Macular choroidal thickness in highly myopic glaucoma patients: a multiple regression analysis
Gai-Yun Li, Wei Chen, Samer abdo Al-wesabi, Li Wang, Hong Zhang
Macular choroidal thickness in highly myopic glaucoma patients: a multiple regression analysis
Gai-Yun Li1, Wei Chen1, Samer abdo Al-wesabi1, Li Wang2, Hong Zhang1
1Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China2Department of Ophthalmology, Baylor College of Medicine, Houston 77030, Texas, USA
Correspondence to:Hong Zhang.Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, No.1095 Liberation Road, Wuhan 430030, Hubei Province, China. dr_zhanghong@vip.163.com
Received: 2015-08-22Accepted: 2016-04-21
目的:探讨高度近视合并原发性开角型青光眼(POAG)患者的黄斑区脉络膜厚度分布特征及影响黄斑区脉络膜厚度的相关因素。
方法:研究共纳入高度近视合并POAG患者18例32眼,POAG患者20例36眼,以及高度近视患者21例33眼。采用频域OCT增强深度扫描测定中心凹下(SFCT)、距中心凹1mm和3mm处上、下、鼻、颞四个方位的脉络膜厚度(S1CT、S3CT、I1CT、I3CT、N1CT、N3CT、T1CT、T3CT)。比较三组间黄斑区脉络膜厚度的差异,并利用多元回归分析对POAG患者的诊断、屈光度、年龄、眼压、角膜厚度、视野缺损等对黄斑区脉络膜厚度的影响进行分析。
结果:高度近视合并POAG患者的黄斑区脉络膜厚度较POAG患者在各测量位置均明显变薄(均P<0.05),但与高度近视患者在各测量点的脉络膜厚度差异均无统计学意义(均P>0.05)。屈光度是POAG患者黄斑区各测量点脉络膜厚度的主要影响因素,颞侧距中心凹3mm处的脉络膜厚度(S3CT)受年龄的影响,而诊断、视野缺损、眼压及角膜厚度不是脉络膜厚度的影响因素。
结论: 高度近视合并POAG患者黄斑区脉络膜厚度比单纯POAG患者薄,但与高度近视患者无统计学差异,黄斑区脉络膜厚度与青光眼性视神经损伤程度无明显关联。
引用:栗改云, 陈威, Al-wesabi S, 王莉, 张虹. 高度近视合并青光眼患者黄斑区脉络膜厚度的多元回归分析.国际眼科杂志2016;16(8):1425-1429
Abstract
•AIM: To evaluate the characteristic of choroidal thickness (CT) in highly myopic glaucoma eyes, and investigate the factors that affect the CT in various regions of the macula.
•METHODS: Thirty-two highly myopic eyes of 18 patients with primary open angle glaucoma (POAG), 36 non-highly myopic eyes of 20 patients with POAG, and 33 non-glaucoma highly myopic eyes of 21 matched volunteers were enrolled. CT at subfoveal, and 1mm and 3mm nasal, temporal, superior, and inferior to the fovea was measured using enhanced depth imaging coherence tomography. Multiple linear regression analyses were performed to detect the effects of diagnosis, spherical equivalent (SE), age, intraocular pressure (IOP), central corneal thickness (CCT), and mean deviation (MD) of visual field defect on CT at all measured points.
•RESULTS: The choroid of highly myopic glaucoma eyes was statistically thinner than non-highly myopic glaucoma eyes at various locations (allP<0.05), while there was no significant difference between highly myopic glaucoma and non-glaucoma high myopia eyes at all locations (allP>0.05). Multiple regression analysis showed that SE was the most influential factor on CT in all regions of the macula, and CT varied significantly with age in 3mm superior to fovea (S3CT), but not with diagnosis, MD of visual field defect, IOP, or CCT.
•CONCLUSION: CT in highly myopic glaucoma is equivalent in comparison with non-glaucoma highly myopia, although it’s thinner than that in glaucoma eyes without high myopia. This implies the lack of association between CT and progression of glaucomatous optic neuropathy.
