Quantification of photosynthetic inorganic carbon utilisation via a bidirectional stable carbon isotope tracer
Hongtao Hang·Yanyou Wu
ORIGINAL ARTICLE
Quantification of photosynthetic inorganic carbon utilisation via a bidirectional stable carbon isotope tracer
Hongtao Hang1,2·Yanyou Wu1
The amount of bicarbonate utilised by plants is usually ignored because of limited measurement methods. Accordingly,this study quantified the photosynthetic assimilation of inorganic carbon(CO2and HCO3-)by plants.The net photosynthetic CO2assimilation(PN),the photosynthetic assimilation of CO2and bicarbonate(PN’),the proportion of increased leaf area(fLA)and the stablecarbonisotopecomposition(δ13C)of Orychophragmus violaceus(Ov)and Brassica juncea(Bj)under three bicarbonate levels(5,10 and 15 mm NaHCO3)were examined to determine the relationship among PN,PN’and fLA.PN’,not PN,changed synchronously with fLA. Moreover,the proportions of exogenous bicarbonate and total bicarbonate(including exogenous bicarbonate and dissolved CO2-generated bicarbonate)utilised by Ov were 2.27%and 5.28%at 5 mm bicarbonate,7.06%and 13.28%at 10 mm bicarbonate,and 8.55%and 17.31%at 15 mm bicarbonate,respectively.Meanwhile,the proportions of exogenous bicarbonate and total bicarbonate utilised by Bj were 1.77%and 3.28%at 5 mm bicarbonate,2.11%and 3.10%at 10 mm bicarbonate,and 2.36%and 3.09%at 15 mm bicarbonate,respectively.Therefore,the dissolvedCO2-generatedbicarbonateandexogenous bicarbonate are important sources of inorganic carbon for plants.
Karst·Bicarbonate·Photosynthesis· Inorganic carbonic utilization·Stable carbon isotope composition
1 Introduction
In general,terrestrial plants prioritize the use of atmospheric CO2as their principal inorganic carbon source for photosynthesis.Inkarstregions,karstrocks mainly develop in limestone(CaCO3)during the dynamic chemicaldissolutionofcalciumcarbonate[CaCO3+H2-O+CO2→Ca2++HCO3-],wherewaterand atmospheric CO2are consumed.A large amount of dissolved inorganic carbon(DIC)in the form of HCO3-exists in the surface runoff.Therefore,plants growing in the karst regions can utilise both atmospheric CO2and dissolved HCO3-for photosynthesis(Waele et al.2009;Palmer 1991;Wu and Xing 2012;Yan et al.2012).The photosynthetic assimilation of CO2in plants and the chemical dissolution of carbonate rocks are important CO2sinks. Thus,research on the photosynthetic assimilation of inorganic carbon(atmospheric CO2and dissolved HCO3-)in plants is crucial for providing evidence on plant productivity and carbon sinks in the karst regions.
Photosynthetic activities,which include the net assimilation of CO2,indicate the potential growth and productivity of plants.The photosynthetic rate reflecting the photosynthetic assimilation of CO2in plants can be accurately determined using an open gas-exchange system(Long and Bernacchi 2003).Several studies indicated that plants can utilise exogenous bicarbonate as an alternative inorganic carbon source for photosynthesis when sources of the exogenous inorganic carbon change(Raven 1970;Shelp and Canvin 1980).However,the photosyntheticassimilation of bicarbonate in plants cannot be determined using an open gas exchange system.Some plants utilise exogenous bicarbonate(Wu and Xing 2012;Raven et al. 1982;Price et al.1985),whereas others utilise bicarbonate generated from dissolved CO2.However,the dissolution of carbonate rocks currently remains unclear.
✉ Yanyou Wu wuyanyou@mail.gyig.ac.cn
1State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550081,China
2University of Chinese Academy of Sciences,Beijing 100049,China
The stable carbon isotope technique is commonly used to identify various carbon sources utilised by plants.The stable carbon isotope ratio(δ13C)can be applied as an index for carbon metabolic processes,such as photosynthesis(Farquhar et al.1989).Labelling the stable carbon isotope in exogenous bicarbonate can trace whether or not plants utilise CO2from the conversion of exogenous bicarbonate for photosynthesis.Therefore,the changes of the δ13C of plant tissues in different culture environments can reflect the sources of inorganic carbon for photosynthesis,as carbon metabolic pathways change when plants are exposed to various environmental conditions.
