Structural Modulation of Gut Microbiota in Rats with Allergic Bronchial Asthma Treated with Recuperating Lung Decoction*
KONG Yan Hua, SHI Qi, HAN Na, ZHANG Ling, ZHANG Yuan Yuan,GAO Tong Xin, CHEN Chen,#, and LI You Lin
1. Beijing University of Chinese Medicine, Beijing 100029, China; 2. Second Department of TCM Pulmonary Disease,The Key Institute of State Administration of Traditional Chinese Medicine (pneumonopathy chronic cough and dyspnea), Beijing Key Laboratory (NO. BZ0321), China-Japan Friendship Hospital, Beijing 100029, China; 3. State Key Laboratory for Infectious Diseases Prevention and Control, and National Institute for Communicable Disease Control and Prevention, Beijing 102206, China, Chinese Center for Disease Control and Prevention. Collaborative Innovation Center Diagnosis and Treatment of Infectious Disease, Hangzhou 310003, Zhejiang, China; 4. Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing Key Laboratory of Emerging Infectious Diseases, Beijing 100015, China; 5. Nephrology Department, Aviation General Hospital of China Medical University, Beijing 100012, China
Original Article
Structural Modulation of Gut Microbiota in Rats with Allergic Bronchial Asthma Treated with Recuperating Lung Decoction*
KONG Yan Hua1,^, SHI Qi2,^, HAN Na3,^, ZHANG Ling1,^, ZHANG Yuan Yuan4,GAO Tong Xin5, CHEN Chen4,#, and LI You Lin2,#
1. Beijing University of Chinese Medicine, Beijing 100029, China; 2. Second Department of TCM Pulmonary Disease,The Key Institute of State Administration of Traditional Chinese Medicine (pneumonopathy chronic cough and dyspnea), Beijing Key Laboratory (NO. BZ0321), China-Japan Friendship Hospital, Beijing 100029, China; 3. State Key Laboratory for Infectious Diseases Prevention and Control, and National Institute for Communicable Disease Control and Prevention, Beijing 102206, China, Chinese Center for Disease Control and Prevention. Collaborative Innovation Center Diagnosis and Treatment of Infectious Disease, Hangzhou 310003, Zhejiang, China; 4. Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing Key Laboratory of Emerging Infectious Diseases, Beijing 100015, China; 5. Nephrology Department, Aviation General Hospital of China Medical University, Beijing 100012, China
Abstract
Objective To investigate whether recuperating lung decoction (RLD) can modulate the composition of gut microbiota in rats during asthma treatment.
Methods Fifteen Sprague-Dawley rats were divided random ly and equally into control group, model group, dexamethasone (DEX) group, RLD medium-dose group, and RLD high-dose group. The asthma model was established in all groups, except for the control group. The rats in the DEX and RLD groups were treated orally with DEX and RLD, respectively. The rats in the control and model groups were treated orally w ith 0.9% saline. The intestinal bacterial communities were compared among groups using 16S rRNA gene amplification and 454 pyrosequencing.
Results The microbial flora differed between the control and model groups, but the flora in the RLD groups was similar to that in the control group. No significant differences were observed between the RLD high-dose and medium-dose groups. RLD treatment resulted in an increase in the level beneficial bacteria in the gut, such as Lactobacillus and Bifidobacterium spp.
Conclusion Oral administration of RLD increased the number of intestinal lactic acid-producing bacteria, such as Lactobacillus and Bifidobacterium, in asthma model rats.
Asthma; Gut microbiota; Recuperating lung decoction
INTRODUCTION
A sthma is characterized by airway inflammation and high airway reactivity[1]. The inflammatory state makes the body susceptible to various factors that lead to hyperresponsiveness and constriction of the airways. The associated eosinophil (EOS) infiltration of the airway causes clinical changes related to asthma,leading to inflammation and other pathological changes in the airway[2-3]. Although incurable,asthma can be controlled with appropriate drugs,self-management education, and by avoiding exposure to allergens[4].
The microflora hypothesis, proposed by Noverr and Huffnagle[5]in 2005, has been widely accepted as a potential explanation of the relationship between intestinal flora and asthma. Based on this hypothesis, the intestinal flora keeps the body healthy by metabolizing drugs and harm ful exogenous compounds, resisting exogenous pathogens and conditioned pathogens inside the body, and regulating the intestinal immune system and metabolism[6]. Long-term antibiotic usage and dietary modifications can disrupt the balance of intestinal flora, possibly leading to the occurrence of allergic diseases. Therefore, it is possible to prevent or even cure existing allergies by probiotic treatment aimed at restoring the normal intestinal flora[7]. The regulation of asthma via immune mechanisms remains unclear; however, oxidized lipids are potential immunomodulatory molecules, and mucosal tolerance can be controlled.
