The Survey of Cronobacter spp. (formerly Enterbacter sakazakii)in lnfant and Follow-up Powdered Formula in China in 2012*
PEI Xiao Yan, YAN Lin, ZHU Jiang Hui, LI Ning, GUO Yun Chang, FU Ping,JIA Hua Yun, ZHANG Xiu Li, YANG Xiao Rong, and YANG Da Jin,#
1. China National Center for Food Safety Risk Assessment, Beijing 100022, China; 2. Hunan Province Center for Disease Control and Prevention, Changsha 410000, Hunan, China; 3. Henan Province Center for Disease Control and Prevention, Zhengzhou 450000, Henan, China; 4. Sichuan Province Center for Disease Control and Prevention, Chengdu 610000, Sichuan, China
Original Article
The Survey of Cronobacter spp. (formerly Enterbacter sakazakii)in lnfant and Follow-up Powdered Formula in China in 2012*
PEI Xiao Yan1, YAN Lin1, ZHU Jiang Hui1, LI Ning1, GUO Yun Chang1, FU Ping1,JIA Hua Yun2, ZHANG Xiu Li3, YANG Xiao Rong4, and YANG Da Jin1,#
1. China National Center for Food Safety Risk Assessment, Beijing 100022, China; 2. Hunan Province Center for Disease Control and Prevention, Changsha 410000, Hunan, China; 3. Henan Province Center for Disease Control and Prevention, Zhengzhou 450000, Henan, China; 4. Sichuan Province Center for Disease Control and Prevention, Chengdu 610000, Sichuan, China
Abstract
Objective To determine Cronobacter spp. contamination in infant and follow-up powdered formula in China.
Methods All of 2282 samples were collected from the retail markets in China from January 2012 to December 2012, and analyzed for Cronobacter spp. by the Chinese National Food Safety Standard. Characterization of the isolates was analyzed by pulsed-field gel electrophoresis (PFGE) with XbaI and SpeI restriction enzymes.
Results Cronobacter spp. strains were isolated from 25 samples, and the positive rates in infant powdered formulas and follow-up powdered formulas were 0.90% (10/1011) and 1.18% (15/1271),respectively. Analysis of variable data regarding different purchasing store formats, seasonality, and production locations as well as comparison of infant versus follow-up formulas did not reveal statistically significant factors. During the sampling period, one of six surveillance zones did exhibit a statistically significant trend towards higher positive rate. PFGE characterization of Cronobacter spp. to elucidate genetic diversity revealed only three pairs of Cronobacter spp. out of 25 having the same PFGE patterns.
Conclusion The current investigation indicated a lower positive rate of Cronobacter spp. in the powdered formula in China. This evidence suggested contamination originating from multiple different sources during the manufacturing process.
Cronobacter spp.; Contamination; PFGE; Powdered formula
www.besjournal.com (full text) CN: 11-2816/Q Copyright ©2016 by China CDC
INTRODUCTION
C ronobacter spp. is a ubiquitous gramnegative, non-spore-forming opportunistic pathogen frequently isolated from food and environmental samples[1-3]. On occasion, it has been associated with sporadic cases or small outbreaks of sepsis, meningitis, cerebritis, and necrotizing enterocolitis. Although Cronobacter spp. is associated with illness in all age groups, analysis of age distribution in reported cases indicates that infants (children <1 year) are at particular risk.Among infants, those at greatest risk for Cronobacter spp. infection are neonates (<28 days), particularly low-birth weight infants or immunocompromised infants. Cronobacter spp. has been identified as one of the microorganisms of greatest concern with infant powdered formula[2,4].