Orychophragmus violaceus(L.)and Brassica juncea(L.)Czern.et Coss.cv.Zangyou No.8 are cruciferous plants commonly used as experimental materials because of their high tolerance to bicarbonate stress in Southwest China(Wu et al.2005;Wang et al.2014).In this study,the photosynthetic and growth parameters of O.violaceus and B.juncea under different levels of exogenous bicarbonate were examined to determine their potential productivity. Moreover,the δ13C values of leaves and culture solutions were measured to determine the photosynthetic assimilation of inorganic carbon(atmospheric CO2or HCO3-)under water culture conditions.Furthermore,the relationship between the δ13C and photosynthesis of the two plant species was studied to explore the capacity of the plants to utilise bicarbonate and the‘missing carbon sink’involved in the chemical dissolution of carbonate rocks.
2 Materials and methods
2.1 Plant materials and experimental treatments
The O.violaceus and B.juncea were obtained from the Institute of Geochemistry,Chinese Academy of Sciences and the Guizhou Institute of Rapeseed,respectively.Seeds of these plants were germinated in 12-hole trays with perlitesinagreenhouseata12 hlightcycle(200 μmol m-2s-1,PPFD),a day/night temperature range of 25°C/18°C,and a relative humidity range of 50%-60%in the laboratory of the Institute of Geochemistry,Chinese Academy of Sciences,Guizhou Province,China(26.57°N,106.72°E,altitude of 1045 m).Seedlings which germinated in a uniform size were selected and cultured with half-strength Hoagland nutrient solution(Hoagland and Arnon 1950).5,10,15 mm NaHCO3labelled with δ13C value of-2.45‰ were added into the Hoagland nutrient solution to simulate three bicarbonate levels to culture two-old seedlings which germinated healthily and uniformly from each plant species.Meanwhile,three levels of bicarbonate labelled with δ13C value of-24.409‰were used to culture two-old seedlings that germinated healthily and uniformly.Eighteen healthy and uniform seedlings from each plant species were subjected to both treatments.The modified Hoagland nutrient solution was changed daily to maintain consistency for each treatment. All measurements were conducted in triplicates.
2.2 Leaf gas exchange
Leaf gas exchange was determined between 09:00 and 11:00 am by using an open gas-exchange system(Li-6400,Li-Cor,Lincoln,NE,USA).Photosynthesis was induced with light(200 μmol m-2s-1,PPFD)and ambient CO2concentration(400 μmol mol-1).The net photosynthetic rate(PN,μmol CO2m-2s-1),the transpiration rate(E,mmol H2O m-2s-1),and the stomatal conductance(gs,mol H2O m-2s-1)were measured on the youngest fully expanded leaf from the top of all the tested plants on day 7 after the onset of bicarbonate treatment.Water use efficiency(WUE)wascalculatedusingthefollowing equation:
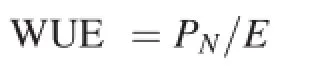
where PNis the net photosynthetic rate and E is the transpiration rate.
2.3 Determination of leaf biomass
The leaf biomass was estimated to determine the leaf area(LA,mm2)of the youngest fully expanded leaf of both plants from each bicarbonate treatment(Evans and Poorter 2001).After 1,3,5,7,9,11,and 13 days of water culture,the leaf length(XL,mm)and the maximum leaf width(XW,mm)of the youngest fully expanded leaves from each bicarbonatetreatmentweredeterminedusinga portable digital caliper.Leaves in varying sizes of each plant species were randomly selected to determine the LA,leaf length,and maximum leaf width on day 7.The values of LA were estimated using the leaf length and the maximum leaf width of all the leaves on the basis of the following power curve equation:

where LA(mm2)is the value of the LA of each plant at different bicarbonate treatments on day 7,and b0and b1are constants.
To eliminate the physiological errors produced by the initial leaf,the initial leaf length and maximum leaf widthof each plant species under different bicarbonate treatments were calibrated using the followed logistic equation:
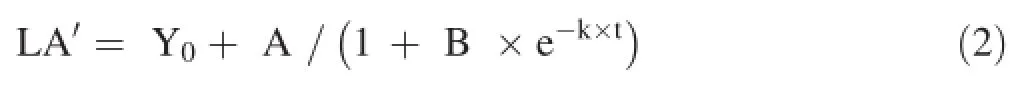
where LA’is the LA of each plant treated with bicarbonate;A,B,and K are constants;and t is the culture time(t=0,1,3,5,7,9,11,13 days).