KEYWORDS:•myopia; primary open-angle glaucoma; choroidal thickness; macula; optical coherence tomography
Citation:Li GY, Chen W,Al-wesabi S, Wang L, Zhang H. Macular choroidal thickness in highly myopic glaucoma patients: a multiple regression analysis.GuojiYankeZazhi(IntEyeSci) 2016;16(8):1425-1429
INTRODUCTION
Myopia is an independent risk factor for having primary open-angle glaucoma (POAG)[1-4]. Epidemiologic evidence suggests that high myopia is a risk factor for not only the development but also the progression of glaucomatous optic neuropathy[4-5]. The pathogenesis in patients with both high myopia and glaucoma is quite equivocal.
Some studies have reported that patients experiencing glaucoma progression have worse ocular hemodynamics than nonprogressors[6-7]. Choroidal thickness (CT) evaluation for detecting choroidal blood flow has been increasingly studied to detect the role of impaired choroidal blood flow to glaucomatous optic neuropathy. Whether CT correlates with glaucomatous damage is still an open question. Some researchers[8-10]found that thinner choroid is associated with glaucoma, while results of other studies[11-14]did not support the view.
High myopia is associated with thinner CT[15-18]. It is not known whether eyes with thinner choroid are more vulnerable to suffer glaucomatous damage than normal eyes. In other words, whether thinner choroid induced by high myopia accelerates the development and progress of glaucomatous damage in highly myopic glaucoma than in non-highly myopic glaucoma eyes. In the current study, we aimed to determine the characteristic of CT in highly myopic glaucoma eyes, and to elucidate the association between CT and glaucomatous optic neuropathy.
SUBJECTS AND METHODS
The study was performed according to the tenets of the Declaration of Helsinki. The ethics committee of Tongji Hospital, in which the work has been held, approved the protocol of the study. Informed consent was obtained from each patient. All subjects received a complete ophthalmic examination which included the best-corrected visual acuity, intraocular pressure (IOP) measurement (Goldmann tonometer), gonioscopy, ocular fundus examination, visual field test (Humphrey, 30-2, SITA - standard), central corneal thickness (CCT; A-mode ultrasound), and dioptometry. Highly myopic glaucoma and POAG subjects were included if they had an established diagnosis made by a glaucoma specialist according to the Chinese glaucoma association guidelines. The high myopia subjects were volunteers without glaucoma. Three groups were involved in the study. Group 1: highly myopic glaucoma [spherical equivalent (SE) > -6.0 diopters (D)], consisted of 32 eyes of 18 patients. Group 2: non-highly myopic glaucoma (SE -6.0D to +0.5D), consisted of 36 eyes of 20 patients. Group 3: consisted of 33 non-glaucoma highly myopic eyes of 21 volunteers. Inclusion criteria were open angles, reliable visual field results, and SE between +0.5 and -18 D to exclude extremely high myopia. The exclusion criteria included: 1) macular abnormalities such as choroidal neovascularization or whitish myopic atrophy; 2) systemic abnormalities such as vascular disease, hypertension, or diabetes mellitus; or 3) a history of intraocular surgery.
Enhanced depth imaging (EDI) mode of spectral domain optical coherence tomography (Spectralis, Heidelberg, Germany) was used to measure CT via the method described previously[17,19]. CT was defined as the layer extended from retinal pigment epithelium (RPE) to the choroid-sclera hyperreflective borderline in the profile of the image. CT was measured at nine points: subfoveal CT (SFCT) and CT at 1mm and 3mm nasal, temporal, superior, and inferior to the fovea. The average CT was calculated by averaging the CTs at the nine points. All measurements were conducted twice by 2 experienced technicians.
Statistical AnalysisSPSS 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. CT is presented as the mean ± standard deviation. Differences among groups were analyzed by One-way ANOVA. Linear correlation analysis was performed to assess the possible association of CT with systemic and ocular parameters in glaucoma individuals. Multiple linear regression analyses were performed to investigate the effects of potential factors such as SE, age, IOP, CCT, and mean deviation (MD) of visual field defect on average CT and CT measured at each point.P<0.05 was considered statistically significant.
RESULTS
The demographics and clinical characteristics of the study population are summarized in Table 1. No significant differences in age and gender among 3 groups. No significant difference in spherical equivalent between Group 1 and Group 3. No significant difference in CCT, IOP and visual field defect MD between Group 1 and Group 2.