In addition, commensal bacteria can modulate the host innate immune system. Infants that were born by cesarean delivery or were adm inistered large doses of antibiotics are prone to develop asthma or other allergic diseases. This may be because cesarean delivery can potentially alter the normal balance of the intestinal flora in infants[9]. Additionally, infants often develop asthma when their mothers have an imbalance of intestinal flora[8]. Björkstén[9]cultured fecal bacterial samples,collected from 62 two-year-old Irish and Swedish children, under anaerobic conditions. He found that the samples from allergic children contained fewer Lactobacilli and anaerobic bacteria and more aerobic bacteria, such as coliform bacteria and Staphylococcus aureus. Bottcher et al.[10]discovered that the bacterial fatty acid profiles differed between allergic and non-allergic Swedish infants, and that allergic infants had higher levels of caproic acid(associated w ith Clostridium difficile). Furthermore,Penders et al.[11]have reported that the presence of Escherichia coli or Clostridium difficile was associated with an increased risk for eczema and allergic sensitization in two-year-old infants. These data,along with many others, suggest the potential effects of gut microbiota on the adaptive and innate immunity, which could affect the development of asthma.
Currently, glucocorticoids are the most effective drugs for controlling asthma due to their action on multiple aspects of the inflammatory response. Glucocorticoids regulate target gene transcription in the respiratory tract cells, inhibit inflammatory cell activation and inflammatory factor production, and increase airway β-receptor sensitivity; this in turn prevents airway inflammation and respiratory remodeling, and reduces bronchial hyperresponsiveness (BHR). However, long-term use of glucocorticoids may have some side-effects, such as toxicity, dependence, and may lead to financial strain due to the cost of long-term treatment[12-13].
Allergies[14]are considered as the primary pathophysiological basis of development of asthma. In traditional Chinese medicine, it is generally believed that allergies weaken the immune system,and that abnormal functioning of lungs and spleen contribute to the occurrence and development of asthma[15-16]. Clinical practice[17-18]has proven that an efficient approach for recovery from asthma is the restoration of normal functioning of the vital organs.
Our study aimed to investigate the effects of recuperating lung decoction (RLD) treatment on the microflora structure in rats with asthma. RLD is a decoction based on a traditional Chinese medicine that includes raw Radix Astragalus, Ramulus Cinnamomi, Suzi, Flos Magnoliae, Rhizoma Zingiberis,Fructus Corni, Fructus Schisandrae Chinensis, Fructus Mune, Flos Inulae, Cortex Magnoliae Offcinalis,Rhizoma Anemarrhenae, and raw Radix Et Rhizoma Glycyrrhizae. Previous studies have demonstrated[19-20]that the lung function of rats with asthma was improved after RLD treatment. The effects include a decreased number of eosinophils in bronchoalveolar lavage fluid (BALF) and reduced serum IgE levels, and improved elimination of oxygen free radicals[21]. RLD use was found to be closely related to changes in seven metabolites(valine, malic acid, gluconic acid, galactose, pyran glucose, 6-deoxidation mannopyranose and stearic acid) present in asthma rabbit serum[22]and stearicacid in asthma rabbit urine[23]. This study used Sprague-Daw ley rats to demonstrate the varying structure and diversity of the intestinal flora in response to various RLD treatments.
METHODS
Preparation of the RLD
All herbal components of RLD were obtained from Beijing Tong Ren Tang Pieces Company, and all the components followed the standard of the Chinese Pharmacopoeia (2010 edition). The herbs were identified and prepared as a fluid extract according to the standard operating procedure of the School of Chinese Materia Medica, Beijing University of Traditional Chinese Medicine, Beijing,People's Republic of China.
OVA-induced Asthma Model and Treatment
Four-week-old male Sprague-Daw ley rats,obtained from Beijing Hua Fu Kang Biological Technology Ltd., China [License Number: SCXK (jing)2009-0007], were used after 1 week of acclimation. All experimental procedures were conducted in accordance with the internationally accepted principles for laboratory animal use and care according to the US guidelines (NIH Publication no. 85-23, revised in 1985) and were approved by the Ethics Committee of the China (Japan Friendship Hospital. The rats were divided into five groups: a control group (N), an asthma model group (M), a positive-control group treated with DEX (W), and two RLD-treated groups (Tb and Tc). Rats in the asthma model group were first sensitized by subcutaneous injection of 0.2 m L of a mixture of 10% ovalbumin (OVA)/Al(OH)3(OVA, A16951, Alfa Aesar,Ward Hill, MS, USA; aluminium hydroxide, A4682,Sigma-Aldrich, St Louis, MO, USA) and 0.0023% whooping cough toxoid (P7208, Sigma-Aldrich) on day 1 to day 8; the injection was administered at five sites (feet, both side of groin, and peritoneum). On days 9-15, after initial sensitization, the rats were challenged with OVA (1%, w/v, in 0.9% saline)for 1 h using an ultrasonic nebulizer (402AI; Yuyue Medical Equipment Co., Jiangsu, China). The rats in the DEX and RLD groups were treated orally with DEX (0.5 g/kg, Sigma-Aldrich) and RLD [3.6 g/kg body weight for the medium-dose (Tb) and 7.26 g/kg bodyweight for the high-dose (Tc) group],respectively, once daily, on days 16-29. The rats in the control and asthma model groups were given 0.9% saline (0.3 m L).