Furthermore,the proportion of increased biomass(fLA)during bicarbonate treatment can be calculated as

2.4 Determination of the stable carbon isotope composition in leaves
The stable carbon isotope ratios(δ13CL)of the first youngest fully expanded leaf from the top of each tested plants at each bicarbonate treatment level was determined via gas isotope ratio mass spectrometry(MAT-252,Finnigan MAT,Bremen,Germany).The δ13C values of leaves from three seedlings in each plant species under each bicarbonate level were determined on day 7 after the bicarbonate treatment.The stable carbon isotope ratios(δ13C)in all samples were calculated using a standard equation(Pee Dee Belemnite,PDB)and expressed as Eq.4.The accuracy of the analysis was±0.1‰.
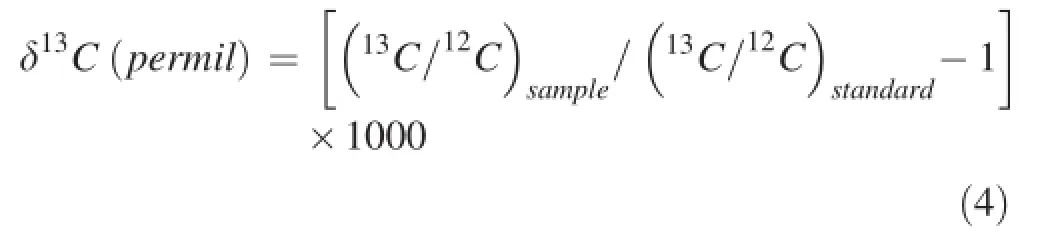
2.5 Determination of the proportion of exogenous bicarbonate in the nutrient solution
The stable carbon isotope ratios(δ13CNS)in the nutrient solution from each plant under each treatment were determined 1 day after the bicarbonate treatment via gas isotope ratio mass spectrometry.
According to the bivariate isotope-mixture model,
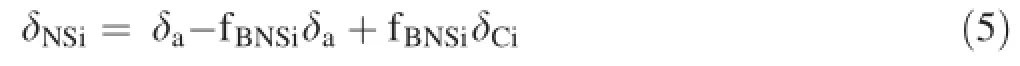
where δNSiis the δ13C value of the nutrient solution,δais the δ13C value of the bicarbonate generated from atmospheric carbon dioxide,δCiis the δ13C value of the initial nutrient solution added with exogenous NaHCO3,and fBNSiis the proportion of exogenous bicarbonate in the total inorganic carbon sources in the nutrient solution.
For the exogenous NaHCO3labelled with a δ13C value of-2.45‰PDB,Eq.5 can be changed to

where δNS1is the δ13C value of the nutrient solution,δais the δ13C value of the bicarbonate generated from atmospheric carbon dioxide,δC1is the δ13C value of the initial nutrient solution added with exogenous NaHCO3and labelled with a δ13C value of-2.45‰PDB,and fBNS1is the proportion of exogenous bicarbonate in the total inorganic carbon sources in the nutrient solution.Similarly,for the exogenous NaHCO3labelled with a δ13C value of -24.409‰PDB,Eq.5 can be changed to
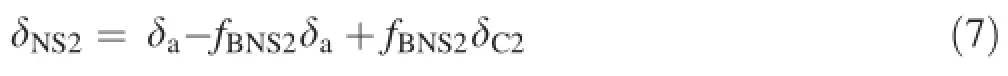
where δNS2is the δ13C value of the nutrient solution,δais the δ13C value of the bicarbonate generated from atmospheric carbon dioxide,δC2is the δ13C value of the initial nutrient solution added with exogenous NaHCO3and labelled with a δ13C value of-24.409‰PDB,and fBNS2is the proportion of exogenous bicarbonate in the total inorganic carbon sources in the nutrient solution.
