以阳性、阴性症状为主的首发精神分裂症患者血清蛋白因子水平与PANSS评分的相关性
戴 南,陈 鹏,曾 勇,熊 鹏*,刘 芳,李 明,郅晋升,储 睿,节会锦
(1. 昆明医科大学第一附属医院,云南 昆明 650031;2. 苏州市广济医院,江苏 苏州 215008*通信作者:熊 鹏,E-mail:xp6945399@163.com)
以阳性、阴性症状为主的首发精神分裂症患者血清蛋白因子水平与PANSS评分的相关性
戴南1,陈鹏2,曾勇1,熊鹏1*,刘芳1,李明1,郅晋升1,储睿1,节会锦1
(1. 昆明医科大学第一附属医院,云南昆明650031;2. 苏州市广济医院,江苏苏州215008*通信作者:熊鹏,E-mail:xp6945399@163.com)
目的探讨以阳性、阴性症状为主的首发精神分裂症患者血清白细胞介素-6(IL-6)、钙结合蛋白S100β(S100β)、神经营养因子-3(NT-3)三种蛋白因子的浓度水平差异以及与其阳性与阴性症状量表(PANSS)评分中阳性症状、阴性症状、认知、兴奋及抑郁情绪评分之间的相关性。方法以2014年1月-2015年11月于昆明医科大学第一附属医院精神科门诊及住院的首发精神分裂症患者为患者组,选取同期来自本院体检中心的健康体检者为对照组。采用酶联免疫吸附技术(ELISA)测定44例以阳性症状为主的首发精神分裂症患者(阳性组)、38例以阴性症状为主的首发精神分裂患者(阴性组)和78名健康对照者(对照组)血清中蛋白因子IL-6、S100β、NT-3的浓度,通过PANSS对患者组和对照组的阳性症状、阴性症状、认知功能、兴奋症状及抑郁情绪进行定量评估。结果①三组血清IL-6浓度比较,差异有统计学意义(F=31.34,P<0.01),两两比较,对照组IL-6浓度低于阳性组和阴性组,阳性组低于阴性组,差异均有统计学意义(P均<0.05);②三组S100β浓度比较,差异有统计学意义(F=9.19,P<0.05),两两比较,阳性组、阴性组的S100β浓度均高于对照组(P均<0.05),两患者组间比较差异无统计学意义(P>0.05);③三组NT-3浓度比较,差异有统计学意义(F=10.45,P<0.05),两两比较,阳性组、阴性组NT-3浓度均低于对照组(P均<0.05),两患者组间比较差异无统计学意义(P>0.05)。阳性组血清NT-3浓度与兴奋评分呈正相关(r=0.38,P<0.05)。结论以阴性症状为主的首发精神分裂症患者的神经炎症反应较以阳性症状为主的患者更强烈,以阳性症状为主的首发精神分裂症患者的异常兴奋可能与其细胞营养不足有关,以阳性症状为主的首发精神分裂症的病理机制可能与以阴性症状为主的首发精神分裂症不尽相同。
精神分裂症;血清蛋白因子;认知功能;兴奋症状;抑郁情绪
精神分裂症(Schizophrenia)是一组复杂多变的临床综合征,可存在阳性、阴性症状[1],认知功能下降[2-3],情绪异常[4],异常兴奋[5-6]等表现。但目前尚缺乏与精神分裂症诊断、病理机制等相关的客观生物学指标,已有研究证实精神分裂症患者的大脑胶质细胞活动存在异常[7-8],而中枢神经系统胶质细胞的异常与神经免疫[9-10]、神经营养[10]以及神经损伤[11]等方面有关。Wallwork等[12]对阳性与阴性症状量表(Positive and Negative Syndrome Scale,PANSS)的深入探索,将PANSS评分划分为阳性症状、阴性症状、认知功能、兴奋症状及抑郁情绪五大方面。