Evaluation of the Model
The evaluation standard of the model included the following five aspects[24-25]. (1) The general condition of the animals: observing the animals for agitation, nose-scratching, sneezing, shortness of breath, abdom inal muscle contraction, lack of luster,etc. (2) After the challenge, their lung function was evaluated using the Flexivent system (Flexivent;SCIREQ, Montreal, QC, Canada) according to the manufacturer's instructions. The system used forced oscillation to discriminate between airway and lung tissue variables, and measured the flow-volume relationship in the respiratory system. The rats were anesthetized by endotracheal intubation and connected to a computer-controlled small-animal ventilator. To avoid spontaneous breathing, rat breathing was stabilized with an automatic ventilator until the interruption wave disappeared. The lung tissue variables of central airway resistance (Rn) and airway hyperreactivity (I) were measured. (3)Collection of BALF: After determining the lung function, BALF was collected by flushing the lungs twice with normal saline through the trachea. Approximately 1 m L of BALF was recovered. Cells were recovered from BALF by centrifugation at 3500 rpm for 15 m in at 4 °C and 8 m L of the cellular components were obtained by centrifugal sedimentation. The supernatant was shaken with phosphate-buffered saline (PBS) three times. A smear (0.1 m L) of the sediment was taken, stained with hematoxylin and eosin (H&E), and examined under an optical microscope (400 ×). The eosinophil count was repeated three times, and the average was calculated. (4) Lung tissue histopathology: The right lungs were fixed in 10% neutral buffered formalin. Each tissue specimen was sliced into 4-μm sections and stained with H&E stain. Tissue lesions and inflammatory cell infiltration were then evaluated m icroscopically. With the groups masked,inflammation was assessed from five fields per section, each graded on a scale of 0-3 (0 = no signs of disease; 3 = severe disease) for each of the follow ing parameters: peribronchial infiltration, epithelial damage, alveolar interval, and bronchospasm for a maximum total score of 12. (5) IgE measurements:At 24 h after the challenge, the rats were anesthetized and dissected. They were then euthanized by aortic exsanguination. The arterial blood was collected in tubes containing either sodium citrate as anticoagulant or a gel procoagulantfor different tests. The level of IgE in serum was assessed using a rat enzyme-linked immunoassay kit(MultiSciences Biotech Co., Ltd., Hangzhou, China)according to the manufacturers' instructions.
Collection and Pretreatment of Specimens
Once the rats were anesthetized, the abdominal cavity was opened. With sterile scissors, the cecum was cut open and, using sterile plastic loops,approximately 200 mg of cecal matter was transferred directly into cryotubes and flash-frozen in liquid nitrogen.
Microbiological culture, isolation, and identification of species were performed by the China CDC Laboratory of Pathogenic Microbiology,according to standard procedures.
DNA Extraction and 16S rDNA Gene Amplification
DNA was extracted from each cecal specimen using the QIAamp DNA Stool Mini Kit (Qiagen Corp.,Valencia, CA, USA) follow ing the manufacturer's recommended protocol. The extracted total bacterial DNA was used for polymerase chain reaction (PCR) amplification and generation of bacterial 16S rDNA sequences. The 16S rDNA hypervariable region 3 to region 5 (V3, V4, and partial V5, approximately 460 bp) was chosen for PCR, as they have previously been proven to be optimal for use in sequence-based assays species-level classification of clinical bacteria. A relatively small fragment was selected for the 454 pyrosequencing technology. A pair of universal bacterial primers directed against the V3-V4 flanking region was used for library preparation: Bakt_341F(5′-CCTACGGGNGGCWGCAG-3′) and Bakt_805R(5′-GACTACHVGGGTATCTAATCC-3′). The forward and reverse primers were synthesized in conjunction w ith a specific barcode immediately upstream of each primer, in order to identify the original sample uniquely. The 16S RNA genes were PCR-amplified under the follow ing cycle conditions: initial denaturation at 95 °C for 5 m in, denaturation at 95 °C for 40 s, annealing at 58 °C for 40 s, extension at 72 °C for 60 s over 30 amplification cycles, and a final elongation step at 72 °C for 7 m in. Amplification products were identified using 1% agarose gel electrophoresis. The target PCR products were purified using Qiagen Gel Extraction Kit (Qiagen Corp.), quantified on a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham,MA, USA), and then pooled using equimolar amounts of each sample. Sequencing was performed using the Roche/454 GS Junior Titanium Series technology platform (Roche, Indianapolis, IN, USA) at the Chinese Center for Disease Control and Prevention, according to the manufacturer's recommendations.