Table 2 shows that the mean CT was significantly different at various locations among three groups (allP<0.05). The choroid of high myopia eyes with glaucoma was found to be thinner than that of non-highly myopic glaucoma eyes at all locations (allP<0.05). But there were no significant differences in CT between highly myopic glaucoma group and non-glaucoma high myopia group at all locations (allP>0.05). Figure 1 shows that the greatest measurement of CT was under the fovea in non-highly myopic glaucoma eyes (323.81μm), whereas at 3mm superior to the fovea in highly myopic glaucoma (214.57μm) and non-glaucoma high myopia (208.18μm) groups. The mean CT had a minimum at 3mm nasal to fovea in all three groups.
Linear correlation analysis demonstrated that the variable associated significantly with CT was SE at all nine locations, and age at some sites (Table 3).
In patients with glaucoma,stepwise multiple regression analysis showed that the SE was the most influential factor on CT in all regions of the macula. The CT varied significantly with age at 3-mm superior to the fovea (P=0.032; not in Table), but not with diagnosis (indicates glaucoma with high myopia vs. glaucoma without high myopia), MD of visual field defect, IOP, or CCT (Table 4).
Table 1Demographic and clinical characteristics of study participants
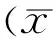
±s)
Group 1: Highly myopic glaucoma; Group 2: Non-highly myopic glaucoma; Group 3: Non-glaucoma high myopia;aANOVA test;bChi-squared test;cIndependentt-test.