本研究从神经免疫、神经营养和神经损伤三方面各选择一种蛋白因子进行定量分析,分别为白细胞介素-6(interleukin-6,IL-6)、神经营养因子-3(neurotrophin-3,NT-3)与钙结合蛋白S100β(S100 calcium-binding protein β,S100β)。应用PANSS评分对被测者的阳性症状、阴性症状、认知功能、兴奋症状及抑郁情绪进行评估[6,12-13],探索首发精神分裂症患者血清中上述蛋白因子浓度与阳性症状、阴性症状、认知、兴奋及抑郁情绪之间的相关性。寻找与精神分裂症诊断、病理及预后评估相关的客观指标,为精神分裂症的诊断治疗提供客观依据[14]。
1 对象与方法
1.1对象
将昆明医科大学第一附属医院精神科2014年1月-2015年11月门诊及住院就诊的精神分裂症患者作为患者组。纳入标准:①符合《国际疾病分类(第10版)》(International Classification of Diseases,tenth edition,ICD-10)精神分裂症的诊断标准;②14~45岁,性别不限;③首次发病,入组前未服用过抗精神病药物;④入组时PANSS总评分≥60分;⑤入组前无电休克治疗史;⑥小学及以上受教育程度,能配合检查,依从性好。排除标准:①有内分泌、免疫或其他代谢障碍等系统性疾病患者;②有癫痫、精神发育迟滞、脑炎等脑器质性疾病或其他神经系统疾病史者;③有药物或精神活性物质滥用史者;④有明显的自杀、危害自身或他人风险者。符合入组标准且不符合排除标准共82例。将患者分为以阳性症状为主的阳性组和以阴性症状为主的阴性组。阳性组入组标准:①PANSS总评分≥70分;②PANSS中的P1(妄)想、P3(幻觉行为)、P6(猜疑/被害)、G9(不寻常思维内容)4个条目中至少2项的单项评分>4分(中度)[15]。阴性组入组标准:①PANSS中与阴性症状和瓦解思维/认知相关的14个条目(P2、N1~N6、G5、G7、G10、G11、G13、G15、G16)总评分>40分;②与阳性症状相关的8个条目(P1、P3、P5、P6、N7、G1、G9、G12)总评分<22分,并且P1、P3、P6、G9这4个条目评分>4分的不超过2项,4个条目评分均≤5分[16]。PANSS评分中P1、P3、P5、G9项为阳性症状相关评分,N1、N2、N3、N4、N6、G7项为阴性症状相关评分,P2、N5、G11项为认知相关评分,P4、P7、G8、G14项为兴奋症状相关评分,G2、G3、G6项为抑郁情绪相关评分[1]。根据上述分组标准,阳性组纳入44例,阴性组纳入38例。选取同期来自本院体检中心的健康体检者为对照组。入组标准:现未患精神疾病,无精神疾病史及家族史;排除标准与患者组相同。对照组共78例。本研究通过昆明医科大学第一附属医院伦理委员会审查,所有被测者均签署知情同意书。
1.2方法
1.2.1血清蛋白因子浓度检测
患者于入组当日或次日清晨,空腹时间≥10 h,抽取空腹肘静脉血5 mL置于非抗凝管,血样静置后以3 000 r/min离心10 min,取血清分装,置于-80 ℃储存待检。采用酶联免疫吸附技术(enzyme-linked immunosorbent assay,ELISA),用美国Merck Millipore公司生产的试剂盒及其配套试剂对NT-3、IL-6和S100β三种血清蛋白因子进行浓度检测,每份血清都进行双份检验,取两次检测的平均值。对照组入组时采血,血液的检测方法同患者组。
1.2.2PANSS评分