Filtering of 454 Pyrosequencing Reads and Microbial Community Diversity Estimates
All pyrosequencing reads were filtered according to the barcodes. The assigned reads were further screened and filtered for quality and length. Sequences less than 50 bp containing ambiguous characters or mononucleotide repeats of more than six nucleotides were excluded. The remaining high-quality sequences were aligned and clustered into operational taxonomic units (OTUs) based on nucleotide sequence identity, with the threshold of 97%. The Ribosomal Database Project Release 10 classifier with a threshold of 0.8 was used to assign representatives of each OTU to a known taxon at the genera level. Alpha diversity (within-samples) and beta diversity among a collection of samples were estimated to evaluate the community diversity and the relationships among the 56 microbial communities. Rarefaction curves were plotted to describe the relationship between sequencing depth and species richness. Unweighted UniFrac distance metric analyses were performed using the OTUs for each sample. These analyses were performed in QIIME v1.8.0 (http://qiime.org/). The heatmap was generated via the heatmap.2 function of the R package (http://www.r-project.org/).
Statistical Analysis
Data were presented as per-group mean±standard error of means (SEM). Statistical analysis was performed using analysis of variance(ANOVA), and data were analyzed using one-factor ANOVA followed by Tukey's multiple-comparison test. Differences were considered significant at P<0.05 unless otherwise stated. Unless otherwise specified, Student's t-test w ith the Benjamini and Hochberg method for significance thresholds of P<0.05 was used for comparing the mean relative abundance of a genus for each pair of treatments. Chi-square tests were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Raw sequence reads for this study are available from NCBI at Sequence Read Archive (http://www.ncbi.nlm. nih.gov/bioproject/), under NCBI Bio Project no. PRJEB5834. Similarity analysis was done using cluster analysis, grouped using the unweighted pair-groupanalysis,grouped using the unweighted pair-group method with arithmetic means(UPGMA)computing system[26].Diversity analysis was performed using richness and biodiversity.
RESULTS
General Condition of Rats and Drug Efficacy Evaluation
The rats in the control group were lively and responsive,w ith smooth breathing and shiny fur. The rats in the asthma group showed scratched facial fur,restless behavior,and other symptoms of allergy.In contrast to the asthma group,the treatment groups(Tb,Tc,and W)showed relief from the symptoms of asthma.However,shortness of breath and dyspnea with mild cyanosis were observed in the treatment groups.
Compared with the control group,the central airway resistance(Rn),airway hyperresponsiveness(I),serum IgE,and eosinophil count in the bronchoalveolar lavage fluid increased in the asthma model group.After medication,the aforementioned indexes had different degrees of improvement.HE staining of the lung tissue in the control group revealed no inflammatory cell infiltration and alveolar expansion,the bronchial mucosa and wall thickness were normal,the epithelial structure of the alveolar and bronchial mucosa was complete and clear,and bronchial spasms were absent. Pathological manifestations,such as infiltration of lung inflammatory cells,disordered arrangement of bronchial mucosal epithelial cells,thickening of the alveolar walls,bronchospasm,and an increase in macrophages were seen in the asthma model group. After treatment(DEX,RLD),these pathological manifestations were reduced,but the change in the RLD medium-dose group was not marked.The asthma model was thus successfully prepared,and drug treatment afforded control of the asthma.The therapeutic effect(high to low)was in the follow ing order:DEX group,RLD high-dose group,RLD medium-dose group.The details are illustrated in Figure 1.
Summary of Cluster Data
First, the structural changes of the gut microbiota in the five groups were analyzed. Raw sequences were demultiplexed and then quality-filtered following the method described. A total of 138,958 high-quality 16S rDNA genes covering the V3-V4 regions derived from the 15 samples represented an average length of 419 bps per sample (Table 1). The sequences were clustered into 31,726 OTUs at a genetic similarity of 97%. Rarefaction and Shannon diversity curves showed a sharp increase in the sampling size of the lower 3000 and gradually reached a plateau, suggesting that most of the diversity was captured at the current sequencing depth (Figure 2A).
Second, to measure the difference in the gut microbiota structure among the five groups, the UniFrac metric was calculated. Unweighted UniFrac PCoA analysis showed that after treatment, the gut microbiota structure of the three treated groups hadsignificantly deviated from the baseline of the model group and had a smaller difference from the healthy group. This suggested that the structure of the gut microbiota of the treated groups showed a drug-dependent deviation and had a microbial shift toward the control group (Figure 2B).