Table 2Choroidal thickness in highly myopic glaucoma, non-highly myopic glaucoma and high myopia subjects
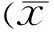
±s)
aCompared with Group 1,P<0.05;bCompared with Group 2,P<0.05;cCompared with Group 3,P<0.05; SFCT: Subfoveal choroidal thickness.
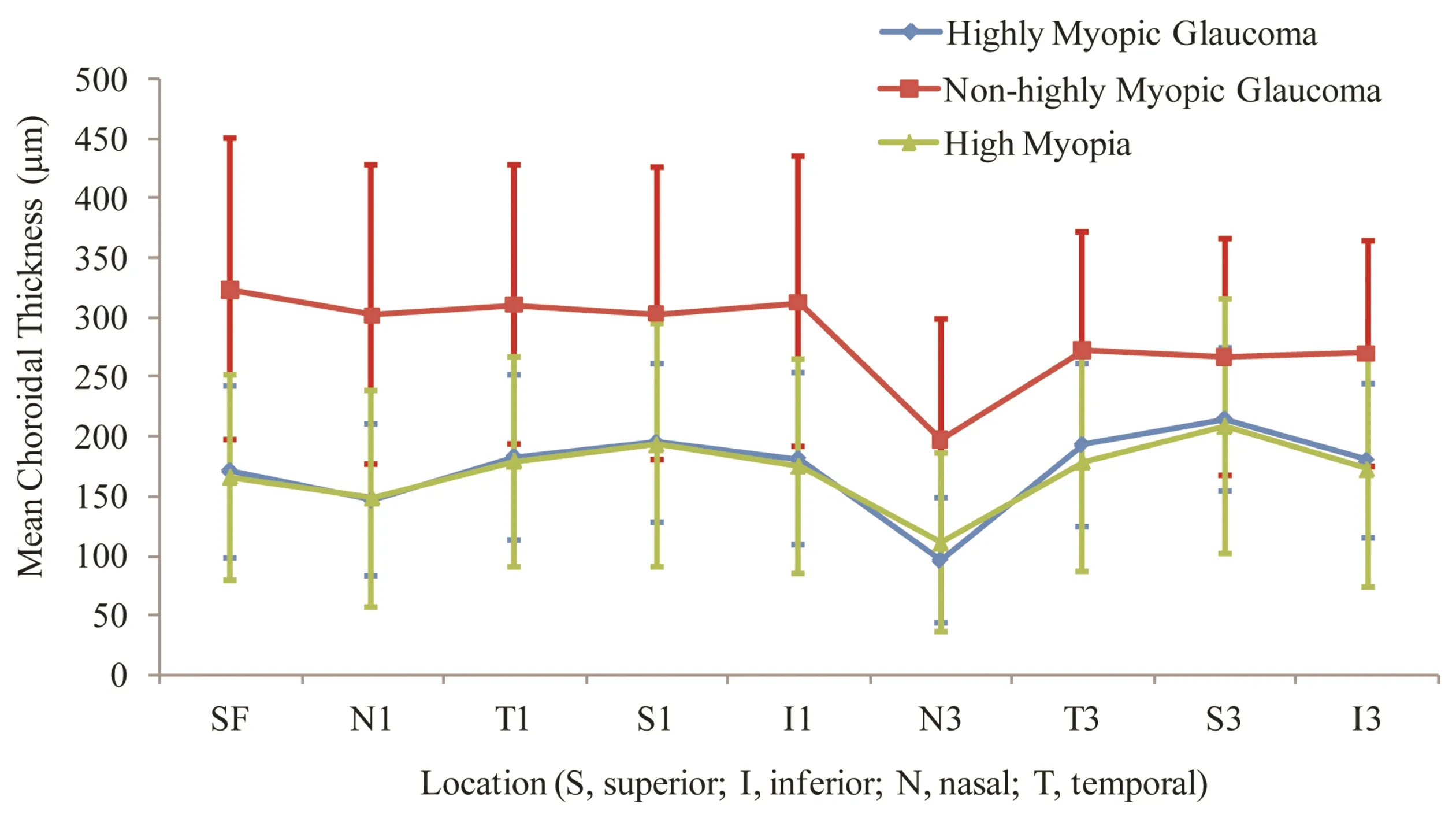
Figure 1Graph showing the mean choroidal thickness measured at 9 locations between highly myopic glaucoma, non-highly myopic glaucoma, and non-glaucoma high myopia subjects.
DISCUSSION
The assessment of the role of CT in the pathogenesis of glaucoma remains an active area of research. It is crucial to know if there is an association between CT and glaucomatous optic neuropathy, and whether thinner choroid induced by high myopia may expose the optic nerve to more glaucomatous damage.
Table 3Linear correlation analysis for variables associated with choroidal thickness in glaucoma
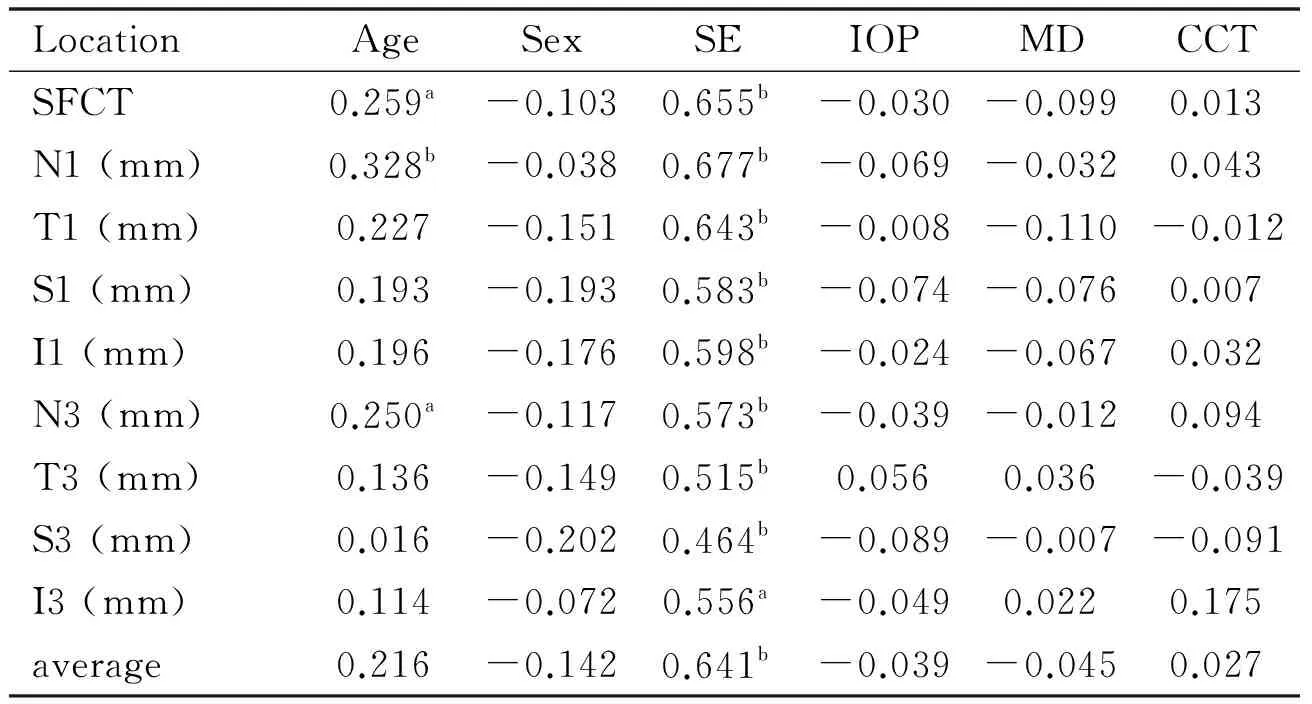
LocationAgeSexSEIOPMDCCTSFCT0.259a-0.1030.655b-0.030-0.0990.013N1(mm)0.328b-0.0380.677b-0.069-0.0320.043T1(mm)0.227-0.1510.643b-0.008-0.110-0.012S1(mm)0.193-0.1930.583b-0.074-0.0760.007I1(mm)0.196-0.1760.598b-0.024-0.0670.032N3(mm)0.250a-0.1170.573b-0.039-0.0120.094T3(mm)0.136-0.1490.515b0.0560.036-0.039S3(mm)0.016-0.2020.464b-0.089-0.007-0.091I3(mm)0.114-0.0720.556a-0.0490.0220.175average0.216-0.1420.641b-0.039-0.0450.027
aP<0.05;bP<0.01; SFCT: Subfoveal choroidal thickness; SE: Spherical equivalent; MD: Mean deviation; IOP: Intraocular pressure; CCT: Central corneal thickness.