The first 2 known cases of Cronobacter spp. neonatal meningitis were reported by Urmenyi and Franklin in 1961[5]. Since 1961, an increasing number of Cronobacter spp. infections have been reported amongst neonates, infants and children with exposure and outbreaks of infection associated with infant formula[1,6]. Prior to 2004, incidence of food contamination and related infection from Cronobacter spp. had not been reported in China[7]. At the inception of field surveillance for this pathogen contamination levels were observed at 12.64% (11/87) in counterfeit and low-quality powdered infant formula in Fuyang, Anhui province in 2004 and 5.19% (11/212) in powdered formula in the Chinese retail market in 2005[8-9]. The Chinese government strengthened the inspection and regulation of powdered infant and follow-up formula factories after the above two surveys[8-9], especially Sanlu milk scandal[10]. This survey was conducted to collect information about the contamination of Cronobacter spp. in powdered infant and follow-up formula sold in national market in all the provinces in order to obtain the contamination level and the effect of government regulation. All these isolates were further characterized using the PFGE to learn the relevance of this contamination.
MATERIALS AND METHODS
Sampling Plan
About 20 cities were investigated in each surveillance area and these cities were selected hierarchically based on economic development as measured by per capita gross domestic product (GDP).
Local retail sales data were used to develop a sampling plan that is representative of daily consumption patterns in both urban and rural markets. The sampling points should include all the retail locations for local residents where these products may be bought. Only one sample can be picked randomly from each batch powdered formula sold in the market. All brands of powdered formula produced within the surveillance area were considered for evaluation. Powdered formulas suitable from birth to 36 months were collected from markets. In total, 2282 powdered formula samples were collected during quarterly collections from January 2012 to December 2012 (Table 1).
Isolation and Identification
For this investigation, laboratories were qualified for participation if they had completed blinded control proficiency testing within the last two years and were capable of having positive isolates re-identified by BioMérieux VITEK 2 GN ID card. All the data were submitted by the National Wed-Based Reporting System of Food Microbiology Surveillance[11].
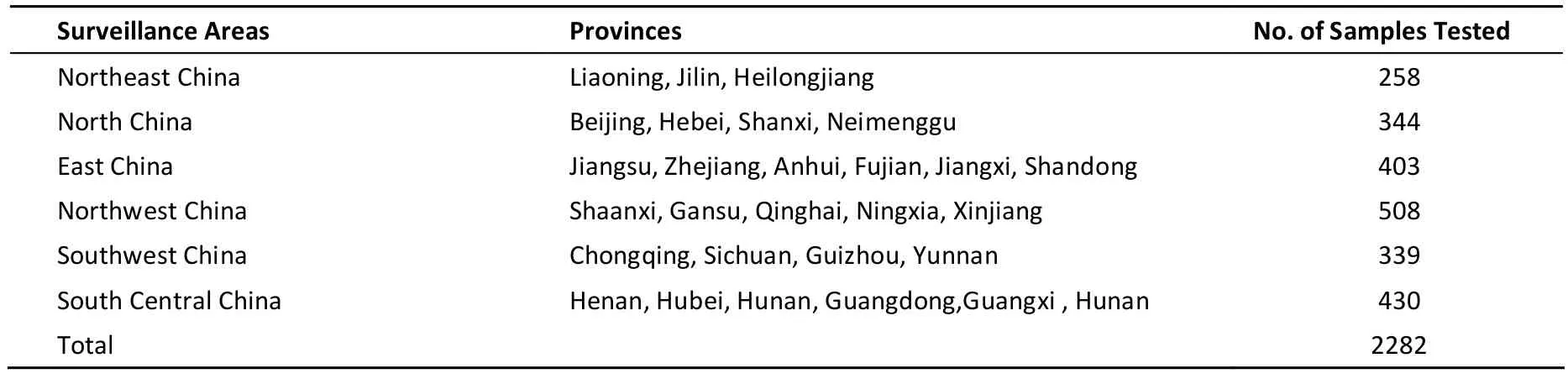
Table 1. Samples in Powdered Formula in Different Surveillance Areas
The examination of the powdered formula was performed according to Chinese National Food Safety Standard-Food Microbiological Examination: Cronobacter spp. GB 4789.40-2010[12]. Using a three-tube most probable number (MPN) procedure,triplicate 100 g, 10 g, and 1 g samples were aseptically weighed out for analysis and transferred into 90 mL culture media and 9 mL sterile buffered peptone water (BPW) (Huankai Micorbiology Technologies Co., Ltd., China), respectively, and incubated at 36 °C for 18±2 h. Then, 1 mL pre-enrichment medium transferred into 10 mL of mLST/vancomycin medium (Luqiao Technologies Co.,Ltd., China) and incubated at 44±0.5 °C for 24±2 h. The incubated broth samples were streaked onto thesurface of the CHROMagar™ Cronobacter spp. agarplate (CHRO Magar, France) and incubated at 37 °C for 24±2 h. The presumptive colonies were streaked onto the surface of the TSA plate (Haibo Technologies Co., Ltd., China), and incubated the plate at 25 °C for 44 h to 48 h. After incubation, only yellow pigmented colonies were selected and confirmed using the VITEK® automatic biochemical identification system according to the manufacturer’s instructions. MPN estimation of Cronobacter spp. cells/100 g of sample was calculated based on the number of ‘positive tubes’ at each dilution in which the presence of Cronobacter spp. was confirmed.