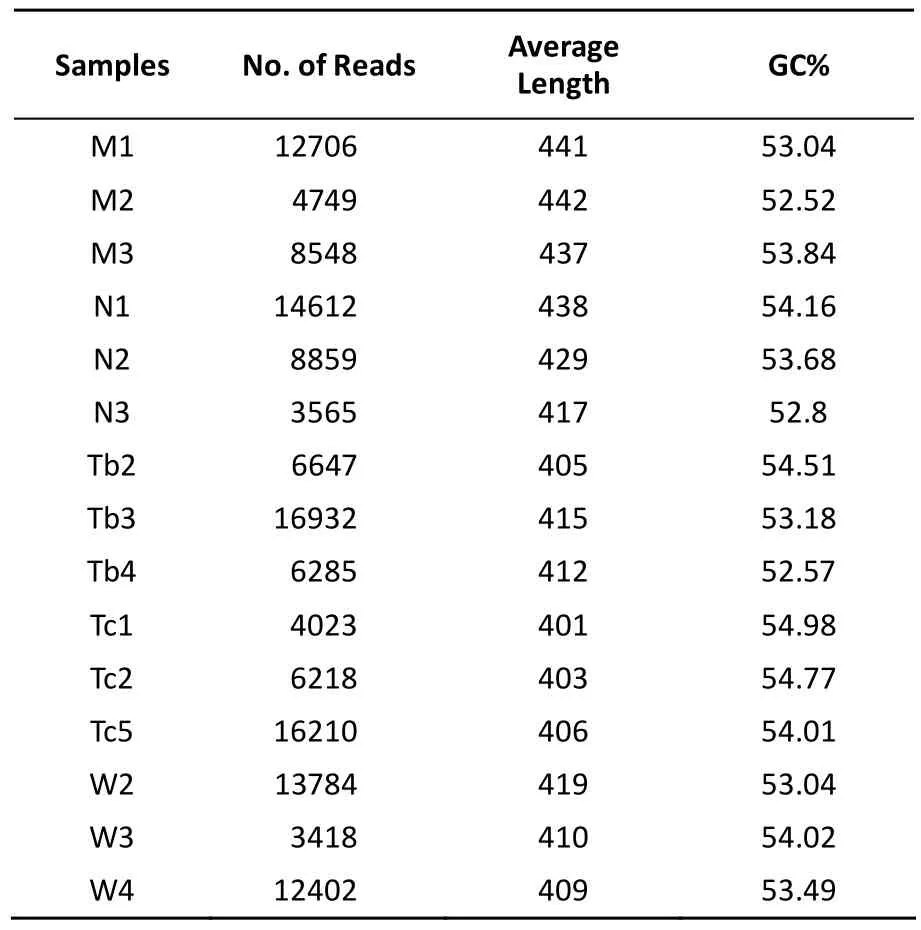
Table 1. Overview of the Distribution of Reads per Samples
Finally, cluster analysis was used to test the similarity of the bacterial communities in the five groups. The bacteria that showed a significant change in relative abundance between the model group and the control group were selected (t-test,P<0.2). The heatmap showed that, based on this bacterial microbiota, the model group and the drug-treated groups could be clearly separated, but the three drug-treated groups could not be separated. This indicated that all drug treatments used in this study could improve the gut microbiota,although at the stage of observation, the gut microbiota balance was not yet stable (Figure 2C).
Microbiota Structural Modulation of Asthma Model Rats
The overall structure of the gut microbiota was analyzed to demonstrate changes in the gut microbiota before and after treatment. Of all the tested flora, 74 genera of flora with a level above 0.01% were used for analysis. A total of 62 genera of flora were shared by all groups, which comprised up to 83.9% of all tested types. These genera mainly belonged to the Proteobacteria, Bacteroidetes, and Firm icutes, and could be considered to comprise the microbiota structural modulation of rats with asthma (Figure 3).
Changes in Microbiota Structure in Asthma Model Rats
To monitor the structural modulation of gut microbiota before and after induction of asthma, the control and the model groups were analyzed (Figure 4). Alpha diversity analysis showed no difference in the relative abundance and richness of microflora between the two groups. However, the bacterial community compositions of these two groups were distinctive (Figure 4A). Compared with the model group, the relative abundance of Prevotella and Lactobacillus in the control group was higher, while that of Bacteroides, Parabacteroides, Paraprevotella,Roseburia, Ruminococcus, Faecalibacterium,Allobaculum, Sutterella, Desulfovibrio, Erwinia, and Escherichia was lower (Figure 4B).