PANSS由7项阳性症状、7项阴性症状以及16项一般精神病理症状组成,每个项目采用7级评分法:1分为无症状,2分为很轻,3分为轻度,4分为中度,5分为偏重,6分为重度,7分为极重度。本研究采用PANSS中的项目P1、P3、P5、G9评估阳性症状,N1、N2、N3、N4、N6、G7评估阴性症状,P2、N5、G11项评估认知功能,P4、P7、G8、G14评估兴奋状态,G2、G3、G6评估抑郁情绪[12],应用上述方法评估中国地区精神分裂症患者的认知、兴奋及情感的有效性和可靠性均已得到验证[13,17]。PANSS评定由两名精神医学专业研究生完成,在进行测查前施测者经过系统的为期1周的一致性培训。
1.3统计方法
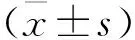
2结果
2.1阳性组、阴性组及对照组的社会人口学资料及PANSS评分比较
阳性组中,男性26例,女性18例。阴性组中,男性22例,女性16例。对照组中,男性40人,女性38人。阳性组、阴性组和对照组的吸烟人数分别为7、5、10人。阳性组、阴性组和对照组三组间的性别(χ2=0.86,P=0.65)、年龄(F=0.93,P=0.40)、BMI指数(F=0.14,P=0.87)、受教育年限(F=0.33,P=0.72)和吸烟人数(χ2=0.24,P=0.89)差异均无统计学意义。阳性组的病程较阴性组短,差异有统计学意义(Z=-3.43,P<0.05)。三组的阳性症状评分(χ2=122.61,P<0.01),阴性症状评分(χ2=124.16,P<0.01),认知评分(χ2=112.44,P<0.01),兴奋评分(χ2=99.26,P<0.01),抑郁情绪评分(χ2=59.11,P<0.01),PANSS总评分(χ2=120.58,P<0.01)差异有统计学意义,进一步两两分析显示,阳性组和阴性组的PANSS总评分、阳性症状评分、阴性症状评分、认知评分、兴奋评分和抑郁情绪评分均高于对照组,阳性组的阳性症状评分高于阴性组,差异均有统计学意义(P均<0.05),而PANSS总评分、阴性症状评分、认知评分、兴奋评分和抑郁情绪评分在两患者组间差异均无统计学意义(P均>0.05)。见表1。
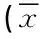
表1 三组的社会人口学资料及PANSS评分比较±s)
注:与阴性组比较,Mann-Whitney U检验,aP<0.05;与对照组比较,Kruskal-Wallis检验,bP<0.05;与阴性组比较,Kruskal-Wallis检验,cP<0.05
2.2三组蛋白因子血清浓度水平
三组血清IL-6浓度比较,差异有统计学意义(F=31.34,P<0.01),两两比较发现,对照组IL-6浓度低于阳性组和阴性组,阴性组高于阳性组,差异均有统计学意义(P均<0.05);三组S100β浓度比较,差异有统计学意义(F=9.19,P<0.05),两两比较,对照组低于阳性组和阴性组,差异有统计学意义(P<0.05),两患者组间S100β浓度差异无统计学意义(P>0.05);三组NT-3浓度比较,差异有统计学意义(F=10.45,P<0.05),两两比较,对照组高于阳性组和阴性组,两患者组间差异无统计学意义(P>0.05)。见表2。
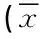
表2 三组血清蛋白因子水平比较±s,ng/L)
注:与对照组比较,经LSD检验,aP<0.05;与阴性组比较,经LSD检验,bP<0.05
2.3三种血清蛋白因子与PANSS评分的相关分析
