内质网应激前后肝癌细胞中C/EBP同源蛋白及X-盒-结合蛋白-1的表达*
李利波, 潘 娅, 陈腾祥
(贵州医科大学, 贵州 贵阳 550004)
内质网应激前后肝癌细胞中C/EBP同源蛋白及X-盒-结合蛋白-1的表达*
李利波, 潘娅**, 陈腾祥**
(贵州医科大学, 贵州 贵阳550004)
目的: 观察内质网应激(ERS)标志蛋白X-盒-结合蛋白-1(XBP-1)和C/EBP同源蛋白(CHOP)在正常肝细胞及不同分化程度肝癌细胞中的表达。方法: 将培养获得的正常肝细胞LO2、高分化肝癌细胞株HepG2及低分化肝癌细胞株SMMC-7721细胞分为无水乙醇未处理组(ERS前组)和无水乙醇处理组(ERS组),ERS组的3种细胞分别给予无水乙醇处理获得ERS细胞模型; 采用Western Blot方法检测3种细胞ERS前后XBP-1、CHOP表达。结果: 在ERS前组中,XBP-1表达从高到低依次为LO2、HepG2、SMMC-7721;ERS组中,XBP-1在3种细胞中的表达都上调,与ERS前组比较差异有统计学意义(P<0.05),增加量从高到低依次为SMMC-7721、LO2及HepG2,3种细胞间两两比较差异无统计学意义(P>0.05);ERS前后,HepG2细胞中均无CHOP表达;ERS前组中,CHOP表达高低依次为SMMC-7721、LO2;ERS组中,CHOP在LO2细胞及SMMC-7721细胞表达均上调,与ERS前组比较差异有统计学意义(P<0.05),而在SMMC-7721细胞中表达上调更明显(与LO2细胞比较,P<0.05)。结论: 发生ERS后,LO2、HepG2及SMMC-7721细胞中XBP-1的表达水平都升高,分化程度低的SMMC-7721细胞中的XBP-1及CHOP的表达升高更明显。
肝肿瘤; 肝细胞; 内质网应激; X-盒-结合蛋白-1; C/EBP同源蛋白
内质网应激(endoplasmic reticulum stress,ERS)是指在多种病理生理因素的干扰下,内质网稳态被打破,错误折叠和未折叠蛋白质在内质网腔内聚集,导致内质网功能和Ca2+平衡紊乱[1]。ERS主要包括未折叠蛋白反应(UPR)、内质网超负荷反应(EOR)和固醇调节级联反应(SREBP),研究认为癌症相关的病理机制与内质网稳态失衡诱导ERS和UPR途径有关[2-4];而ERS反应过强或持续时间过久,这些反应不足以恢复内质网稳态,则最终引起细胞凋亡[5-6]。诱导内质网中应激蛋白表达是UPR 的主要效应之一, 这些蛋白包括内质网分子伴侣, 例如CHOP、XBP-1等,因此这些蛋白质水平的变化可提示ERS的发生[7-9]。本研究通过观察XBP-1、CHOP在正常肝细胞、肝癌HepG2及SMMC-7721细胞在ERS前后表达水平的变化,探讨不同分化程度肝癌细胞与ERS的关系。
1 材料与方法
1.1细胞及试剂
正常肝细胞LO2、高分化肝癌细胞株HepG2和低分化肝癌细胞株SMMC-7721购买于上海生命科学研究所,XBP-1和CHOP抗体为ABCAM公司产品,BCA Protein Assay KitP0010S、RIPA裂解液(强)P0013B购买于上海碧云天公司。Prestained protein marker 00161543、ECL-PLUS/KitM3121/1859022购买于thermo公司,医用X光片038401501购买于carestream公司,SDS-PAGE 蛋白电泳仪 VE-180,蛋白转膜仪VE-186为上海天能公司产品。
1.2方法
1.2.1细胞培养及处理人正常肝细胞LO2及高分化人肝癌细胞系HepG2细胞用DMEM加10%胎牛血清、37 ℃、5% CO2细胞培养箱中培养,培养1周后传代至第3代,细胞贴壁生长。低分化人肝癌细胞系SMMC-7721细胞用RMPI1640加10%胎牛血清、37 ℃、5% CO2细胞培养箱中培养,首次传代23 d,2周后传至第3代。将收集的3种细胞分成两组,一组进行ERS处理(ERS组),一组不予ERS处理(ERS前组)。诱导ERS的方法为用150 mmol/L无水乙醇作用于HepG2、SMMC-7721及L-02细胞48 h,弃去无水乙醇,PBS 洗涤2 次,获得ERS后的3种细胞。
