Inf uence of chelator and near-infrared dye labeling on biocharacteristics of dual-labeled trastuzumab-based imaging agents
Xuejuan Wang, Melissa B Aldrich, Zhi Yang, Nina Zhou, Qing Xie, Chen Liu, Eva Sevick-MuracaKey Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Nuclear Medicine, Peking University Cancer Hospital & Institute, Beijing 004, China;Center for Molecular Imaging, the Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center at Houston, Houston TX 77030, USA
Inf uence of chelator and near-infrared dye labeling on biocharacteristics of dual-labeled trastuzumab-based imaging agents
Xuejuan Wang1, Melissa B Aldrich2, Zhi Yang1, Nina Zhou1, Qing Xie1, Chen Liu1, Eva Sevick-Muraca21Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Nuclear Medicine, Peking University Cancer Hospital & Institute, Beijing 100142, China;2Center for Molecular Imaging, the Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center at Houston, Houston TX 77030, USA
Correspondence to: Xuejuan Wang. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Nuclear Medicine, Peking University Cancer Hospital & Institute, No. 52, Fucheng Road, Haidian District, Beijing 100142,China. Email: xuejuan_wang@hotmail.com.
Abstract
Objective: To investigate the effect of fluorescent dye labeling on the targeting capabilities of111In-(DTPA)n-trastuzumab-(IRDye 800)m.
Methods: Trastuzumab-based conjugates were synthesized and conjugated with diethylenetriaminepentaacetic acid (DTPA) at molar ratios of 1, 2, 3 and 5 and with a fluorescent dye (IRDye 800CW) at molar ratios of 1, 3 and 5. Immunoreactivity and internalization were assessed on SKBR-3 cells, overexpressing human epidermal growth factor receptor 2. The stability in human serum and phosphate-buffered saline (PBS) was evaluated. The biodistribution of dual-labeled conjugates was compared with that of111In-(DTPA)2-trastuzumab in a SKBR-3 xenograft model to evaluate the effect of dye-to-protein ratio.
Results: All trastuzumab-based conjugates exhibited a high level of chemical and optical purity. Flow cytometry results showed that increasing dye-to-protein ratios were associated with decreased immunoreactivity. Stability studies revealed that the conjugate was stable in PBS, while in human serum,increased degradation and protein precipitation were observed with increasing dye-to-protein ratios. At 4 h, the percentages of internalization of dual-labeled conjugates normalized by dye-to-protein ratio (m)were 24.88%±2.10%, 19.99%±0.59%, and 17.47%±1.26% for “m” equal to 1, 3, and 5, respectively. A biodistribution study revealed a progressive decrease in tumor uptake with an increase in the dye-to-protein ratios. The liver, spleen and kidney showed a marked uptake with increased dye-to-protein ratios, particularly in the latter.
Conclusions: With non-specific-site conjugation of the fluorescent dye with a protein based on imaging agent, the increase in dye-to-protein ratios negatively impacted the immunoreactivity and stability, indicating a reduced tumor uptake.
Keywords:Influence; near infrared dye; trastuzumab; human epidermal growth factor receptor 2; duallabeled
Submitted Dec 29, 2015. Accepted for publication May 23, 2016.
View this article at: http://dx.doi.org/10.21147/j.issn.1000-9604.2016.03.11
Introduction
Precision medicine, selecting appropriate and optimal therapies based on the context of a patient's genetic content or other molecular analysis, has become increasingly important in the treatment strategies of cancer (1). Nuclear medicine and optical f uorescence imaging techniques provide powerful non-invasive imaging capability for the assessment of biomarker overexpression in tumor cells, including tissues that may not be amenable to detect with conventional imaging modalities (2,3). However, the low spatial resolution at the cellular level limits the ability of nuclear medicine techniques to detect microscopic lesions. Furthermore, low penetration depth is a key limitation of optical imaging modalities (4). The use of dual-modality imaging probes confers an unprecedented leverage by harnessing the complementary advantages and outweighing the respective limitations of these techniques (5,6).
Trastuzumab is a humanized anti-human epidermal growth factor receptor 2 (HER2) antibody that is approved for the treatment of breast cancer (7,8). HER2 overexpression,reported in 25%—30% of all breast cancers, is associated with a poor prognosis and aggressive tumor attributes (9). The reported conservation and upregulation of HER2 make trastuzumab a promising therapeutic modality for targeting metastatic disease in patients with HER2(+) breast cancer (10).