Molecular Subtyping by PFGE
The method for PFGE subtyping of Cronobacter spp. was derived from a modified Salmonella PulseNet protocol with modification: XbaI and SpeI as primary and secondary restriction enzymes,respectively[13]. The PFGE method has been proven to be a stable high resolution method, and the XbaI restriction enzyme is recommended for this type of investigation[14-16]. Following overnight incubation,Cronobacter spp. were removed from TSA plate (Oxoid, Hampshire, UK) with a cotton swab, and cell suspensions were adjusted to 14%-15% transmittance (measured in Falcon 2054 tubes) as measured by a bioMérieuxVitek colorimeter. Agarose plugs [0.5% (w/v)] were prepared in plug molds (Bio-Rad Laboratories, Hercules, CA) and allowed to solidify. The plugs were lysed at 54 °C for 2 h in 5 mL cell lysis buffer [50 mmol/L Tris-HCl (pH 8.0), 50 mmol/L EDTA (pH 8.0), 1% (w/v) sodium lauroyl-sarcosine, 20 mg/mL proteinase K] with gentle shaking. Agarose plugs were then washed twice with 15 mL deionized water and a further four times with 10 mL TE buffer [10 mmol/L Tris (pH 8.0),1 mmol/L EDTA (pH 8.0)] at 50 °C with vigorous shaking. The prepared plugs containing the purified DNA were cut into 3 mm slices and digested with XbaI for 3-4 h. DNA fragments were separated by electrophoresis (CHEF Mapper, Bio-Rad Laboratories)through a 1% (w/v) agarose gel (Seakem Gold,Rockland, Maine) in 0.5× TBE buffer at 14 °C for 18 h at 6.0 V/cm with pulse times ramped linearly from 2.16 to 63.80 s. Cronobacter spp. with the same patterns digested with XbaI were also characterized with SpeI for 4-hours digestion, DNA fragments were separated by electrophoresis through a 1% (w/v)agarose gel in 0.5× TBE buffer at 14 °C for 20 h at 6.0 V/cm with pulse times ramped linearly from 2.16 to 40.01 s.
Statistical Analysis
Cronobacter spp. positive rate was analyzed by logistic regression due to the low positive samples. DNA fingerprints were analyzed by BioNumerics software version 6.6 (Applied Maths, NV, Belgium)where the dendrogram was generated using the DICE coefficient and the unweighted pair group method with arithmetic mean (UPGMA).
RESULTS
Cronobacter spp. Positive Rate in Powdered Formula
Overall, 1.10% (25/2282) samples were positive for the presence of Cronobacter spp. Twenty of 25 positive samples were in low-level contamination of Cronobacter spp. between 0.3 MPN/100 g and 0.94 MPN/100 g, and the maximum contamination level was 24 MPN/100 g. 0.90% (10/1011) positive rate of Cronobacter spp. was observed with slightly lower incidences in infant powdered formula than that in follow-up products 1.18% (15/1271). However, a significant difference was failed to observed between the two kinds of powdered formula neither using Chi-square test (χ2=0.054, P=0.816) nor Fisher exact test (P=0.692).