阳性组中血清NT-3浓度与其兴奋评分散点图显示两者有线性趋势,见图1。进一步相关分析发现两者呈正相关(r=0.38,P<0.05),与其他评分未发现统计学相关;S100β和IL-6浓度与上述各项评分均未发现统计学相关。阴性组和对照组中的NT-3、S100β和IL-6浓度与其上述各评分均未发现统计学相关。见表3。
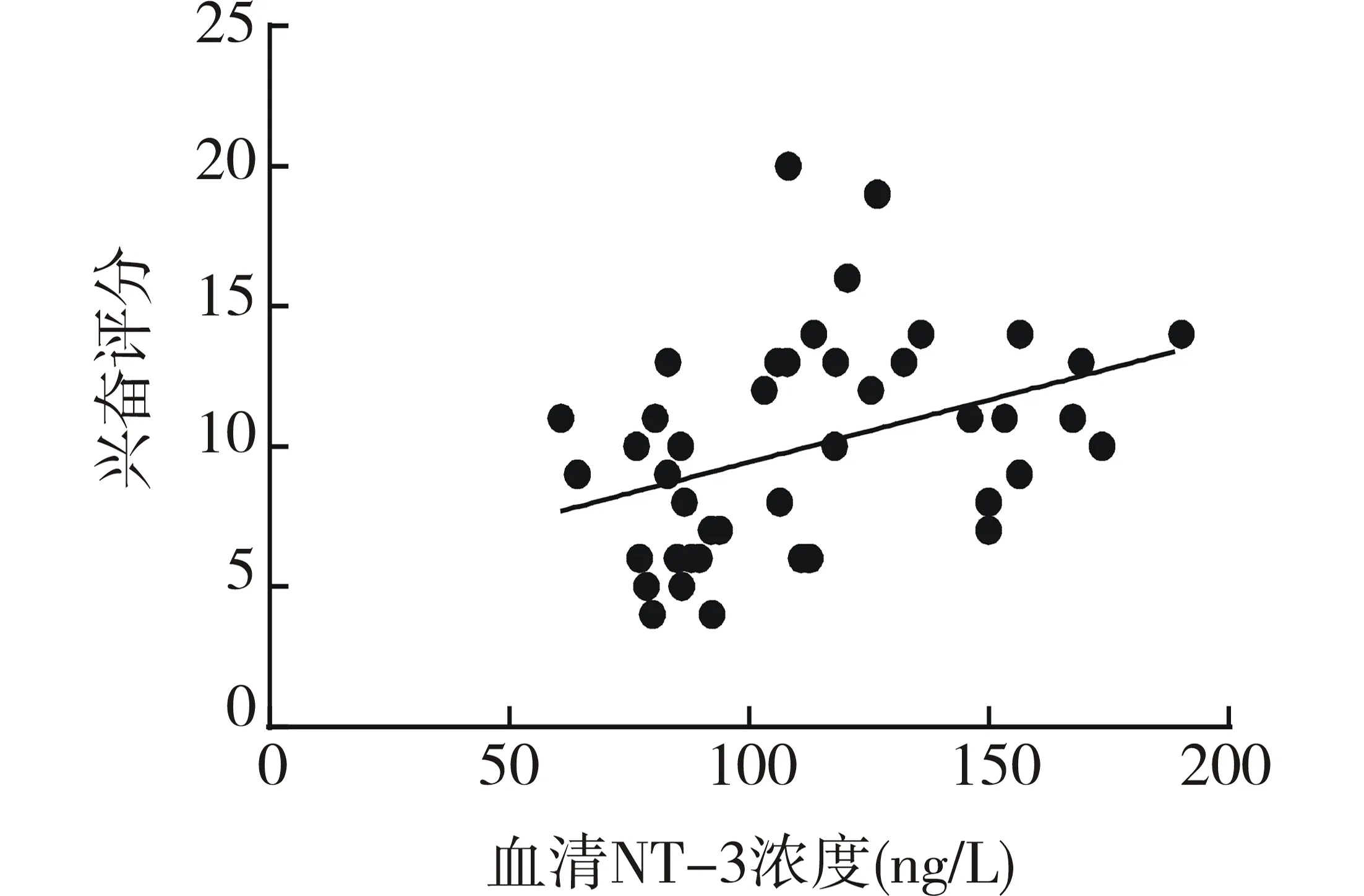
图1 血清NT-3浓度水平与兴奋评分散点图
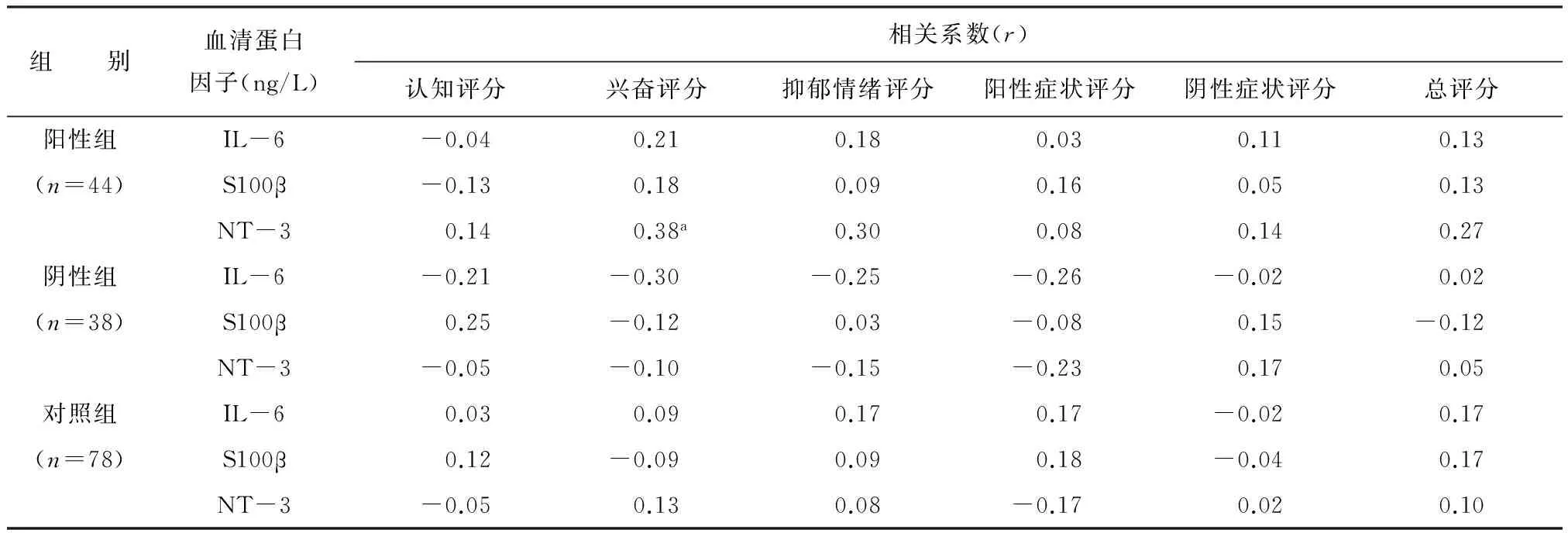
组 别血清蛋白因子(ng/L)相关系数(r)认知评分兴奋评分抑郁情绪评分阳性症状评分阴性症状评分总评分阳性组IL-6-0.040.210.180.030.110.13(n=44)S100β-0.130.180.090.160.050.13NT-30.140.38a0.300.080.140.27阴性组IL-6-0.21-0.30-0.25-0.26-0.020.02(n=38)S100β0.25-0.120.03-0.080.15-0.12NT-3-0.05-0.10-0.15-0.230.170.05对照组IL-60.030.090.170.17-0.020.17(n=78)S100β0.12-0.090.090.18-0.040.17NT-3-0.050.130.08-0.170.020.10
注:aP<0.05
3 讨 论
本实验将研究对象分为阳性组、阴性组及健康对照组,从神经免疫、神经营养和神经损伤三方面各选取一种具有代表性的蛋白因子进行检测。发现两患者组血清S100β、IL-6浓度水平高于对照组,两患者组血清NT-3浓度低于对照组,与既往多个研究结果一致[15-16,18]。进一步分析发现,阳性组血清IL-6浓度水平低于阴性组。提示首发精神分裂症患者已经存在中枢神经免疫异常活跃,神经营养不足以及神经细胞损伤表现,而阴性组患者较阳性组患者的神经免疫激活程度更高,这可以部分解释临床上精神分裂症患者阴性症状很难在短期明显改善和预后不佳的现象。三组认知评分、兴奋评分及抑郁情绪评分比较,阳性组和阴性组的认知、兴奋和抑郁情绪评分均高于对照组,而阳性组和阴性组之间此三项评分差异无统计学意义,可推测精神分裂症患者在疾病早期存在认知功能损害,兴奋异常以及情感障碍,同时以阳性症状为主和以阴性症状为主的精神分裂症患者在疾病早期此三方面的损害程度相当,与既往研究结论一致[2-6]。三种蛋白因子与阳性症状评分、阴性症状评分、认知评分,兴奋评分及抑郁情绪评分的相关分析显示,阳性组中首发精神分裂症患者的外周血清NT-3浓度与其兴奋评分呈正相关(r=0.38,P<0.05),NT-3的生理作用与抑制性神经元γ-氨基丁酸的营养有关,γ-氨基丁酸的神经营养不足和N-甲基-D-天冬氨酸(N-methyl-D-aspartic acid,NMDA)的受体功能不足被认为与精神分裂症的阴性症状有一定关系,可损害神经可塑性,影响神经细胞的增殖、分化等功能[19-20]。而PANSS评分中兴奋评分可较好地反映患者的躁狂样兴奋状态[6],包括不合作性、冲动控制障碍、兴奋状态及敌对性四个方面[5]。提示以阳性症状为主的首发精神分裂症患者的异常兴奋状态与中枢神经系统的神经营养不足有关。
有研究发现个人饮食[21]和早期病毒感染[22]均可能影响精神分裂症的血清蛋白因子,同时本研究样本量相对较小,上述因素均可能影响本研究结果。在今后的研究中期待加大样本量,收集被测者更全面的信息,进一步探索蛋白因子与精神分裂症症状学之间的联系。