Recent studies have evaluated dual-labeling of trastuzumab for nuclear imaging using positron emission tomography (PET)or single photon emission computed tomography (SPECT)radionuclides as well as for near-infrared (NIR) fluorescence imaging at excitation wave lengths >750 nm (11-13). A comparison of the nuclear and NIR fluorescence images of xenografts following the administration of dual-labeled peptide and antibody showed comparable target-to-background ratios (indicative of comparable sensitivities and stable dual-labeling)but a higher signal-to-noise ratio (SNR) of NIR fluorescence,which is consistent with the increased photon count rates possible with fluorescence. However, the synthetic complexity and the effect of the fluorescent dye on the biodistribution of the imaging agent are two major challenges to the use of dualmodality imaging (14).
Here, we describe a method for dual-labeling of an antibody with a NIR fluorophore, IRDye 800CW, and diethylenetriaminepentaacetic acid (DTPA) for the chelation of111In. The stearic effect of conjugation of a biomodal imaging tag to trastuzumab was investigated by altering the molar ratios of chelator/near-infrared dye to protein. The stability, binding affinity and targeting property of the dual-labeled imaging agent were assessed both in vitro and in vivo.
Materials and methods
Materials
Trastuzumab (HerceptinTM, Genentech South, CA) was purchased from clinical pharmacy for research purposes. IRDye 800CW was purchased from LI-COR Bioscience (Lincoln,NE) and p-SCN-Bn-DTPA (Dallas, TX).111InCl3was obtained from Perkin-Elmer Life Sciences (Waltham, MA).
Cell culture
Human breast cancer SKBR-3 cells with a stable overexpression of HER2 (American Type Culture Collection) were cultured in Dulbecco's minimal essential medium (DMEM)/F-12 (Invitrogen) with 10% fetal bovine serum (Hyclone) in a humidif ed incubator at 37 °C with 5% CO2.
Preparation of trastuzumab-based conjugate
Conjugation of DTPA to trastuzumab
Trastuzumab was dissolved in 50 mmol/L NaHCO3(pH 8.0) at a concentration of 20 mg/mL and incubated with p-SCN-Bn-DTPA at room temperature (RT) for 1 h in varying DTPA-to-trastuzumab molar ratios (1:1 to 10:1). The final molar ratio of DTPA to protein, “n”, was determined as described by Hnatowich et al. (15). Unbound DTPA was removed using ultrafiltration (molecular weight cut-off 50 kD, Millipore). Final protein concentrations were determined by ultraviolet absorbance at 280 nm.
Conjugation of IRDye 800CW to DTPA-trastuzumab
(DTPA)2-trastuzumab was conjugated with IRDye 800CW in varying IRDye 800CW-to-antibody molar ratios of 1:1 to 10:1 as per the instructions provided by LI-COR Biosciences. The number of final dye molecules per protein molecule (dye-toprotein ratio, “m”) and protein concentration were calculated by measuring absorbance with a UV-Vis spectrophotometer (Beckman Coulter) at 280 nm and 780 nm (11).
Assessment of purity of (DTPA)n-trastuzumab-(IRDye 800)m
Aliquots of 0.1—0.2 ng conjugates mixed with Laemmli sample buffer (without beta-mercaptoethanol) were loaded onto 4%—12% Tris-glycine acrylamide gels (Bio-Rad). Gels were run until the bromophenol blue reached approximately 10 mm from the bottom of gels, and subsequently imaged using a LICOR Odyssey infrared imaging system, and 16-bit tif files were analyzed using ImageJ software (NIH, MD). The identification of the region of interest (ROI) of a pre-defined pixel size foreach gel band and quantif cation of f uorescent intensity levels were performed.
Flow cytometry
In brief, 0.3×106SKBR-3 cells were incubated in 100 μL of culture medium that contained 5 μg of trastuzumab conjugate. After 30 min on ice, the cells were washed once with 4 mL of medium and incubated for an additional 30 min with f uorescein isothiocyanate (FITC)-conjugated mouse anti-human IgG antibody (BD Bioscience) on ice. After a final wash, the cells were resuspended in 2.5 μg/mL of 7-amino-actinomycin D solution. The mean fluorescence intensity (MFI) was assessed with flow cytometry (FACS-Calibur, Becton Dickinson). As a control for specific binding, trastuzumab was tested at equivalent molar concentrations to provide the measurement of MFIpositive, whereas cells treated with phosphate-buf ered saline (PBS) served as a negative control for the measurement of MFInegative. The percentage of immunoreactivity was calculated as follows: [(MFIsample—MFInegative)/(MFIpositive—MFInegative)]×100%.111In labeling and serum stability
The111In labeling procedure was similar to that described by Lub-de Hooge et al. (16). In brief,111InCl3(0.1 mol/L sodium acetate, pH 7.0) was incubated with conjugate solution for 30 min at RT; 20 mmol/L DTPA in 0.1 mol/L sodium acetate was added to quench free111In. The resulting111In-DTPA was removed by ultrafiltration.111In radiolabeling yields were monitored by silica gel instant thin-layer chromatography (SGITLC, EMD Chemicals) using a Bioscan System 200 Imaging Scanner. The radiolabeling efficiency was calculated using WinScan software.