Plant seedlings with uniform sizes were randomly selected for analysis;thus,fBNS1can be equal to fBNS2.-Comparing Eq.6 with Eq.7,we can calculate fBNSas
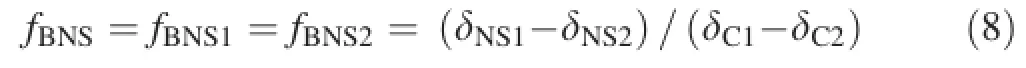
where δNS1is the δ13C value of the bicarbonate treatment solution added with NaHCO3and labelled with a δ13C value of-2.45‰PDB,δNS2is the δ13C value of the bicarbonate treatment solution added with NaHCO3and labelled with a δ13C value of-24.409‰PDB,and fBNSis the proportion of exogenous bicarbonate in the total inorganic carbon sources in the nutrient solution.
2.6 Calculations of the bicarbonate utilisation proportion and corrected photosynthetic rate
For the bivariate isotope-mixture model,

δLis the δ13C value of the leaves in the tested plants cultivated with NaHCO3and labelled with an δ13C value of -2.45‰or-24.409‰PDB,δAis the δ13C value of the leaf in the tested plants with atmospheric CO2as the sole carbon source,δBis the δ13C value of the leaf of the tested plants with exogenous NaHCO3as the sole carbon source,and fBLis the proportion of exogenous bicarbonate utilised by the tested plants under each bicarbonate treatment.
For the exogenous NaHCO3labelled with a δ13C value of-2.45‰PDB,Eq.9 can be changed to

where δL1is the δ13C value of the leaves in the tested plants cultivated with NaHCO3and labelled with a δ13C value of-2.45‰PDB,δA1is the δ13C value of the leaf in the tested plants with atmospheric CO2as the sole carbon source,δB1is the δ13C value of the leaf of the tested plants with exogenous NaHCO3as the sole carbon source,and fBL1is the proportion of exogenous bicarbonate utilised by the tested plants under each bicarbonate treatment.
Similarly,for the exogenous NaHCO3labelled with a δ13C value of-24.409‰PDB,Eq.9 can be changed to
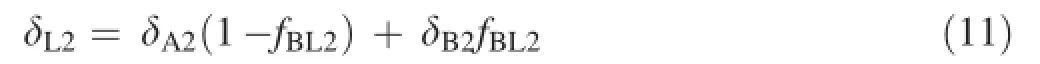
where δL2is the δ13C value of the leaves in the tested plants cultivated with NaHCO3and labelled with a δ13C value of-24.409‰PDB,δA2is the δ13C value of the leaf in the tested plants with atmospheric CO2as the sole carbon source,δB2is the δ13C value of the leaf in the tested plants with exogenous NaHCO3as the sole carbon source,and fBL2is the proportion of exogenous bicarbonate utilised by the tested plants under each bicarbonate treatment.
In this study,plant seedlings with uniform sizes were randomly selected for analysis.Thus,δA1could be equal to δA2,and fBL1could be equal to fBL2.Comparing Eq.10 with Eq.11,we can calculate the proportion of utilised exogenous bicarbonate(fBL)as

For(δB1-δB2)in Eq.12,the difference can be replaced with(δC1-δC2),where δC1and δC2represent the δ13C valuesofNaHCO3labelledwith-2.45‰and -24.409‰PDB,respectively.Thus,Eq.12 can be changed to
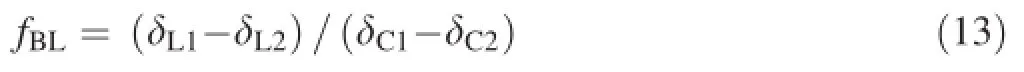
where fBLonly represents the proportion of exogenous bicarbonate used by the plants(in total leaf biomass)as a photosynthetic substance during the bicarbonate treatment. Thus,the bicarbonate utilisation proportion(fb)of the increased leaf biomass of each plant during the bicarbonate treatment can be calculated as

where fbis the proportion of utilised exogenous bicarbonate with increased leaf biomass(LA’i-LA’0)during the bicarbonate treatment,fBLis the proportion of exogenous bicarbonate used by the plants(in total leaf biomass)as a photosynthetic substance during the bicarbonate treatment,and fLAis the proportion of increased biomass during the bicarbonate treatment.