综上所述,本研究发现精神分裂症患者在疾病早期便存在神经免疫异常,神经营养不足及神经细胞损害,以阴性症状为主的首发精神分裂症患者的神经炎症反应较以阳性症状为主的患者更强烈。以阳性症状为主的首发精神分裂症患者的异常兴奋状态与神经营养不足有关,提示首发精神分裂症患者的蛋白因子稳态失衡,而且此稳态的异常可能与首发精神分裂症的异常兴奋有一定关系,结合以上发现可推测以阳性症状为主的首发精神分裂症的病理途径可能与以阴性症状为主的首发精神分裂症不尽相同。
[1]Magomedov RA, Garakh ZhV, Orekhov IuV, et al. Gamma-rhythm, positive, negative symptoms and cognitive dysfunction in schizophrenia[J]. Zh Nevrol Psikhiatr Im S S Korsakova, 2010, 110(1): 78-83.
[2]Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia[J]. Biol Psychiatry, 2015, 77(12): 1010-1019.
[3]Arnsten AF, Girgis RR, Gray DL, et al. Novel dopamine therapeutics for cognitive deficits in schizophrenia[J]. Biol Psychiatry, 2016, pii: S0006-3223(16)00044-5.
[4]Rajkumar RP. Depressive symptoms during an acute schizophrenic episode: frequency and clinical correlates[J]. Depress Res Treat, 2015: 674641.
[5]Lindenmayer JP, Brown E, Baker RW, et al. An excitement subscale of the Positive and Negative Syndrome Scale[J]. Schizophr Res, 2004, 68(2-3): 331-337.
[6]Montoya A, Valladares A, Lizán L, et al. Validation of the excited component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room[J]. Health Qual Life Outcomes, 2011, 9: 18.[7]Laskaris LE, Di Biase MA, Everall I,et al. Microglial activation and progressive brain changes in schizophrenia[J].Br J Pharmacol, 2016, 173(4): 666-680.
[8]Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2013, 42: 115-121.