Microbiota Structural Modulation in Asthma Model Rats by Treatment
The microbial community structure of the RLD medium-dose and high-dose groups was compared to determine whether the Chinese herbs had a dose-dependent effect on asthma. The bacterial community structure was considered to be similar in the Tb (medium-dose) and Tc (high-dose) groups,although Aggregatibacter and Akkermansia were unique to Tc. Further, Prevotella was more common in the Tb group (P-value=0.005). The high-dose RLD group showed a higher level of Enterococcus and Proteus and a lower level of Lactobacillus and Bifidobacterium than did the medium-dose RLD group, but no statistically significant dose-dependent effect was found.
There was, however, a pronounced difference between the RLD and DEX groups. Similar to the Tb and Tc groups, the majority of the bacterial community structure was shared between the RLD and DEX groups. However, Adlercreutzia was more prevalent in the RLD groups (P-value=0.036) and Erysipelotrichaceae was more prevalent in the DEX group (P-value=0.038). Compared with the model group, the relative abundance of Lactobacillus was clearly increased in the DEX group, while the relative abundance of Escherichia was markedly increased in the RLD groups. The details of this structural modulation are illustrated in Figure 5.
DISCUSSION
Our study investigate the relationship between intestinal flora diversity and the treatment of asthmawith DEX and a traditional Chinese medicine, RLD. Our results suggested that RLD was effective in treating asthma, and that the RLD high-dose group showed more similarities with the DEX group with respect to treatment outcomes. The treatment also changed the diversity of intestinal flora in rats and alleviated their symptoms. The intestinal flora diversity of the control group was higher than that of the model group, and differed in terms of species and abundance, both of which were increased in the treatment group as well (Tb, Tc, and W) compared with that in the model group. This indicated a clear imbalance of intestinal flora in the model group rats.
In recent years, the economy-related change in human dietary patterns and the increased use of antibiotics has resulted in a change in intestinal flora and a higher risk of asthma[8,27-28]. A reduction in Bacteroidetes, Lactobacilli, and Bifidobacteria has been associated with the asthma phenotype[29]. A recent study on patients with asthma showed that the biodiversity in the patients with airway hyperresponsiveness was higher than that in the healthy subjects[30].
Traditional Chinese medicine holds that meridians link the lungs and the large intestine in an interior-exterior dyad. Based on this theory,facilitating bowel movement while treating asthma can relieve the patient's symptoms. This could be achieved by improving the microcirculation and reducing pulmonary hypertension, which relieves bronchial smooth muscle spasm, reduces capillary permeability and inflammatory exudate. Additionally,lowering abdominal pressure by promoting bowel movement to release feces and gas, increasing the range of motion of the diaphragm, the resulting improved lung respiratory function may also contribute to this effect. Although the mechanism underlying the pathological changes in asthma is extremely complex w ith respect to the onset, regulation, and prognosisof the condition, various studies have recently made tremendous progress in understanding this mechanism. This may change the future treatment strategies for asthma.
In recent years, there has been increasing number of reports on the modulatory effect of single herbs or compound herbal preparations on the intestinal flora[31-32]. The effective components in single herbs, such as Astragalus polysaccharide (APS)in Astragalus, have been proven[33]to have a positive effect on the intestinal dysbiosis in rats w ith ulcerative colitis. For example, reports have shown that, after APS treatment for 7 d, the number of Bifidobacteria and Lactobacilli significantly increased,and that of Enterococci and Enterobacteria decreased. APS has been confirmed[34]to facilitate the grow th of harm less bacteria and inhibit the grow th of pathogenic bacteria, thus maintaining the intestinal microecological balance. Schisandra has also been proven[35]to have a similar effect on microbial flora and a protective effect on the liver. ShenLingBaiShuSan has been reported[36]to correct intestinal dysbiosis caused by the use of antibiotics in m ice; increase in the level of probiotic bacteria in the intestine, and the levels of IgG, endotoxin, and vasoactive intestinal peptide (VIP) in the serum have also been reported.
CONCLUSIONS
This study has provided an explanation for the Chinese theory in which ‘meridians link the lungs and the large intestine' and has aimed to give a stronger experimental basis for future studies on its cryptic micro-ecology mechanism. The intestina flora structure of rats with asthma was different from that of the control group. After treatment, the intestinal flora structure of the RLD groups was similar to that of the DEX group, and the flora structure of the three treatment groups tended to be similar to that of the control group. Hence, this study demonstrated that oral administration of RLD increased the number of intestinal probiotic bacteria such as Lactobacillus and Bifidobacterium in rats with asthma, but this effect may be due to complicated interactions among various components. It remains unclear whether changes in gut microbiota caused by RLD contribute directly to the treatment outcomes in asthma. The beneficial effect is thought to be associated with an immunoregulatory effect of RLD; this possible association needs to be explored in further studies.
CONFLICT OF INTEREST
The authors declare that they have no actual or potential conflicts of interest.