We used Eq.14 to determine the proportion of exogenous NaHCO3used by the plants(in increased leaf biomass)as a photosynthetic inorganic carbon during the bicarbonate treatment.However,the addition of both exogenous bicarbonate and dissolved atmospheric CO2in the nutrient solution can trigger a reversible chemical reaction because of the bicarbonate treatment.We assumed that fBNS0and fBNSare the proportions of exogenous bicarbonate in the initial and final nutrient solutions,respectively.In this study,the value of fBNS0can be considered as 1,and the value of fBNScan be calculated using Eq.8.Furthermore,the proportion of total bicarbonate(fb’)utilised by each plant during the bicarbonate treatment can be calculated with

The bicarbonate utilisation capacity(BUC)and the corrected photosynthetic rate(PN’)were calculated with
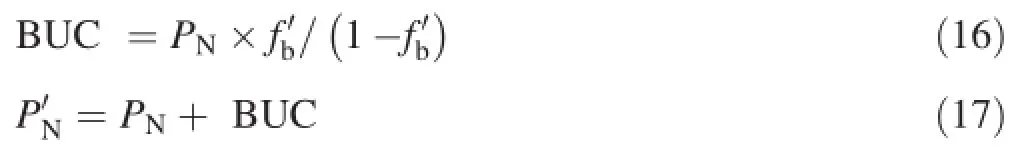
where PNis the net photosynthetic rate of the plants with CO2as the sole carbon source for photosynthesis,fb’is the proportion of total bicarbonate utilised by the plants,and BUC is the photosynthetic rate of the plants which catalysed bicarbonate into CO2for photosynthesis7.
2.7 Data analysis
Data were subjected to ANOVA to determine significant differences(defined as P≤0.05)between group means. Data are shown as mean±standard error(SE)via factorial analysis with SPSS(version 20.0).The mean results were compared via a Duncan post hoc test at the 5%significance level(P≤0.05).
3 Results
3.1 Gas exchange
TheO.violaceusandB.junceaexhibiteddifferentchangesin gas exchangecharacteristics,suchasPN,gs,andWUE,under variousbicarbonatelevels(Fig.1).PNsignificantly decreased in O.violaceus but increased in B.juncea after the bicarbonate treatment.However,both plants showed no significant changes in gsafter treatments with different bicarbonate levels.Furthermore,the WUE values of both plants significantly increased after the bicarbonate treatment,and the highest WUE was achieved at 10 mm bicarbonate.
3.2 Leaf biomass
To estimate accurately the leaf biomass,15 leaves of varying sizes from each plant species were randomly selected to determine the LA,leaf length and maximumleaf width.The LAs can be estimated using the power curve equation(Table 1).Furthermore,the leaf area of both plants in the hydroponic culture with bicarbonates for various culturing times can be fitted using the logistic growth model equation(Table 2).The initial leaf,initial leaf length,and maximum leaf width of each plant species under various bicarbonate treatments were calibrated on the basis of the logistic growth equation to eliminate physiological errors.
The O.violaceus and B.juncea exhibited different changes in fLAvalues under different bicarbonate levels(Fig.2).The highest and lowest fLAvalues in O.violaceus were under 10 and 15 mm bicarbonate levels,respectively. Those of B.juncea were under 10 and 5 mm bicarbonate levels,respectively.
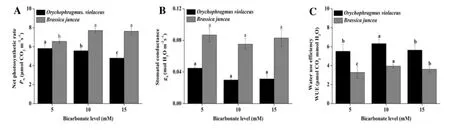
Fig.1 Net photosynthetic rate(a,PN),stomatal conductance(b,gs)and water use efficiency(c,WUE)of Orychophragmus violaceus and Brassica juncea under bicarbonate treatments.The mean±SE(n=9)followed by different letters in the same plant species differ significantly at p≤0.05 subjected to one-way ANOVA and t-test

Table 1 Leaf area estimation model

Table 2 The model of growth rate(leaf area)with time(t)
3.3 Leaf stable carbon isotope ratios
The δ13C values of the leaves varied with plant species and bicarbonate treatments(Table 3).The δ13C values of O. violaceus were higher than those of B.juncea.Moreover,the δ13C values of O.violaceus and B.juncea were higher under treatment with 10 mm bicarbonate than under treatments with other bicarbonate levels.