Our finding that CT of both highly myopic glaucoma and high myopia without glaucoma was significantly thinner than that of non-highly myopic glaucoma agrees with other previous studies[15-18,20]. Patients with highly myopic glaucoma sometimes have greater progression of visual field loss and severe loss of central visual function[21]. Due to the ambiguous relationship between CT, SE, and POAG of highly myopic glaucoma, we set both non-glaucoma high myopia and non-highly myopic glaucoma as control groups for further comparison and better understanding.
Table 4Stepwise multivariate regression for association with subfoveal and average choroidal chicknesses

ParameterSubfovealThicknessCoefficientPAverageThicknessCoefficientPDiagnosisa0.0810.689-0.0350.865Age(a)-0.0710.513-0.1180.284SE(D)17.9860.00013.7340.000MD(dB)-0.0870.359-0.0330.732IOP(mmHg)0.0290.758-0.0390.688CCT(μm)0.0560.556-0.0690.477
aIndicates glaucoma with high myopiavsglaucoma without high myopia; SE: Spherical equivalent; MD: Mean deviation; IOP: Intraocular pressure; CCT: Central corneal thickness.
In this clinical study, subfoveal CT was measured in highly myopic glaucoma, non-highly myopic glaucoma, and non-glaucoma high myopia subjects. The results were compared to assess the angiopathic effect of high myopia on CT and subsequently its role in glaucomatous optic neuropathy. We found there is no significant difference between highly myopic glaucoma patients and age matched high myopia controls, and CT was not associated with damage severity as estimated by MD of visual field defect and IOP. It suggests that choroidal thinning is not an important component of glaucomatous optic neuropathy.