1.2.2CHOP及XBP-1蛋白的表达用Western Blot方法检测,分别提取培养获得的ERS前后的3种细胞样品,PBS洗涤两次后刮下细胞转移入EP管中,用预冷的2×Lysis Buffer裂解,冰上裂解10~15 min后超声破碎细胞,离心15 min,取上清液提取蛋白样品。根据CHOP及XBP-1的蛋白分子量制胶,样品上样,恒压80 V,电泳2 h,在4 ℃及300 mA恒流条件下电转150 min,将蛋白转移到PVDF膜上,用封闭液(含5%脱脂牛奶的TBST溶液)室温封闭PVDF膜1 h、4 ℃过夜;TBST洗膜3次、10 min/次,然后显色。将胶片进行扫描、拍照,用凝胶图像处理系统分析目标条带的分子量和净光密度值,检测3种细胞在发生ERS前后细胞裂解液中的CHOP及XBP-1蛋白的表达。
1.3统计学处理
采用SPSS 21软件进行统计分析,采用one-way ANOVA法、重复测量方差分析法分析3种细胞中这CHOP及XBP-1蛋白在ERS前后水平的差异。P<0.05为差异有统计学意义。
2 结果
2.1XBP-1表达
ERS前组:3种细胞中,XBP-1在LO2细胞中表达最高,其次为HepG2细胞,在SMMC-7721细胞中表达最低。ERS组:3种细胞中,XBP-1表达均上调,与ERS前组比较差异有统计学意义(P<0.05),增加量从高到低依次为SMMC-7721、LO2及hepG2细胞,但增加量在3种细胞间两两比较,差异无统计学意义(P>0.05)。见图1、表1。

图1 ERS前后XBP-1在SMMC-7721、LO2及hepG2细胞中的表达(Western Blot)Fig.1 The expression of XBP-1 in three kinds of cells before and after ethanol treatment

细胞光密度值ERS前组ERS组变化量LO21.16±0.221.29±0.25(1)0.13±0.02HepG20.96±0.571.07±0.64(1)0.12±0.005SMMC-77210.62±0.530.90±0.77(1)0.28±0.17
(1)与ERS前组比较,P<0.05
2.2CHOP表达
ERS前后,CHOP在HepG2细胞中均无表达;ERS前组中CHOP在LO2细胞中表达较低,而在SMMC-7721细胞中表达较高;ERS组中,CHOP在LO2细胞及SMMC-7721细胞表达均上调,与ERS前组比较差异有统计学意义(P<0.05);CHOP在ERS前后表达水平的增加量在SMMC-7721细胞中变化较大,在LO2细胞中变化较小,差异有统计学意义(P<0.05)。见图2、表2。
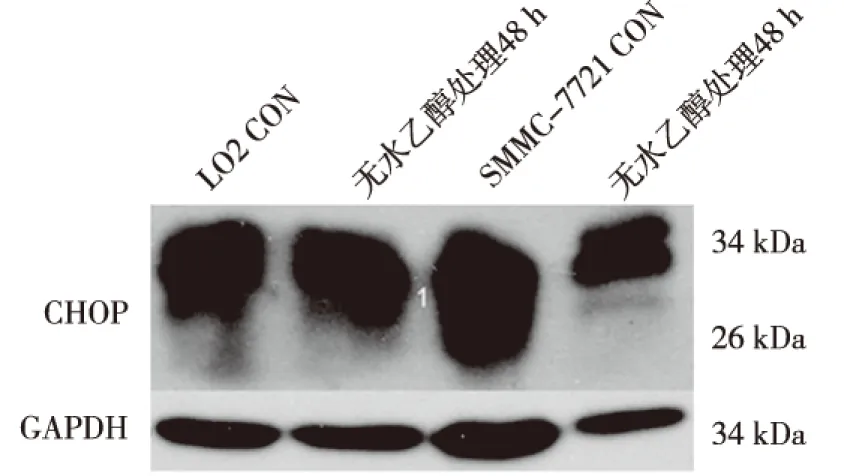
图2 ERS前后LO2及SMMC-7721细胞中CHOP的表达(Western Blot)Fig.2 The altered expression of CHOP in two kinds of cells before and after ethanol treatment

细胞光密度值ERS前组ERS组变化量LO20.97±0.420.99±0.40(1)0.019±0.0005SMMC-77210.89±0.160.95±0.17(1)0.066±0.008(2)
(1)与ERS前组比较,P<0.05;(2)与LO2比较,P<0.05
3 讨论
当ER内通路被阻断时,ER所具有的调节蛋白质折叠、转录后修饰、脂质和类固醇合成、基因表达、调节细胞代谢和钙信号等多种功能将不能发挥,ER腔内错误折叠蛋白质的累积将最终导致 ERS[10]。ERS可通过促凋亡反应诱导肿瘤细胞的凋亡,亦可通过抗凋亡反应导致肿瘤细胞的无限增殖,这体现了ERS对肿瘤细胞作用的矛盾方面,提示ERS的反应程度或反应类型对肿瘤的作用不同。近年来,ERS诱导肿瘤细胞凋亡的研究已经成为肿瘤治疗研究的热点。诱导内质网的应激蛋白表达是UPR 的主要效应之一, 这些蛋白包括内质网分子伴侣, 例如GRP78、CHOP、XBP-1、PDI等。所以ERS一般用参与UPR的标志性分子来提示ERS的发生。研究发现,ERS时CHOP的表达量在转录水平上大大增加,认为CHOP参与调节下游凋亡相关基因的表达,CHOP高表达时ER对蛋白质折叠修饰功能受到影响,引起细胞分裂周期停滞及DNA损伤,促进凋亡发生,因此往往将CHOP作为ERS凋亡途径被激活的主要标志之一[11-13]。