3.4 Stable carbon isotope rations in the nutrient solution
Similarly,the δ13C values in the bicarbonate treatment solutions varied with plant species and bicarbonate levels(Table 4).Whenthebicarbonateconcentrationwasincreased,the δ13C values in the initial nutrient solutions(δ1and δ2)used to culture the plants for 1 day were similar to those of the exogenous bicarbonate(δC1and δC2).
3.5 BUC and corrected photosynthetic rates
The seedlings of the two plants were cultured for 7 d under treatments with different levels of exogenous NaHCO3and labelledwithδ13Cvaluesof-24.409‰and -2.45‰PDB.The fBLand fb’of both plants were calculated using Eqs.13 and 15,respectively.The fBLand fb’values of O.violaceus were significantly increased with the increasing concentration of bicarbonate,while those in B. juncea had no significant change(Fig.3a).The fBLand fb’values of B.juncea were lower than those of O.violaceus under each bicarbonate treatment.The photosynthetic inorganic carbon assimilation capacities(BUC and PN’)were calculated using Eqs.16 and 17,respectively.O. violaceus had higher BUC than B.juncea under the same bicarbonate treatment(Fig.3b).However,the PN’of O. violaceus were lower than that of B.juncea under each treatment.Moreover,both plants had the highest PN’values under the 10 mm bicarbonate level.
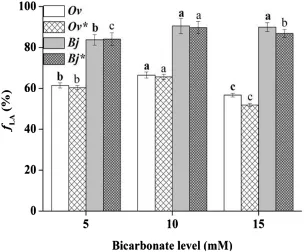
Fig.2 The proportion of the increased leaf biomass(fLA)during bicarbonate treatment,asterisk represents the calibrated fLAvalues in the same parameters.Ov-Orychophragmus violaceus,Bj-Brassica juncea.The mean±SE(n=9)followed by different letters in the same plant species differ significantly at p≤0.05 subjected to oneway ANOVA and t-test
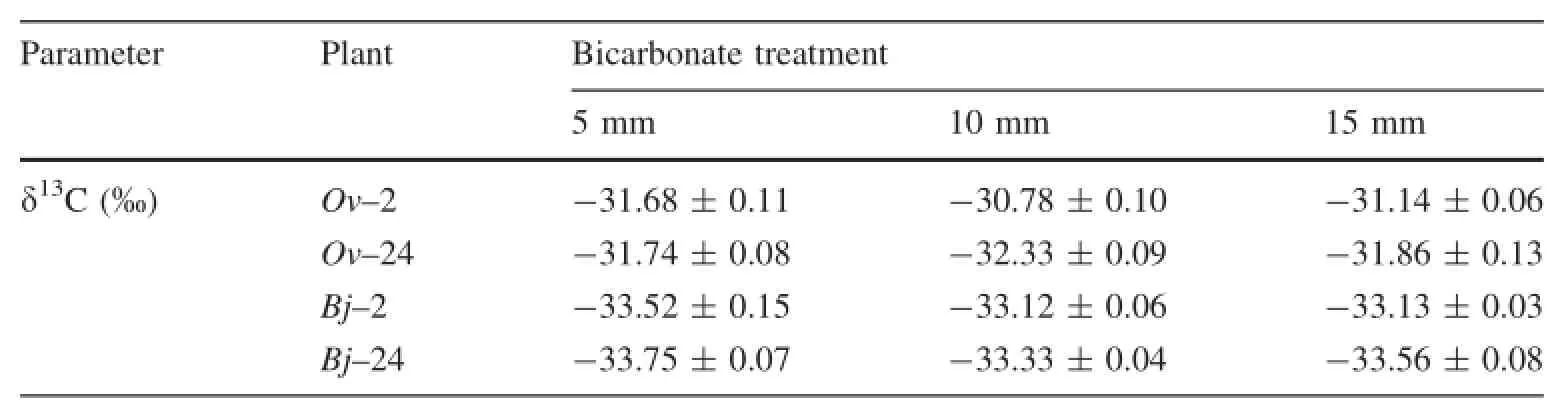
Table 3 δ13C values of the leaves of Orychophragmus violaceus and Brassica juncea under bicarbonate treatments
4 Discussion
TerrestrialplantsutiliseatmosphericCO2astheir principal inorganic carbon source for photosynthesis. However,theseplantscanalsoutiliseexogenous bicarbonate as an alternative inorganic carbon source for photosynthesis when sources of exogenous inorganic carbon change(Raven 1970;Shelp and Canvin 1980).In karst regions,during the chemical dissolution ofcarbonaterocks(CaCO3+H2O+CO2→-Ca2++HCO3-),the majority of DIC is involved in the formation of bicarbonate.In the presence of plants,the dissolution of carbonate rocks can be accelerated by various biological effects.Therefore,plants growing in karst regions have access to both atmospheric CO2and bicarbonate for photosynthesis(Waele et al.2009;Yan et al.2012;Raven 1970).
