癌组织中,癌细胞可以产生细胞因子(如TGF-β、IL-10和IL-6),又可以表达表面分子(如VEGF、Fas-L、PD-L1和IDO)[11-13],肿瘤微环境内还存在许多免疫抑制细胞(如CAFs、tolerogenic DCs、MDSCs、immunosuppressive TAMs和Treg cells)[14],这些免疫抑制细胞可通过消耗精氨酸、产生ROS和NO来抑制抗肿瘤免疫,与Treg细胞分泌的转化生长因子 β(transforming growth factor β, TGF-β)和白细胞介素10(interleukin-10,IL-10)等共同形成一个免疫抑制环境,影响DCs分子表型和功能。我们通过研究人胰腺癌细胞培养液上清对DCs上TIM-3表达的影响,结果发现含有Capan-2、SW1990和Panc-1 3种胰腺癌细胞株上清的培养液对DCs上TIM-3表达均有不同程度上调作用;当在Capan-2细胞上清中联合加入VEGFR2 mAb、IL-10 mAb和PGE2受体EP2阻断剂时, DCs上TIM-3表达量有明显下调,但并未完全抑制其表达,而分别单独加入VEGFR2 mAb、IL-10 mAb和PGE2受体EP2阻断剂后,TIM-3表达则并无明显下调,提示胰腺癌微环境中参与DCs表达TIM-3调节的因素可能是多方面的,其中VEGF、IL-10及炎症因子PGE2等可能共同参与了对DCs上TIM-3表达的正性调节。且已有研究表明在体内肿瘤免疫抑制微环境中这些细胞因子相互影响,对DCs的调节作用更为广泛。VEGF是一种重要的血管生成刺激因子,由肿瘤细胞释放后,对肿瘤的血管生成起着关键作用,还可以抑制CD34+前体细胞向DCs的分化, 在抑制肿瘤微环境中TIDC 的成熟过程中发挥着重要作用,导致肿瘤逃避机体的免疫监视[15]。而IL-10能抑制DCs 的分化成熟及抗原提呈能力、表达免疫抑制性受体[16],同时IL-10 的分泌又受到TGF-β的影响,TGF-β可显著促进IL-10 的分泌[17]。另外免疫炎性反应也是肿瘤微环境的重要特征之一,在人和小鼠肿瘤内肿瘤相关巨噬细胞(tumor-assocciated macrophages,TAMs),能够产生大量的炎性细胞因子,肿瘤本身在 Toll样受体(Toll-like recepters,TLRs)配体激活后也会产生大量炎性细胞因子[18],PGE2在大肠癌、原发性胃癌、乳腺癌等多种肿瘤组织中均发现高表达[19-21],是肿瘤微环境中重要的炎症因子,参与了TIDC免疫抑制的发生[22]并且PGE2可通过EGFR-MAPK信号通路上调VEGF的表达[22-23];复杂的肿瘤免疫抑制的微环境[24-25],使得肿瘤局部免疫应答逐渐趋于负性调节,形成免疫耐受是导致肿瘤细胞免疫逃逸的重要原因。
综上所述,肿瘤微环境中负性免疫抑制因子及炎症因子可能是造成TIDC上TIM-3表达上调的原因之一。
本研究还初步探讨了TIM-3对TIDC功能的影响。IL-12是一种Th1型细胞因子,主要由成熟树突状细胞产生,是Th0细胞向Th1细胞分化的主要诱导因子,参与肿瘤细胞免疫,与NK细胞、T淋巴细胞等协同发挥抗肿瘤作用[26-29]。INF-β属于Ⅰ型干扰素,能促进CD4+T细胞分泌IFN-γ,可激活NK细胞,进一步增强NK细胞非特异性杀伤靶细胞的作用。检测IFN-β和IL-12水平,可以反映DCs固有免疫功能。细胞毒性药物DDP是胰腺癌化疗的一线用药,能引起肿瘤细胞凋亡,同时会释放内源性核酸分子(DNA或RNA)导抗肿瘤的免疫应答[30]。我们通过实验发现TIM-3+DCs与凋亡胰腺癌细胞Capan-2共培养后上清中细胞因子IL-12和IFN-β水平较对照组水平低,而阻断TIM-3后,IFN-β和IL-12水平明显升高,但加入DNase和RNase后,这种升高趋势被消除,提示胰腺癌微环境中TIDC上表达的TIM-3可能参与了核酸介导的固有免疫应答的负性调节,TIDC上TIM-3 的高表达可能抑制了化疗诱导的肿瘤细胞死亡后释放的内源性核酸对DCs固有免疫免疫应答的激活,这也可能是导致胰腺癌对化疗产生耐药原因之一。而阻断TIM-3是否可以增强胰腺癌化疗的效果、促进胰腺癌核酸疫苗抗肿瘤免疫应答有待于体内实验的验证。
本研究结果表明:TIM-3是胰腺癌肿瘤微环境中DCs固有免疫激活的负性调节因子之一;肿瘤细胞分泌的VEGF、IL-10和PGE2可能参与了对TIM-3表达的调控。研究为胰腺癌以DCs为基础的免疫治疗提供新的思路。但是,引起TIM-3高表达的转录因子及其下游的信号通路如何?在不同组织环境TIM-3通过什么途径对固有免疫、适应性免疫进行调节?如何改善免疫抑制性的微环境提高DCs抗肿瘤效应?这一系列问题都有待进一步探索。
[1]Tanemura M, Miyoshi E, Nagano H, et al.Cancer immunotherapy for pancreatic cancer utilizing α-gal epitope/natural anti-Gal antibody reaction[J].World J Gastroenterol, 2015,21(40):11396-11410.