There were statistically significant differences between different surveillance areas using logistic regression analysis and the odds ratio was 1.491 with 95% confidence interval (CI) from 1.141-2.001 (Table 2). The contamination in South Central China was much higher than other areas. Five samples with different lots from one production factory in South Central China were found positive for Cronobacter spp. by three different laboratories.
Sample collection occurred at supermarkets,retail stores, whole sale markets and production factories. Supermarkets were the primary source for samples (87.86%; 2005/2282), followed by retail stores (9.99%; 228/2282). The present study did not show statistically significant difference of Cronobacter spp. positive rate in powdered formula from different sampling sites including (odds ratio=1.277, 95% CI: 0.499-2.461) and excluding processing factory (odds ratio=1.419, 95% CI: 0.531-2.939) by logistic regression analysis (Table 2).
There was no statistically significant difference found for Cronobacter spp. positive rate in powdered formula among different productionquarters neither by Chi-square test (χ2=0.683,P=0.823) nor logistic regression analysis (odds ratio=1.040, 95% CI: 0.721-1.482).
The present study failed to find significant difference of Cronobacter spp. positive rate in powdered formula among different sampling quarters using Chi-square test (χ2=1.962, P=0.580)and logistic regression analysis (odds ratio=1.208,95% CI: 0.803-1.863).
Based on the labeling production locations,Cronobacter spp. positive rate in powdered formula from different production areas was shown in Table 2. Using Chi-square test (χ2=5.752,P=0.451) and logistic regression analysis (odds ratio=1.147, 95% CI: 0.938-1.397), the present study failed to find the statistical difference of Cronobacter spp. positive rate in powdered formula from different production areas, and the same as comparison between samples produced at home and abroad by Fisher exact probability method (odds ratio=0.882, 95% CI: 0.140-36.716).
A multiple factors logistic regression was performed to measure the association between the
Cronobacter spp. positive rate in powdered formula and its potential factors, including surveillance area, sampling site, production quarters and sampling quarters. It found significant variation of the contamination among different surveillance areas (odds rate=1.491, 95% CI: 1.141-2.008), but failed to detect the statistical associations for sampling site (odds rate=1.058, 95% CI: 0.386-3.663),production quarters (odds rate=1.011, 95% CI: 0.697-1.448) and sampling quarters (odds rate=1.215, 95% CI: 0.806-1.886).
Molecular Characteristics of Cronobacter spp. Isolates

Table 2. Cronobacter spp. Positive Rate in Powdered Formula
A dendrogram was compiled showing genetic relationships among Cronobacter spp. isolates (Figure 1). Twenty-five isolates generated 21 unique PFGE patterns digested with XbaI, and four pairs of isolates had indistinguishable PFGE patterns,respectively. Three pairs of positive isolates produced indistinguishable PFGE patterns after digestion with SpeI (Figure 2). The positive sampleswere isolated from different products in one processing factory in North China. C122, C125, C124,and C139 were all from the same factory in South Central China, but the production date was very different and at least 3 months separated production of these four samples. There was only one difference in DNA banding between C016 and C101 with 96.6% similarity after digestion with SpeI. Interestingly,these two positive samples were produced from different factories located in Northwest China and Northeast China.
DISCUSSION
Contamination of Cronobacter spp. in Powdered Formula
Only 40 grams of sample was tested for the qualitative analysis of counterfeit and low-quality infant formula in Fuyang, Anhui province in 2004[8]and the powdered formula in Chinese retail market from in 2005[9], but 333 g sample were tested for quantitative analysis in this surveillance. There have been reported Cronobacter spp. Contaminations in2.4% samples of powdered infant formulas[17], and 0.7% of follow up formulas assessed qualitatively using 25 g samples in the United Kingdom[18]and 5.3% powdered infant formulas with 333 g total sample by quantitative analysis in Korea[19]. The current investigation indicated a lower positive rate than in the above referenced studies.