[9]Zhang XY, Tang W, Xiu MH, et al. Interleukin 18 and cognitive impairment in first episode and drug naïve schizophrenia versus healthy controls[J]. Brain Behav Immun, 2013, 32: 105-111.
[10] Zhang XY, Tan YL, Chen DC, et al. Interaction of BDNF with cytokines in chronic schizophrenia[J]. Brain Behav Immun, 2016, 51: 169-175.
[11] Ehrenreich H, Hinze-Selch D, Stawicki S, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin[J]. Mol Psychiatry, 2007, 12(2): 206-220.
[12] Wallwork RS, Fortgang R, Hashimoto R, et al. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia[J]. Schizophr Res, 2012, 137(1-3): 246-250.
[13] Rodriguez-Jimenez R, Bagney A, Mezquita L, et al. Cognition and the five-factor model of the positive and negative syndrome scale in schizophrenia[J]. Schizophr Res, 2013, 143(1): 77-83.
[14] Owen MJ, Sawa A, Mortensen PB. Schizophrenia[J]. Lancet, 2016, 388(10039): 86-97.
[15] Petrikis P, Voulgari PV, Tzallas AT, et al. Cytokine profile in drug-naïve, first episode patients with psychosis[J]. J Psychosom Res, 2015, 79(4): 324-327.
[16] Stefanovics EA, Elkis H, Zhening L, et al. A cross-national factor analytic comparison of three models of PANSS symptoms in schizophrenia[J].Psychiatry Res, 2014, 219(2): 283-289.
[17] Zhang XY, Xiu MH, Song C, et al. Increased serum S100B in never-medicated and medicated schizophrenic patients[J]. J Psychiatr Res, 2010, 44(16): 1236-1240.
[18] Vargas HE, Gama CS, Andreazza AC, et al. Decreased serum neurotrophin 3 in chronically medicated schizophrenic males[J]. Neurosci Lett, 2008, 440(3): 197-201.