[2]Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells[J]. Annu Rev Immunol,2007, 25:267-296.
[3]巴俊慧, 吴本权,王艳红,等.MUC1 mRNA 体外负载树突状细胞诱导细胞毒性T 细胞对非小细胞肺癌的杀伤作用[J].中国病理生理杂志,2014,30(9):1574-1579.
[4]Golden-Mason L, Palmer BE, Kassam N, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+and CD8+T cells[J]. J Virol, 2009, 83(18):9122-9130.
[5]Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1[J]. Nat Immunol,2012,13(9):832-842.
[6]刘锡娟,丁慧荣,田志华,等. FACSAria流式细胞仪无菌操作分选高纯度细胞亚群[J]. 安徽医科大学学报,2014,49(12):1811-1814.
[7]Monney L,Sabatos CA,Gaglia JL,et al.Th1-specific cell surface protein TIM-3 regulatesmacrophage activation and severity of an autoimmune disease[J]. Nature,2002,415(6871):536-541.
[8]Tembhre MK, Parihar AS, Sharma A,et al.Participation of T cell immunoglobulin and mucin domain-3 (TIM-3) and its ligand (galectin-9) in the pathogenesis of active generalized vitiligo[J].Immunol Res,2015,62(1):23-34.
[9]Anderson AC, Anderson DE, Bregoli L,et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells[J]. Science, 2007, 318(5853):1141-1143.
[10]Han G,Chen G,Shen B,et al. Tim-3: an activation mar-ker and activation limiter of innate immune cells[J]. Front Immunol,2013,4:449.
[11]Teraoka H, Sawada T,Nishihara T,et al. Enhanced VEGF production and decreased immunogenicity induced by TGF-beta 1 promote liver metastasis of pancreatic cancer[J]. Br J Cancer,2001, 85(4):612-617.
[12]Morse MA, Hall JR, Plate JM. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer[J]. Expert Opin Biol Ther,2009,9(3):331-339.
[13]Loos M, Giese NA, Kleeff J,et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer[J].Cancer Lett, 2008,268(1):98-109.
[14]Koido S, Homma S, Hara E, et al. Regulation of tumor immunity by tumor/dendritic cell fusions[J]. Clin Dev Immunol, 2010,2010:516768.
[15]Gabrilovich DI,Ishida T, Ohm JE,et al. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function[J]. Clin Cancer Res,1999,5(10):2963-2970.
[16]刘峥嵘,张敏,黎纬明,等.IL-10诱导小鼠树突状细胞耐受的分子机制[J].中国病理生理杂志,2008, 24(2):374-378.
[17]Allavena P, Piemonti L, Longoni D, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophage[J]. Eur J Immunol,1998,28(1):359-369.
[18]He W,Liu Q,Wang L,et al. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance[J].Mol Immunol,2007,44(11): 2850-2859.
[19]Jaffe BM,Parker CW,Philpott GW.Immunochemical measurement of prostaglandin or prostaglandin-like activity from normal and neoplastic cultured tissue[J].Surg Forum,1971,22:90-92.
[20]Uefuji K,Ichikura T,Mochizuki H.Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer[J].Clin Cancer Res,2000,6(1):135-138.
[21]Watson J,Chuah SY.Prostaglandins,steroids and human mammary cancer[J].Eur J Cancer Clin Oncol,1985,21(9):1051-1055.
[22]Obermajer N,Muthuswamy R,Lesnock J,et al.Positive feedback between PGE2and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells[J].Blood,2011,118(20):5498-5505.
[23]Ding YB,Shi RH,Tong JD,et al.PGE2 up-regulates vascular endothelial growth factor expression in MKN28 gastric cancer cells via epidermal growth factor receptor signaling system[J].Exp Oncol,2005,27(2):108-113.
[24]Shevach EM. Mechanisms of Foxp3+T regulatory cell-mediated suppression[J]. Immunity, 2009,30(5):636-645.
[25]Mougiakakos D, Choudhury A, Lladser A, et al. Regulatory T cells in cancer[J]. Adv Cancer Res,2010,107:57-117.