To our knowledge, in published studies that assessed the link between CT and POAG, few reports involved highly myopic glaucoma eyes. Usuietal[8]reported that CT in highly myopic normal-tension glaucoma(NTG) is significantly thinner than that in myopic volunteers without glaucoma. In a similar manner, Chebiletal[22]reported that foveal choroidal thickness is reduced in highly myopic eyes with glaucoma. However, our results showed that CT in eyes with highly myopic glaucoma was not significantly different from that of high myopic eyes without glaucoma, and there is no association between severity of glaucomatous optic neuropathy and CT. Many more recent studies showed POAG was not significantly associated with a marked thinning or a thickening of the choroid subfovea[11-14,23]. In the studies measuring peripapillary CT, the authors found similar results between glaucoma patients and control subject[24-25]. After adjustment for age and refractive error, Zhangetal[24]concluded that POAG was not significantly associated with a marked thinning of the choroid in both the foveal and parafoveal region, which is consistent with our findings. We also found diagnosis (indicates glaucoma with high myopiavs. glaucoma without high myopia) was not the significantly influential factor on CT in all regions of the macula. So we think that choroidal thinning is not sufficient evidence for the diagnosis and follow-up of highly myopic patients with glaucoma. The hypothesis that a thin choroid corresponds to less choroidal blood flow to the optic nerve head may not be true.
Our findings and previous studies[15-18,20]showed that SE is the most influential factor on the thickness of choroid, which assumed that the vascular changes in glaucoma are more likely a result of the myopia process rather than of the glaucomatous. Thus, these results suggest that choroidal thinning is not an important component of glaucomatous optic neuropathy. In addition, choroid provides blood supply to the 5 layers of outer retina, and glaucomatous damage is mainly at nerve fiber layer, this supports the illation that no correlation was found between CT and glaucoma damage.
The choroid of glaucoma patients without high myopia was the thickest underneath the fovea in our study. This is consistent with that observed in healthy subjects and patients with POAG by previous studies[11-12,16,26-27]. However, among patients with highly myopic glaucoma, the choroid was thicker in the superior sectors compared with the central choroid. Similar pattern was observed in subjects with high myopia, suggesting that the location pattern of CT may have been affected by the fact that highly myopic eyes have preexisting thinner choroids; since the elongation of the globe in myopic eyes leads to mechanical stretching and thinning of the choroid in the posterior pole.
Despite of lack of difference between CT of highly myopic glaucoma and non-glaucoma high myopic eyes, the former may have ischemia and hypoxia of the choroid for the following factors: 1) CT acquired by EDI-OCT cannot reflect the real vascular density of choroid; 2) CT acquired by EDI-OCT cannot reflect hemodynamic alteration of choroid; and 3) CT does not reflect oxygen-carrying capacity of hemoglobin. Therefore, further molecular biological studies are suggested for better identification of the relationship of choroidal blood supply and glaucomatous damage.
Our work deduced important findings in exploring some of POAG pathogenesis in highly myopia glaucoma eyes; several limitations of our study should be mentioned. First,CT only indicates the choroidal blood flow indirectly. We need three-dimensional and anatomical information to reveal the characteristics of choroidal blood flow in highly myopic glaucoma patients in the future. Second, the possible effect of the antiglaucoma medication on CT was not considered in our patients, although other studies assumed that vascular changes in glaucoma are more likely a result of the glaucomatous process rather than of its treatment[28-29].
In conclusion, this study provides additional evidence that choroidal thickness in eyes with highly myopic glaucoma is thinner than that in glaucoma eyes without high myopia, but comparable with that in high myopic eyes without glaucoma. It also confirms the lack of association between choroidal thickness and glaucomatous optic neuropathy.