Stability analysis was performed by adding 1 mCi111In-(DTPA)2-trastuzumab-(IRDye 800)mto 1 mL of PBS or human serum (Millipore). The mixtures were then incubated at 37 °C for 24 h. Aliquots at each time point were run on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system described above. The radiochemical and fluorescent purity were analyzed using a Bioscan System 200 Imaging Scanner and a LI-COR Odyssey infrared imaging system, respectively.
Lindmo assay
The binding affinity of111In-(DTPA)2-trastuzumab-(IRDye 800)mand111In-(DTPA)2-transtuzumab was compared via the Lindmo assay as described previously (11). In brief, a fixed amount of trastuzumab-based conjugate (5 pmol) was added to increasing concentrations of SKBR-3 cells (from 0.1×106to 2×106in 0.5 mL) and incubated for 1 h. To determine nonspecific cell binding, aliquots of cells were incubated with radiolabeled conjugates in the presence of 0.5 μmol unlabeled trastuzumab. The cell pellets were measured using a gamma counter. Specific binding was calculated as the ratio of the cell bound to the total radioactivity applied minus nonspecific binding. The immunoreactive fraction was calculated using OriginLab Microcal software.
Internalization studies
In brief, approximately 2.5 pmol of trastuzumab-based conjugate was added to pretreated SKBR-3 cells with an overexpression of HER2 and incubated (in triplicate) for 4h and 24 h at 37 °C. A 1,000-fold excess of each blocking agent was used to determine nonspecific internalization. At each time point, the internalization was stopped by the removal of the medium followed by washing the cells with ice-cold 0.01 mol/L PBS (pH 7.4). The cells were then treated for 5 min (three times) with ice-cold glycine buffer (0.05 mol/L glycine solution, and pH adjusted to 2.8 with 1 mol/L HCl) to distinguish between cell surface-bound (acidreleasable) and internalized (acid-resistant) radiolabeled antibodies. The internalized fluorescence intensity was measured using Odyssey infrared imaging system and the results were plotted with ImageJ software.
In vivo biodistribution of111In labeled trastuzumab-based conjugates
All protocols followed for animal experiments were approved by the Institutional Animal Care and Use Committees of Peking University Cancer Hospital & Institute and University of Texas Health Science Center at Houston. Female nude BALB/c (nu/nu) mice (age, 4—5 weeks) were subcutaneously implanted with 10 million SKBR-3 cells, and the tumor was allowed to grow to a size of approximately 5 mm diameter.
The biodistribution profiles of111In-(DTPA)2-trastuzumab-(IRDye 800)2,111In-(DTPA)2-trastuzumab-(IRDye 800)3, and111In-(DTPA)2-trastuzumab-(IRDye 800)5were compared to that of111In-(DTPA)2-trastuzumab. Groups of 3—4 athymic female nude mice bearing SKBR-3 cell tumors were used for all experiments. The mice were administered 20 μg of111In-labeled conjugates (approximately 1.48 mBq) in 0.1 mL of 0.9% NaCl via the lateral tail vein. For the determination of nonspecific uptake in the tumor tissue, another group of 3 mice were preinjected with 1 mg of trastuzumab dissolved in 0.1 mL of 0.9% NaCl solution, 1 h prior to the administration of the imaging agent. At 48 h after the administration of the imaging agent,mice were anesthetized with isoflurane (2.5%, 0.4 L/min oxygen) and subsequently euthanized using carbon dioxide. The organs of interest were collected, excess blood was rinsed of , weighed, and assessed on a gamma-counter together with injection standards. The percentage of injected per gram (%ID/ g) was calculated for each tissue. The tumor-to-tissue ratios were also determined.
Data analysis
All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) software. The data are expressed as±s. Between-group differences were evaluated using Student's t test, and P<0.05 was considered statistically signif cant.
Results
Preparation of dual-labeled imaging conjugate
A series of (DTPA)n-trastuzumab and (DTPA)2-trastuzumab-(IRDye 800)mwere successfully prepared as described in this section. The chelator-to-trastuzumab ratios used for (DTPA)n-trastuzumab were 1.03, 2.12, 3.05 and 5.18; the dyeto-protein ratios used for (DTPA)2-trastuzumab-(IRDye 800)mwere 1.06, 3.03 and 4.71.