PNreflects the photosynthetic CO2assimilation and thus the potential growth and productivity of plants.This index can be determined using the open gas exchange system. Several studies determined the bicarbonate utilisation capacities of particular plants under hydroponic culture conditions via the stable carbon isotope technique;however,the fact that the bicarbonate generated from the dissolved CO2is utilised by the plants was ignored(Long and Bernacchi 2003;Wu and Xing 2012).In natural environments,quantifying the photosynthetic assimilation of bicarbonate in plants is difficult.Based on previous studies,the present study developed a new and improved method to quantify the bicarbonate utilisation capacity of plants under various bicarbonate levels via the stable carbon isotope technique in hydroponic culture.
The PNand LA of both plants under various bicarbonate levels were examined in this study(Figs.1a,2).When the bicarbonate levels increased,the PNof O.violaceus significantly decreased,whereas that of the B.junceasignificantly increased.The increased fLAin O.violaceus changed non-synchronously with PNas the bicarbonate treatment intensified(Fig.4a).Meanwhile,the fLAvalues in the leaves of the B.juncea changed synchronously with the PNin response to various bicarbonate treatments.The deviation between the fLAand PNof the O.violaceus revealed that the PNvalues determined using the open gas exchange system did not reflect the true response to bicarbonate treatments and that some errors of the fLAvalues were caused by the difference in the properties of the initial leaves.Thus,an LA growth logistical model was established to eliminate these errors under each bicarbonate level.To eliminate the physiological errors caused by the initial leaf,we assumed that the calibrated values of LA in the initial leaves were 287 mm2in O.violaceus and 152 mm2in B.juncea.Similarly,the fLA*of O.violaceus still changed non-synchronously with PN.Therefore,we hypothesised that the PNdoes not reflect the real growth state(fLAor fLA*)under bicarbonate treatments.Furthermore,the change in δ13C values in the leaves and culture solutions treated with different bicarbonate levels can reflect that the plants can utilise both exogenous bicarbonate(NaHCO3)and dissolved atmospheric CO2-generated bicarbonate.

Table 4 δ13C values in the nutrient solutions under bicarbonate treatments
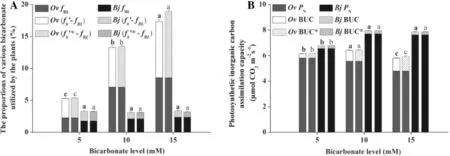
Fig.3 The various forms and proportions of bicarbonate utilized by the plants(a)and photosynthetic inorganic carbon assimilation capacities(b)of both plants among bicarbonate treatment.fBLis the proportion of exogenous bicarbonate utilised by the plants,fb’is the proportion of total bicarbonate utilised by the plants,(fb’-fBL)is the proportion of the bicarbonate generated by the dissolved CO2utilized by the plants.PNis net photosynthetic rate and BUC is bicarbonate-utilisation capacity of the plants.Asterik represents the calibrated values in the same parameters. Ov-Orychophragmus violaceus,Bj-Brassica juncea.The mean±SE(n=9)followed by different letters in the same plant species differ significantly at p≤0.05 subjected to one-way ANOVA and t-test

Fig.4 The relationship of photosynthetic rate(PN,PN’or PN’*)and the proportion of increased leaf biomass(fLAor fLA*)of Orychophragmus violaceus(a)and Brassica juncea(b)during the bicarbonate treatment
The PN'and PN'*changed synchronously with fLAand
fLA*in the leaves of both plants under various bicarbonate levels(Fig.4).During the bicarbonate treatment period(7 days),the proportions of exogenous NaHCO3and total bicarbonate(including exogenous bicarbonate and the bicarbonate generated by the dissolved CO2)utilised by O. violaceus were 2.27%and 5.28%at 5 mm bicarbonate,7.06%and 13.28%at 10 mm bicarbonate,and 8.55% and 17.31%at 15 mm bicarbonate,respectively.Meanwhile,the proportions of exogenous NaHCO3and total bicarbonate utilised by B.Juncea were 1.77%and 3.28% at 5 mm bicarbonate,2.11%and 3.10%at 10 mm bicarbonate,and 2.36%and 3.09%at 15 mm bicarbonate,respectively.When the amount of exogenous bicarbonate was increased,the amounts of exogenous bicarbonate and total bicarbonate utilised increased in O.violaceus but did not significantly increase in B.juncea.The plants consumed a considerably large amount of bicarbonate generated from the dissolved CO2.The results of this study can be used to explore the potential productivity of the plants and the‘missing carbon sink’produced by the dissolution of carbonate rocks.