REFERENCES
1 Suzuki Y, Iwase A, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, Inoue Y, Kitazawa Y;Tajimi Study Group. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study.Ophthalmology2006;113(9): 1613-1617
2 Detry-Morel M. Is myopia a risk factor for glaucoma?JFrOphtalmol2011;34(6): 392-395
3 Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population.InvestOphthalmolVisSci2013;54(1): 830-835
4 Chen SJ, Lu P, Zhang WF, Lu JH. High myopia as a risk factor in primary open angle glaucoma.IntJOphthalmol2012;5(6):750-753
5 Marcus MW, de Vries MM, Junoy Montolio FG,Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis.Ophthalmology2011;118(10): 1989-1994.e2
6 Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study.ArchOphthalmol2003;121(12):1711-1715
7 Satilmis M, Orgül S, Doubler B, Flammer J. Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure.AmJOphthalmol2003;135(5):664-669
8 Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma.AmJOphthalmol2012;153(1):10-16
9 Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma.BMCOphthalmol2012;12:29
10 Hirooka K, Fujiwara A, Shiragami C, Baba T, Shiraga F. Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma.ClinExperimentOphthalmol2012;40(6):576-582
11 Wang W, Zhang X. Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis.InvestOphthalmolVisSci2014;55(9): 6007-6014
12 Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma.InvestOphthalmolVisSci2012;53(10): 6695-6701
13 Fénolland JR, Giraud JM, May F, Mouinga A, Seck S, Renard JP. Enhanced depth imaging of the choroid in open-angle glaucoma: a preliminary study.JFrOphtalmol2011;34(5): 313-317
14 Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S,Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography.ExpEyeRes2011;92(3):189-194
15 Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects.InvestOphthalmolVisSci2010;51(4):2173-2176
16 Shin JW, Shin YU, Lee BR. Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography.Ophthalmology2012;119(5):1017-1023
17 Chen W, Wang Z, Zhou X, Li B, Zhang H. Choroidal and photoreceptor layer thickness in myopic population.EurJOphthalmol2012;22(4):590-597
18 Tan CS, Cheong KX. Macular choroidal thicknesses in healthy adults-relationship with ocular and demographic factors.InvestOphthalmolVisSci2014;55(10):6452-6458
19 Chen W, Wang ZT, Zhang H. Comparison of choroidal thickness measured by two methods.IntJOphthalmol2012;5(3):348-353
20 Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, Quigley HA. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients.Ophthalmology2011;118(8):1571-1579
21 Lee YA, Shih YF, Lin LL, Huang JY, Wang TH. Association between high myopia and progression of visual field loss in primary open-angle glaucoma.JFormosMedAssoc2008;107(12): 952-957
22 Chebil A, Maamouri R, Ben Abdallah M, Ouderni M, Chaker N, El Matri L. Foveal choroidal thickness assessment with SD-OCT in high myopic glaucoma.JFrOphtalmol2015;38(5):440-444
23 Jonas JB, Steinmetz P, Forster TM, Schlichtenbrede FC, Harder BC. Choroidal Thickness in Open-angle Glaucoma.JGlaucoma2015;24(8):619-623
24 Zhang Z, Yu M, Wang F, Dai Y, Wu Z. Choroidal Thickness and Open-Angle Glaucoma: A Meta-Analysis and Systematic Review.JGlaucoma2016;25(5):e446-e454
25 Van Keer K, Abegao Pinto L, Willekens K, Stalmans I, Vandewalle E. Correlation Between Peripapillary Choroidal Thickness and Retinal Vessel Oxygen Saturation in Young Healthy Individuals and Glaucoma Patients.InvestOphthalmolVisSci2015;56(6):3758-3762
26 Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems.Ophthalmology2012;119(1):119-123
27 Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes.AmJOphthalmol2009;147(5): 811-815
28 Fuchsjäger-Mayrl G, Wally B, Georgopoulos M, Rainer G, Kircher K, Buehl W, Amoako-Mensah T, Eichler HG, Vass C, Schmetterer L. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension.InvestOphthalmolVisSci2004;45(3):834-839
29 Zeitz O, Matthiessen ET, Wiermann A, Reuss J, Richard G, Klemm M. Ocular hemodynamics in normal tension glaucoma: effect of bimatoprost.KlinMonblAugenheilkd2004;221(7):550-554
(作者单位:1430030中国湖北省武汉市,华中科技大学同济医学院附属同济医院眼科;277030美国德克萨斯州休斯顿贝勒医学院眼科)
作者简介:栗改云,毕业于华中科技大学附属同济医院,博士研究生,副主任医师,研究方向:青光眼 。
通讯作者:张虹,毕业于华中科技大学附属同济医院,博士研究生,主任医师,研究方向:青光眼. dr_zhanghong@vip.163.com
DOI:10.3980/j.issn.1672-5123.2016.8.06
关键词:高度近视;原发性开角型青光眼;脉络膜厚度;黄斑;光学相干断层成像术
高度近视合并青光眼患者黄斑区脉络膜厚度的多元回归分析
栗改云1,陈威1,Samer abdo Al-wesabi1,王莉2,张虹1
摘要