Since 2004, serial intervention measures have been carried out for the production and sale of infant and follow-up powdered formula by Chinese Government. Measures included standardization of dairy cattle breeding, encouraging reorganization and modernization of enterprises, raising the threshold of market entry, mandating whole process traceability, and implementation of factory inspections for all sites. In addition, harmonization with international standards, higher frequency in process and market supervision, and release of all the related alert information to the public have led to the rapid quality improvement for powdered formula and the significant reduction in Cronobacter spp. contamination that has been observed in the past ten years of ongoing surveillance[9,20]. However,it was worthwhile to note that some factories were occasionally contaminated by this pathogen. The national disease surveillance for Cronobacter spp. has been conducted in China since 2012 after infant and follow-up food was found to be contaminated by this pathogen[21]. Four children aged 4-10 were reported for foodborne disease of Cronobacter spp. for drinking spoiled milk beverage in Chongqing in 2013[22], and an infant with persistent diarrhea from environmental contamination was reported in Sichuan province in 2014[23]. There have been no reports of foodborne Cronobacter spp. related infection caused by contaminated infant and follow-up powdered formula in contrast to the worldwide distribution of reported cases[4].
Cronobacter spp. was detected in several studies with positive rates ranging from 2% to 12.5% reported around 2000[24]. However, positive isolates were seldom obtained from the infant powdered formula in developed countries in the most recent 10 years[25-27]. There was little observed difference concerning the average positive rates of powdered formula among different provinces in China from 0% to 0.8%[28-30]. However, the positive rates of Cronobacter spp. in several Chinese cities were still high (above 5%) and were associated with persistent contamination within individual local factories[20,31].
Cronobacter spp. was detected at levels from 0.3 MPN/100 g up to 66 MPN /100 g in some studies[1,24,26,32]. In our surveillance, the quantitative data of 25 positive samples ranged from 0.3 MPN /100 g up to 24 MPN /100 g and the contamination levels were similar to the above reports. All of these data showed low contamination level of Cronobacter spp. in most infant and follow-up powdered formula in China.
Analysis of Contaminated Powdered Formula
There were no statistically significant differences concerning Cronobacter spp. occurrence among the infant powdered formula and follow-up products,four kinds of sampling sites, production and sampling quarters. These low positive rates are anticipated since all the facilities can produce both kinds of products in confined and sanitary environments with critical control of raw materials,hygiene, temperature, and humidity. Furthermore,each production facility can source and use raw materials on production lines that maintain sanitary standards over similar periods of time, and all of these powdered products are produced and pre-packed with long shelf life and quite stable microbial colony control as dehydrated powdered foods[2].
The positive rate of Cronobacter spp. in South Central China was much higher than other areas because of the severe contamination of a local production factory. A total of six positive samples from this factory were detected by 4 laboratories in this survey. Further investigation showed that this factory used a dry-mix process, which suggests there was not a high-temperature sterilization process step, and the production line was not strictly controlled by a HACCP system resulting in a lack of critical controls for raw materials and the manufacturing environment. Four PFGE patterns for six isolates proved most of contaminations occurred from different sources. Together, these data demonstrated a serious microbiological hazard in this factory. Products from this facility were mainly sold within the South Central China regional markets, and exposure in the other five markets was limited.
Another brand of products was also found to have 5 samples contaminated by Cronobacter spp. Two positive samples, isolates C114 and C185, were produced in the parent factory in North China, and three positive samples, isolates C153, C154, and C062,were from the subsidiary factory in Northeast China. Analysis of the types of powdered formula,production data, production factories and PFGEpatterns, it was deduced that different contamination sources of Cronobacter spp. were responsible for contamination of these samples with the exception of C153 and C154. Analysis of the sources of the contamination was more complex than the dry-mix investigation since these samples were produced using a wet-mix process that included pasteurization and high-temperature sterilization processing steps.
The isolates with higher similarity, such as C111 and C165, C044 and C153 (or C154), were from different factories separated by considerable distance. Strain C111 was isolated from a sample produced in Holland, and no known epidemiological link could be identified. Three pairs of isolates with the same XbaI and SpeI PFGE pattern were isolated from different products made in the same production factory. Two of these had similar production dates but the third occurrence happened after a 10 month gap (Figure 2). The PFGE method was a useful tool in identifying clusters of Cronobacter spp.