[26]Minkis K, Kavannagh DG, Alter G, et al.Type 2 bias of T cell expanded from the blood of melanoma patients switched to type1 by IL-12p70 mRNA-transfected dendritic cells[J]. Cacer Res, 2008, 68(22): 9441-9450.
[27]Sinigaglia F, D’Ambrosio D, Panina-Bordignon P,et al. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function[J]. Immunol Rev, 1999, 170(8):65-72.
[28]Dowell AC, Oldham KA, Bhatt RI, et al. Long-term proliferation of functional human NK cells, with conversion of CD56dimNK cells to a CD56brightphenotype, induced by carcinoma cells co-expressing 4-1BBL and IL-12[J].Cancer Immunol Immunother, 2012,61(5):615-628.
[29]Tugues S, Burkhard SH, Ohs I,et al.New insights into IL-12-mediated tumor suppression[J].Cell Death Differ, 2015,22(2):237-246.
[30]Emens LA.Chemotherapy and tumor immunity: an unexpected collaboration[J].Front Biosci, 2008,13(1):249-257.
(责任编辑: 林白霜, 罗森)
Effect of human pancreatic cancer cell supernatant on expression of TIM-3 and function of dendritic cells
XIA Yuan-yuan1,2, CAI Chang-qing2, LU Ya-nan3, GAN Hui-quan4,ZHOU Quan-bo5
(1GuangdongMedicalCollege,Zhanjiang524023,China;2DepartmentⅡofOncology,TheSecondPeople’sHospitalofGuangdongProvince,Guangzhou510317,China;3DepartmentofAnesthesiology,SunYat-senMemorialHospital,SunYat-senUniversity,Guangzhou510120,China;4DepartmentofClinicalLaboratory,GuangdongGeneralHospital,Guangzhou510080,China;5DepartmentofHepatopancreatobiliarySurgery,SunYat-senMemorialHospital,SunYat-senUniversity,Guangzhou510120,China.E-mail:cchangq@163.com;zhquanbo@126.com)
AIM: To investigate the influence and mechanisms of human pancreatic cancer tumor microenvironments on T-cell immunoglobulin mucin-3 (TIM-3) expression and function of dendritic cells (DCs). METHODS: Tumor-infiltrating dendritic cells (TIDC) and para-carcinoma tissue DCs were isolated by Ficoll-Hypaque density centrifugation from trypsinized pancreatic carcinoma tissues, and the peripheral blood mononuclear cells were isolated from pancreatic cancer patients or healthy people. The expression of TIM-3 on DCs was detected by flow cytometry. DCs isolated from healthy people peripheral blood mononuclear cells were induced by rhGM-CSF and IL-4. The expression of TIM-3 in the DCs treated with the culture supernatants of Capan-2, SW1990 and Panc-1 pancreatic cancer cells or human skin fibroblast (Hs27) cells for 48 h, and in the DCs treated with supernatants of Capan-2 cells, anti-VEGF-R2, anti-IL-10 and EP2 receptor blocking peptide were evaluated by flow cytometry. The releases of IFN-β and IL-12 in the culture supernatants of DCs pretreated with monoclonal antibody (mAb) to TIM-3 or DNase+RNase, followed by stimulation with apoptotic Capan-2 cells, were detected by ELISA. RESULTS: DCs in tumor microenvironments had higher expression of TIM-3 than the DCs from para-carcinoma tissues and pancreatic cancer patient or healthy people peripheral blood (P<0.01). TIM-3 expression in the DCs treated with the culture supernatants of Capan-2, SW1990 and Panc-1 pancreatic cancer cells for 48 h was much higher than that in Hs27 cells (P<0.05). Treatment with a combination of anti-VEGF-R2, anti-IL-10 and EP2 receptor blocking peptide largely diminished the upregulation of TIM-3 on the DCs mediated by Capan-2 cell supernatants (P<0.05). The concentrations of IFN-β and IL-12 in the DCs with high expression level of TIM-3 were lower than those in the DCs with low TIM-3 expression level. Treatment with mAb to TIM-3 resulted in much more IFN-β and IL-12 releases (P<0.01), but DNase+RNase made this effect disappear. CONCLUSION: TIM-3 serves as a negative regulator of DCs innate immune responses in the pancreatic cancer microenvironments. The secretion of soluble factors to tumor microenvironment by pancreatic cancer cells, including IL-10, VEGF and PGE2, may contribute to the regulation of TIM-3 expression.
Pancreatic cancer; Dendritic cells; Tumor microenvironment; T-cell immunoglobulin and mucin-3
1000- 4718(2016)04- 0628- 09
2015- 11- 02
2016- 01- 13
国家自然科学基金资助项目(No.81000917;No.81370059)
Tel: 020-89168114; E-mail: cchangq@163.com; zhquanbo@126.com
R730.23
A
10.3969/j.issn.1000- 4718.2016.04.009
杂志网址: http://www.cjpp.net