XBP-1是ER中蛋白质折叠能力的主要调控者之一,它通过调控蛋白陪衬分子和ER中的相关降解蛋白表达来发挥其增加ER对蛋白质折叠能力的作用[14-17]。当ERS发生时,ER网腔中未折叠或错误折叠的蛋白质堆积,刺激定位于ER膜的肌醇酶1(inositolrequiring1,IRE1)二聚化而具有蛋白激酶和核糖核酸酶的活性,将XBP1-U剪接成具有高度转录活性的XBP1-S。因此,XBP-1的表达上调也常常作为ERS标志。
本研究发现,在无水乙醇未处理时,XBP-1在正常肝细胞中表达最高,其次为HepG2细胞,在SMMC-7721细胞中表达最低,经无水乙醇处理后表达均上调在SMMC-7721细胞中XBP-1表达水平变化最大,其次为LO2细胞,变化最小的为hepG2细胞。提示XBP-1 ERS后XBP-1表达变化与细胞分化程度不相关。正常肝细胞中CHOP表达较SMMC-7721细胞中高,HepG2细胞未见CHOP表达,ERS后两种细胞中CHOP上调,在SMMC-7721细胞中CHOP表达水平变化较大,而LO2细胞变化较小。提示CHOP在ERS前后的表达水平变化与细胞分化程度相关,细胞分化程度越低,CHOP 表达水平变化越大。
本研究证实了无水乙醇处理诱导发生ERS后,低分化肝癌SMMC-7721细胞中XBP-1及CHOP表达水平升高。ERS后,CHOP、XBP-1等ERS标志物的表达水平变化是与恶性肝癌细胞的分化程度相关的,分化程度越低,CHOP、XBP-1等ERS标志物表达水平变化越大。
[1] kaufman RJ.Orchestrating the unfolded protein response inhealth and disease[J].J Clin Invest, 2002(10):1389-1398.
[2] Xu Y, Chiu L F, He QY, et al. Tubeimoside-1 exerts cytotoxicity in Hela cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways [J]. J Proteome Res, 2009(3): 1585-1593.
[3] Mujcic H, Rzymski T, Rouschop KM, et al. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3[J]. Radiother Oncol, 2009(3): 450-459.
[4] Sun SY, Liu XG, Zou W, et al. The farnesyl transferase inhibitor lonafarnib induces CCAAT/enhancer-binding protein homologous protein-dependent expression of death receptor 5, leading to induction of apoptosis in human cancer cells[J]. Biol Chem, 2007(26): 18800-18809.
[5] Volmer R,van der Ploeg K,Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains[J]. Proc Natl Acad Sci USA, 2013(12):4628-4633.
[6] Hetz C,Chevet E,Harding HP. Targeting the unfolded protein response in disease[J]. Nat Rev Drug Discov, 2013( 9) : 703-719.
[7] Jauhiainen A, Thomsen C, Strömbom L, et al. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153[J]. PLoS One, 2012(4):e33208.