Accepted: July 2, 2016
REFERENCES
1. Masoli M, Fabian D, Holt S, et al, The global burden of asthma:executive summary of the GINA Dissem ination Comm ittee report. Allergy, 2004; 59, 469-78.
2. Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med, 2005; 11, 148-52.
3. Kulkarni NS, Hollins F, Sutcliffe A, et al, Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients w ith asthma. J Allergy Clin Immunol,2010; 126, 61-9.e3.
4. Kliegman RM, R Behrman, H Jenson, et al. Nelson textbook of pediatrics. 2007, Germany: Elsevier Health Sciences.
5. Noverr MC, GB Huffnagle. The 'm icroflora hypothesis' of allergic diseases. Clin Exp Allergy, 2005; 35, 1511-20.
6. Blaut M, T Clavel. Metabolic diversity of the intestinal m icrobiota: implications for health and disease. J Nutr, 2007;137, 751s-5s.
7. Sun D, Yang X. Progress in the mechanism of action and application of microecological preparations. International journal of pediatrics, 2009; 36, 48-50.
8. Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med, 2011; 364, 701-9.
9. Björkstén B, Naaber P, Sepp E, et al. The intestinal m icroflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy, 1999; 29, 342-6.
10. Böttcher M, Nordin EK, Sandin A, et al. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin Exp Allergy, 2000; 30, 1591-6.
11. Penders J, Thijs C, van den Brandt PA, et al. Gut m icrobiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut, 2007; 56, 661-7.
12. New ton R. Molecular mechanisms of glucocorticoid action:what is important? Thorax, 2000; 55, 603-13.
13. Lesovaya E, Yemelyanov A, Swart AC, et al, Discovery of Compound A-a selective activator of the glucocorticoid receptor w ith anti-inflammatory and anti-cancer activity.Oncotarget, 2015; 6, 30730-44.
14. Lee MY, Seo CS, Lee JA, et al. Anti-asthmatic effects of Angelica dahurica against ovalbum in-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem Toxicol, 2011;49, 829-37.
15.Shi Q, Song Q, Yan Y, et al. Treatment of allerfic diseases from the lung and spleen aspects. China Journal of Traditional Chinese Medicine and Pharmacy, 2013; 28, 3265-8. (In Chinese)
16. Zhang L, Li YL. Experience of treating chronic persistent bronchial asthma based on 'warm ing and moisturize lung w ith supplementing spleen' method. China Journal of Traditional Chinese medicine and Pharmacy, 2013; 28, 1525-8. (In Chinese)
17. Han J. Efficacy analysis of warming and moisturizing lung with supplementing spleen for the patients suffering from bronchial asthma with allergic rhinitis. Chinese Archives of Traditional Chinese Medicine, 2015; 33, 1341-3. (In Chinese)
18. Yan Y. Impact factors of wenren xuantong method in treating stable chronic obstructive pulmonary diseases and bronchial asthma in chronic persistent period. China Journal of Traditional Chinese medicine and Pharmacy, 2014; 29, 1954-6.(In Chinese)
19. Li SW. Research on the mechanism of bronchial asthma treatment by Qiweilifei Decoction regulating transcription factors activate protein-1 (AP-1), Beijing university of Chinese medicine, 2014.
20. Li CL. Research on the antioxidant mechanism of bronchial asthma treatment by Qiweilifei Decoction, Beijing university of Chinese medicine, 2014.
21. Li CL. Infuence of Qiwei Lifei Decoction on oxygen free radical scavenging capacity in asthma rats serum. China Journal of Traditional Chinese medicine and Pharmacy, 2015; 30, 1644-7.(In Chinese)
22. Shi Q, Chen XX, Kong YH, et al. Metabolomics study on urine of allergic bronchial asthma rabbits treated by Recuperating Lung Decotion. RSC Adv, 2015; 5, 13768-76.
23. Shi Q, Kong YH, He B, et al. Metabolomics study on serum of allergic bronchial asthma rabbits treated by Recuperating Lung Decotion. RSC Adv, 2015; 5, 13768-76.
24. Qin Q, Chen X, Feng J, et al. Low-intensity aerobic exercise training attenuates airway inflammation and remodeling in a rat model of steroid-resistant asthma. Chinese Medical Journal, 2014; 127, 3058-64. (In Chinese)
25. Kim Y, Lee MY, Kim OS, et al. Acute oral toxicity of Insampaedok-san, a traditional herbal formula, in rats and its protective effects against ovalbumin-induced asthma via anti-inflammatory and antioxidant properties. BMC Complement Altern Med, 2014; 14, 365.
26. From in N, Hamelin J, Tarnawski S, et al. Statistical analysis of denaturing gel electrophoresis (DGE) fingerprinting patterns. Environ M icrobiol, 2002; 4, 634-43.