[19] Lewin GR, Carter BD. Neurotrophic factors. Preface[J]. Handb Exp Pharmacol, 2014, 220: v-vi.
[20] Wysokiński A. Serum levels of brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in depressed patients with schizophrenia[J]. Nord J Psychiatry, 2016, 70(4): 267-271.
[21] Seo WH, Choi BM, Lee H, et al. Effect of the prenatal maternal environments and diets on cord blood interleukin-4 and interferon-gamma: a pilot study[J]. Asian Pac J Allergy Immunol, 2016, [Epub ahead of print].
[22] Dickerson F, Adamos MB, Katsafanas E, et al. The association among smoking, HSV-1 exposure, and cognitive functioning in schizophrenia, bipolar disorder, and non-psychiatric controls[J]. Schizophr Res, 2016, pii: S0920-9964(16)30246-8.
(本文编辑:唐雪莉)
Association of serum protein factors levels with scores of Positive and Negative Syndrome Scale in first-episode schizophrenia patients characterized by positive or negative symptoms
DAINan1,CHENPeng2,ZENGYong1,XIONGPeng1*,LIUFang1,LIMing1,ZHIJin-Sheng1,CHURui1,JIEHui-Jin1
(1.TheFirstAffiliatedHospitalofKunmingMedicalUniversity,Kunming650031,China;2.SuzhouPsychiatricHospital,Suzhou215008,China*Correspondingauthor:XIONGPeng,E-mail:xp6945399@163.com)
ObjectiveTo explore serum levels of interleukin 6 (IL-6), S100 calcium-binding proteinβ(S100β), neurotrophin-3 (NT-3) in first-episode schizophrenia patients characterized by positive or negative symptoms and the association of IL-6, S100 βand NT-3 serum levels with scores of positivesymptom, negativesymptom, cognitive function, excited symptom and depressive emotion from Positive and Negative Syndrome Scale (PANSS) in these patients.MethodsCases (first-episode schizophrenia patients) were collected from outpatient and inpatient departments of the First Affiliated Hospital of Kunming Medical University from January 2014 to November 2015. Controls were collected from Health Examination Centre of the same hospital during the same period.44 first-episode schizophrenia patients characterized by positive symptoms (positive group), 38 first-episode schizophrenia patients characterized by negative symptoms (negative group) and 78 healthy controls (control group) were collected. The serum levels of IL-6, S100β and NT-3 were measured by enzyme-linked immunosorbent assay (ELISA). Positive symptom, negative symptom, cognitive function, excited symptom and depressive emotion scores were measured by Positive and Negative Syndrome Scale (PANSS) in patients and controls.Results①The difference of IL-6 serum level among the three groups was statistically significant (F=31.34,P<0.01), IL-6 serum level in control group was lower than those of positive or negative groups and the differences were statistically significant (P<0.05). IL-6 serum level in the positive group was lower than that of the negative group and the difference was statistically significant (P<0.05). ②The difference of S100β serum level among the three groups was statistically significant (F=9.19,P<0.05), S100β serum level in control group was lower than those of positive or negative groups and the differences were significant (P<0.05). The difference of S100β serum level between the positive and negative groups was not statistically significant(P>0.05). ③The difference of NT-3 serum level among the three groups was statistically significant (F=10.45,P<0.05), NT-3 serum level in the control group was higher than those of positive or negative groups and the differences were significant (P<0.05). However, the difference of NT-3 serum level between the positive and negative groups was not statistically significant (P>0.05). In positive group, NT-3 serum level was associated with the score of excited symptom (r=0.38,P<0.05).ConclusionFirst-episode schizophrenia patients characterized by negative symptoms have more severe neuroinflammatory responses than those characterized by positive symptoms.The excitement in first-episode schizophrenia patients characterized by positive symptoms may be associated with malnutrition in neuro cells. The pathophysiology between the first-episode schizophrenia patients characterized by positive symptoms and those characterized by negative symptoms may be different.
Schizophrenia;Serum protein factor; Cognitive function;Excitement symptom;Depression
国家自然科学基金(81360210)
R749.3
A
10.11886/j.issn.1007-3256.2016.04.009
2016-06-20)