[8] Irie Y, Saeki M, Tanaka H, et al. Methamphetamine induces endoplasmic reticulum stress related gene CHOP/Gadd153/ddit3 in dopaminergic cells[J]. Cell Tissue Res, 2011(2):231-241.
[9] Benham AM. The protein disulfide isomerase family: key players in health and disease[J]. Antioxid Redox Signal, 2012(16):781-789.
[10]Zhou M, Jacob A, Ho N, et al. Downregulation of protein disulfide isomerase in sepsis and its role in tumor necrosis factor-alpha release[J]. Crit Care, 2008(12):100.
[11]Robinson A,Hines V,Eiden K,et al.Protein disfide isomerase over expression increase secretion of foreign protein in sac-charomyces cerevisiae [J].Biotechnology, 1994(12):381-384.
[12]Goplen D, Wang J, Enger PO, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma[J]. Cancer Res, 2006(66):9895-9902.
[13]Henders hot LM.The ER function BiP is a master regulat or of ER funct ion[J].Mt Sinai J Med, 2004(5):289-297.
[14]Hsu TA,Watson S, Eiden JJ,et al.Rescue of immunoglobulins from insolubility is facilitated by PDI in the baculovirus expression system[J].Protein Expr Purif, 1996(7):281-288.
[15]Tabata Y, Takano K, Ito T, et al. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation[J]. Am J Physiol Cell Physiol, 2007(293):C411-C418.
[16]Hsu TA,Watson S, Eiden JJ,et al.Rescue of immunoglobulins from insolubility is facilitated by PDI in the baculovirus expression system[J].Protein Expr Purif, 1996(7):281-288.
[17]Xu S, Sankar S, Neamati N. Protein disulfide isomerase: a promising target for cancer therapy[J]. Drug Discov Today, 2014(19): 222-240.
(2016-05-21收稿,2016-08-31修回)
中文编辑: 文箐颍; 英文编辑: 赵毅
The Expression of XBP-1 and CHOP Alteration in the Normal Liver Cells and Liver Cancer Cells before and after RES
LI Libo, PAN Ya, CHEN Tengxiang
(GuizhouMedicalUniversity,Guiyang550004,Guizhou)
Objective: To observe the alteration of ERS marker protein X-box-binding protein 1 (XBP -1) and C/EBP homologous protein (CHOP) in normal liver cells LO2, expression of high differentiation of primary liver cancer cell line HepG2 and low expression in the differentiation of primary liver cancer cell line SMMC-7721. Methods: Cultivation of LO2 cells and HepG2, SMMC-7721 cells was divided into anhydrous ethanol untreated group before (ERS) and anhydrous ethanol treatment group (ERS), ERS group of three kinds of cells were given anhydrous ethanol processing to gain ERS model. Western blot method was adopted to detect the expression level of CHOP and XBP-1 before and after anhydrous ethanol intervention. Results: Before and after ERS, XBP-1 was expressed in three kinds of cells which had not been given anhydrous ethanol processing. Expression level from high to low in turn for LO2 cells, HepG2 cells, SMMC - 7721 cells. After treated with anhydrous ethanol, expression of XBP-1 in three kinds of cells rose; compared with before ERS, of which there was statistically significant difference in the expression of XBP-1 in three kinds of cells between before ERS and after ERS (P<0.05). The increase was not statistically significant compared between among the three (P>0.05). Before and after ERS, CHOP in HepG2 cells had no expression. Its expression in LO2 cells is higher, SMMC-7721 cells expression is lower. Compared with before ERS, the expression of CHOP in LO2 and SMMC-7721 cell lysate significantly up-regulated after ERS (P<0.05). The alteration of Expression levels varied considerably in SMMC-7721 cells which is more than LO2 cells,it was statistically significant (P<0.05). Conclusion: After ERS, CHOP, XBP-1 is associated with malignant cancer of the liver cell differentiation degree, the lower of the degree of differentiation, the greater the expression level changes.
liver carcinoma; hepatocellular; endoplasmic reticulum stress; X box-binding protein-1; C/EBP homologous protein
贵州省科技厅科技项目[黔科合LG(2001)008]; 贵州省教育厅自然科学研究项目[黔教合(2008)022]
E-mail:710232517@qq.com; 371251826@qq.com
R735.7;R363
A
1000-2707(2016)09-1033-04
10.19367/j.cnki.1000-2707.2016.09.010
**
网络出版时间:2016-09-13网络出版地址:http://www.cnki.net/kcms/detail/52.5012.R.20160913.2240.050.html