In conclusion, the positive rate of Cronobacter spp. was lower than previous observations in China. However, the presence of these foodborne pathogens in powder products is still a potential threat to the health of infant and young children,particularly for infants under 6 months of age and vulnerable infant. Greater attention should be paid to the contamination of infant and follow-up powdered formula.
Accepted: February 2, 2016
REFERENCES
1. Yan QQ, Condell O, Power K, et al. Cronobacter species (formerly known as enterobacter sakazakii) in powdered infant formula: A review of our current understanding of the biology of this bacterium. J App Microbiol, 2012; 113, 1-15.
2. FAO/WHO. Enterobacter sakazakii and other microorganisms in powdered infant formula. In Microbiological risk assessment series 6, meeting report, 2007; p51.
3. Mozrova V, Brenova N, Mrazek J, et al. Surveillance and characterisation of Cronobacter spp. In czech retail food and environmental samples. Folia Microbiol(Praha), 2014; 59, 63-8.
4. Hunter CJ, Bean JF. Cronobacter: An emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J Perinatol, 2013; 33, 581-5.
5. Urmenyi AM, Franklin AW. Neonatal death from pigmented coliform infection. Lancet, 1961; 1, 313-5.
6. Hunter CJ, Petrosyan M, Ford HR, et al. Enterobacter sakazakii: An emerging pathogen in infants and neonates. Surg Infect(Larchmt), 2008; 9, 533-9.
7. PEI X, LIU X. Biological characteristics and health hazards of Enterobacter sakazakii: A review. Chin J Food Hygiene, 2004;550-5. (In Chinese)
8. LIU X, PEI X, GUO Y. Isolation of Enterobacter sakazakii from fake and low-quality infant fomular powder samples collected from fuyang , anhui province, china. Chin J Food Hygiene, 2005;10-2. (In Chinese)
9. PEI X, LIU X. Survey of Enterobacter sakazakii and other enterobacteriaceae from powdered formula in chinese marker. Journal of Chinese institute of food science and technology,2006; 6-10. (In Chinese)
10. Xin H, Stone R. Tainted milk scandal. Chinese probe unmasks high-tech adulteration with melamine. Science, 2008; 322,1310-11.
11. PEI X, YANG D, GUO Y, et al. Design and realization of network system of national food microorganism risk surveillance. China Digital Medicine, 2014; 90-3. (In Chinese)
12. MOH. National food safety standard food microbiological examination: Enterobacter sakazakii. In the Second Method: Enumerition of Enterobacter sakazakii, Standards Press of China, 2010; Vol. 2010, p 10. (In Chinese)
13. PulseNet. Standard operating procedure for pulsenet pfge ofEscherichia coli O157:H7, Escherichia coli non-o157 (STEC),Salmonella serotypes, Shigella sonnei and Shigella flexneri. http://www.pulsenetinternational.org/protocols/ (24 March 2013).
14. PEI X, GUO Y, LIU X. Study on the molecular typing of Enterobacter sakazakii with pulsed-field gel electrophoreses. Journal of Hygiene Research, 2008; 179-82+86. (In Chinese)
15. Cui J, Du X, Liu H, et al. The genotypic characterization of Cronobacter spp. Isolated in china. PloS One, 2014; 9, e102179.
16. Brengi SP, O'Brien SB, Pichel M, et al. Development and validation of a pulsenet standardized protocol for subtyping isolates of Cronobacter species. Foodborne Pathog Dis, 2012; 9,861-7.
17. Iversen C, Forsythe S. Isolation of Enterobacter sakazakii and other enterobacteriaceae from powdered infant formula milk and related products. Food Microbiology, 2004; 21, 771-7.
18. Chap J, Jackson P, Siqueira R, et al. International survey of Cronobacter sakazakii and otherCronobacter spp. In follow up formulas and infant foods. Int J Food Microbiol, 2009; 136,185-8.
19. KimA, Oh SW, Lee YM, et al. Microbial contamination of food products consumed by infants and babies in korea. Lett Appl Microbiol, 2011; 53, 532-8.