Purity of (DTPA)2-trastuzumab-(IRDye 800)m
The relative amount of unconjugated IRDye 800CW was determined via SDS-PAGE and fluorescence imaging system. The location of free IRDye 800CW in the gel (approximately 1 kD)was similar to that of bromophenol blue (approximately 0.5 kD). The purity of (DTPA)2-trastuzumab-(IRDye 800)1, (DTPA)2-trastuzumab-(IRDye 800)3and (DTPA)2-trastuzumab-(IRDye 800)5is presented in Figure 1, no free dye band was observed in the purified conjugates, and <3% of the fluorescent signal was associated with larger molecules (>250 kD).
Radiolabeling and stability
The radiolabeling yield of trastuzumab-based radiotracers ranged between 85% and 100% at a specific activity of approximately 370—740 mBq per mg protein as demonstrated by SG-ITLC analysis. After the purification of radiotracers, no free radioactive band was detected on gel scanning with Bioscan System (Figure 2), and a main radioactive band (approximately 150 kD) was displayed for each radiolabeled trastuzumab-based conjugate.

Figure 1 (DTPA)2-trastuzumab-(IRDye 800)mwith varying dye to protein ratios for purified conjugates. Fluorescence intensity of SDS-PAGE gels was imaged using a LI-COR Odyssey infrared imaging system. The gel to the left is a molecular weight ladder in kD. After purification, the band for each dual-labeled conjugate was found in approximately 145 kD. No free dye bands are observed in the purified conjugates. M, protein molecular weight ladder.

Figure 2 Radioactive purity of111In-(DTPA)2-trastuzumab-(IRDye 800)mwith varying dye to protein ratios. The gel to the left represents the molecular weight ladder in kD. The band for each dual-labeled conjugate was found in approximately 145 kD. No radioactive band was found in the f nal dual-labeled conjugates. M,protein molecular weight ladder.
The optical stability of111In-(DTPA)2-trastuzumab-(IRDye 800)min PBS and human serum was demonstrated using a LICOR Odyssey near-infrared imaging system. The IRDye 800signal remained constant in PBS after 24 h but not in human serum (Figure 3). After 24 h, large proteins (>150 kD) were found on the gel for all three conjugates (Figure 3). Similar results were obtained on scanning of SDS-PAGE gels with the Bioscan system (Figure 4). The extra radioactive peak, which corresponded to the higher molecular weight proteins, can be observed in the histograms of dual-labeled conjugates. The larger the dye-to-protein ratio, the greater the amount of >150 kD proteins were found in the conjugate solution.
Immunoreactivity assay
The FACS histogram for SKBR-3 cells incubated with (DTPA)1-trastuzumab, (DTPA)2-trastuzumab, (DTPA)3-trastuzumab and (DTPA)5-trastuzumab at the same concentration was comparable to that of trastuzumab, which suggests that the DTPA-to-trastuzumab ratios of 1 to 5 had minimal ef ect on immunoreactivity (Figure 5).

Figure 3 SDS-PAGE/Odysse analysis of111In-(DTPA)2-trastuzumab-(IRDye 800)m, m=1, 3 and 5, at 24 h after incubation in PBS (A—C) and human serum (D—F) at 37 °C. The ladder on the right represents the molecular weight. M, protein molecular weight ladder; (A, D)111In-(DTPA)2-trastuzumab-(IRDye 800)1; (B, E)111In-(DTPA)2-trastuzumab-(IRDye 800)3; (C, F)111In-(DTPA)2-trastuzumab-(IRDye 800)5.

Figure 4 Representative histograms indicating radioactivity of111In-(DTPA)2-trastuzumab-(IRDye 800)mat different m values (0, 1, 3 and 5),after incubation with human serum at 37 °C for 24 h. The larger the dye-to-protein ratio, the greater the amount of >150 kD proteins that were found in the conjugate solution. (A)111In-(DTPA)2-trastuzumab-(IRDye 800)1; (B)111In-(DTPA)2-trastuzumab-(IRDye 800)3; (C)111In-(DTPA)2-trastuzumab-(IRDye 800)5.