AcknowledgmentsThe study was supported by the National Key Basic Research Program of China(2013CB956701),the National Natural Science Foundation of China(No.31070365),the project on social development of Guizhou Province(SY[2010]3043),and the StateKeyLaboratoryofEnvironmentalGeochemistry(SKLEG2014909).
Evans JR,Poorter H(2001)Photosynthetic acclimation of plants to growth irradiance:the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain.Plant Cell Environ 24:755-767
Farquhar GD,Ehleringer JR,Hubick KT(1989)Carbon isotope discrimination and photosynthesis.Annul Rev Plant Biol 40:503-537
Hoagland DR,Arnon DI(1950)The water-culture method for growing plants without soil.Calif Agric Exp St 347:1-32
Long SP,Bernacchi CJ(2003)Gas exchange measurements,what can they tell us about the underlying limitations to photosynthesis?Procedures and sources of error.J Exp Bot 54:2393-2401
Palmer A(1991)Origin and morphology of limestone caves.Geol Soc Am Bull 103:1-21
Price GD,Badger MR,Bassett ME,Whitecross MI(1985)Involvement of plasmalemmasomes and carbonic anhydrase in photosynthetic utilization of bicarbonate in Chara corallina.Funct Plant Biol 12:241-256
Raven J(1970)Exogenous inorganic carbon sources in plant photosynthesis.Biol Rev 45:167-220
Raven J,Beardall J,Griffiths H(1982)Inorganic C-sources for Lemanea,Cladophora and Ranunculus in a fast-flowing stream:measurements of gas exchange and of carbon isotope ratio and their ecological implications.Oecologia 53:68-78
Shelp BJ,Canvin DT(1980)Utilization of exogenous inorganic carbon species in photosynthesis by Chlorella pyrenoidosa.Plant Physiol 65:774-779
Waele JD,Plan L,Audra P(2009)Recent developments in surface and subsurface karst geomorphology:an introduction.Geomorphology 106:1-8
Wang R,Wu Y,Hang H,Liu Y,Xie T,Zhang K,Li H(2014)Orychophragmus violaceus L.,a marginal land-based plant for biodiesel feedstock:heterogeneous catalysis,fuel properties,and potential.Energ Convers Manag 84:497-502
Wu YY,Xing DK (2012)Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae.Photosynthetica 50:587-594
Wu YY,Wu XM,Li PP,Zhao YG,Li XT,Zhao XZ(2005)Comparison of photosynthetic activity of Orychophragmus violaceus and oil-seed rape.Photosynthetica 43:299-302
Yan J,Li J,Ye Q,Li K(2012)Concentrations and exports of solutes from surface runoff in Houzhai Karst Basin,southwest China. Chem Geol 304:1-9
18 September 2015/Revised:30 November 2015/Accepted:29 December 2015/Published online:12 January 2016ⒸScience Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2016
- Acta Geochimica的其它文章
- Transmission electron microscopic study of the fine-grained vein matrix in the Suizhou L6 meteorite
- An optimized sequential extraction scheme for molybdenum association in environmental samples
- Nutrient uptake by mulberry and Chinese prickly ash associated with arbuscular mycorrhizal fungi
- Tin partition behavior and implications for the Furong tin ore formation associated with peralkaline intrusive granite in Hunan Province,China
- Improvement of saponification extraction method for fatty acids separation from geological samples
- Zircon U-Pb dating of Pubei granite and strontium isotope from sphalerite of the Xinhua Pb-Zn-(Ag)deposit,Yunkai Area of Guangxi Province,South China