27. Riedler J, Braun-Fahrländer C, Eder W, et al. Exposure to farm ing in early life and development of asthma and allergy: a cross-sectional survey. Lancet, 2001; 358, 1129-33.
28. Om land O, Hjort C, Pedersen OF, et al. New-onset asthma and the effect of environment and occupation among farm ing and nonfarm ing rural subjects. J Allergy Clin Immunol, 2011; 128,761-5.
29. Ouwehand AC, Isolauri E, He F, et al. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol, 2001; 108, 144-5.
30. Gollw itzer ES, BJ Marsland. M icrobiota abnormalities in inflammatory airway diseases-Potential for therapy. Pharmacol Ther, 2014; 141, 32-9.
31. Yin X, Peng J, Zhao L, et al. Structural changes of gut m icrobiota in a rat non-alcoholic fatty liver disease model treated w ith a Chinese herbal formula. Syst Appl M icrobiol, 2013; 36, 188-96. 32. Xu J, Lian F, Zhao L, et al. Structural modulation of gut m icrobiota during alleviation of type 2 diabetes w ith a Chinese herbal formula. Isme j, 2015; 9, 552-62.
33. Liang JH, Zheng KW, Sun LQ. Explore the Regulative Action of Astragalus Polysaccharide for Intestinal Dysbacteriosis in Ulcerative Colitis Rat Models. Studies of Trace Elements and Health, 2013; 2, 003.
34. Zhang Y, Shen Y, Hu XJ, et al. Research on material bases of m icroecological adjustment of Astragalus polysaccharides. Chinese Journal of M icroecology, 2012; 2, 003. (In Chinese)
35. Cheng Y, Wang HH, Yi-Yang HU. Effect of Jianpi Huoxue Recipe on Gut Flora in rats w ith alcoholic fatty licer induced by LieberDeCarli liquid diet. Chinese Journal of Integrated Traditiona & Western Medicine, 2011; 31, 73-9. (In Chinese)
36. Dong K, Gao Y, Qin N, et al. Effects of shenlinbaizhusan on the dysbacteriosis induced by antbiotics in m ices. Chinese Journal of Experimental Traditional Medical Fomulae, 2015; 1,154-7. (In Chinese).
Biomed Environ Sci, 2016; 29(8): 574-583 10.3967/bes2016.076 ISSN: 0895-3988 www.besjournal.com (full text) CN: 11-2816/Q Copyright ©2016 by China CDC
CONTRIBUTIONS
January 26, 2016;
*This study was supported by Young Scientists Fund of National Science Foundation of China [No.81302943,No.81302941]; This was a project aimed at promoting the talents of young scientists in 2015 [No.2015-QNYC-A-01].
^These authors contributed equally to the work.
#Correspondence should be addressed to LI You Lin, Tel: 86-10-84206023, Fax: 86-10-84205823, E-mail:lyl19610721@163.com; CHEN Chen, E-mail: chenchen.bj2008@163.com
Biographical notes for the first authors: KONG Yan Hua, born in 1987, PhD, clinical and applied basic research on prevention and treatment of lung diseases with traditional Chinese medicine; SHI Qi, female, born in 1983, PhD, hoase physician, clinical and applied basic research on prevention and treatment of lung diseases with traditional Chinese medicine; HAN Na, female, born in 1984, PhD, research assistantidentification of pathogenic microorganisms,analysis of clinical microbial community structure and species diversity; ZHANG Ling, female, born in 1986, PhD, clinical and applied basic research on prevention and treatment of lung diseases with traditional Chinese medicine.
KONG Yan Hua, SHI Qi, ZHANG Ling, and LI You Lin contributed in conducting the study, collecting the data and drafting the manuscript. HAN Na and CHEN Chen performed the statistical analysis and participated in its design. GAO Tong Xin and ZHANG Yuan Yuan helped in drafting the manuscript. All authors have read and approved the final manuscript.
 Biomedical and Environmental Sciences2016年8期
Biomedical and Environmental Sciences2016年8期
- Biomedical and Environmental Sciences的其它文章
- Circulating MicroRNAs as Novel Diagnostic Biomarkers for Very Early-onset (≤40 years) Coronary Artery Disease*
- Expression of Peroxiredoxins and Pulmonary Surfactant Protein A Induced by Silica in Rat Lung Tissue*
- Distribution Characteristics of Spermophilus dauricus in Manchuria City in China in 2015 th rough ‘3S' Techno logy*
- Alcohol Drinking, Dyslipidemia, and Diabetes: A Population-based Prospective Cohort Study among lnner Mongolians in China*
- Bio logical Effec ts o f Clo th Con taining Specific Ore Pow der in Patien ts w ith Po llen Allergy
- 8-isop rostane as Oxidative Stress Marker in Coal Mine Wo rkers