20. Pan Z, Cui J, Lyu G, et al. Isolation and molecular typing of Cronobacter spp. In commercial powdered infant formula and follow-up formula. Foodborne Pathog Dis, 2014; 11, 456-61.
21. Pei X, Guo Y, Li N, et al. Overview on food microbiological risk monitoring in china,2012. Chinese Journal of Public Health Management, 2015; 25-8. (In Chinese)
22. Chen K, Wen X, Hu X, et al. Laboratory analysis of a foodborne poison caused by Enterobacter sakazakii. Strait Journal of Preventive Medicine, 2013; 51-2. (In Chinese)
23. Yang X, Huang W, Huang X, et al. Source trace ofEnterobacter sakazakii infection in an infant with repeated diarrhea. Disease Surveillance, 2014; 794-96. (In Chinese)
24. FAO/WHO. Enterobacter sakazakii and salmonella in powdered infant formula; 2006.
25.NZFSA. Infant formula and Cronobacter sakazakii survey report;2009.
26. EFSA. Microbiological contaminants in food in the european union in 2004-2009; 2012.
27. Holy O, Forsythe S. Cronobacter spp. As emerging causes of healthcare-associated infection. J Hosp Infect, 2014; 86,169-77.
28. Li M, Ye L, Chen W. Survey of Enterobacter sakazakii in formula and cereal-based complementary foods for infants and young children from fujian province in 2011. Strait Journal of Preventive Medicine, 2012; 18, 55-6. (In Chinese)
29. Yao X, Tang Z, Liu Z, et al. Surveilance and analysis of food borne pathogens in foods for infants and young children in guangxi markets in 2012. Chinese Journal of Food Hygiene,2015; 27, 85-8. (In Chinese)
30. Zhou S, Deng X, Zhu H, et al. Survey of Enterobacter sakazakii pollution in infant food in guangdong province. Chinese Journal of Health Laboratory Technology, 2014; 2248-51. (In Chinese)
31. Quan Y, Hu X, Lan G, et al. Survey on contamination by Enterobacter sakazakii in infant formula milk powder produced in gansu. Chinese Journal of Health Laboratory Technology,2013; 990-1+3. (In Chinese)
32. Siqueira Santos RF, da Silva N, Amstalden Junqueira VC, et al. Screening for Cronobacter species in powdered and reconstituted infant formulas and from equipment used in formula preparation in maternity hospitals. Ann Nutr Metab,2013; 63, 62-8.
Biomed Environ Sci, 2016; 29(2): 99-106 10.3967/bes2016.011 ISSN: 0895-3988
November 29, 2015;
*This study was supported by National High-tech R&D Program of China (863 Program) (2012AA101603).
#Correspondence should be addressed to YANG Da Jin, Tel: 86-10-52165572; Fax: 86-10-52165573, E-mail: yangdajin@ cfsa.net.cn
Biographical note of the first auhtor: PEI Xiao Yan, female, born in 1979, PhD, majoring in food safety.
 Biomedical and Environmental Sciences2016年2期
Biomedical and Environmental Sciences2016年2期
- Biomedical and Environmental Sciences的其它文章
- Dimethylacetamide-induced Hepatic Injury in Vitro: Mechanism and Potential Preventive Strategy*
- Evaluation of Protective Effects of Bioactive Phytochemicals Against Methotrexate in Salmonella typhimurium TA1535/pSK1002 Coupled with Micronucleus Assay*
- Protective Effects of Lycopene on Furan-treated Diabetic and Non-diabetic Rat Lung
- Gene Knockdown in Human Rhinovirus 1B Using 2′-OMe-modified siRNAs Results in the Reactivation of the Interferon Response
- Bioremediation of Hexavalent Chromium Pollution by Sporosarcina saromensis M52 lsolated from Offshore Sediments in Xiamen, China*
- Cadmium Activates Reactive Oxygen Species-dependent AKT/mTOR and Mitochondrial Apoptotic Pathways in Neuronal Cells*