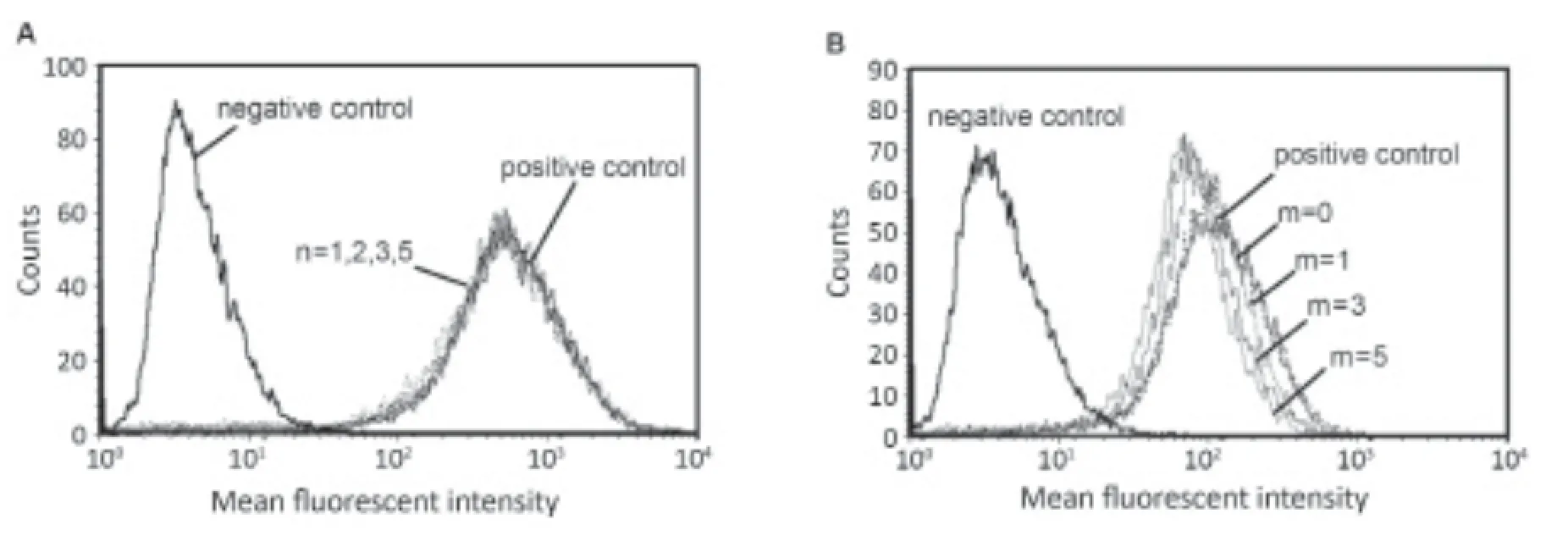
Figure 5 FACS histogram of trastuzumab-based conjugates showing counts vs. the mean fluorescent intensity using anti-human IgG as a secondary antibody. (A) The histogram to the left represents SKBR-3 cells incubated without trastuzumab. The overlaying peaks represent FACS histograms of SKBR-3 cells incubated with 5 μg of trastuzumab (DTPA)n-trastuzumab with n=1, 2, 3, and 5; (B) Cytofluorimetric comparison of cultured SKBR-3 cells without trastuzumab and after treatment with 5 μg of (DTPA)2-trastuzumab, (DTPA)2-trastuzumab-(IRDye 800)1, (DTPA)2-trastuzumab-(IRDye 800)3and (DTPA)2-trastuzumab-(IRDye 800)5.
However, the FACS histogram of (DTPA)2-trastuzumab-(IRDye 800)1showed a slight reduction in f uorescent intensity,which indicates reduced immunoreactivity as compared to that of (DTPA)2-trastuzumab. In contrast, the immunoreactivity of (DTPA)2-trastuzumab-(IRDye 800)5was moderately impaired, as evidenced in the clear shift of the histogram toward the negative control population of SKBR-3 cells. The immunoreactivity of (DTPA)2-trastuzumab-(IRDye 800)3was in between those of the other two conjugates. Immunoreactive fractions of trastuzumab conjugates corresponding to “m”values of 0, 1, 3 and 5 were 99.74%±4.56%, 83.14%±1.43%,63.96%±4.00% and 57.71%±4.41%, respectively.
The Lindmo assay was performed to determine the immunoreactivity fraction of111In-(DTPA)2-trastuzumab-(IRDye 800)m. A linear relationship between TA/SB and 1/cells was established. The average fraction of immunoreactivity of the radiolabeled conjugates corresponding to “m” values of 0, 1,3 and 5 was 90.72%±3.77%, 80.42%±2.28%, 60.74%±5.41% and 50.71%±1.45%, respectively.
Internalization study
Figure 6 illustrates the specific internalization of111In-(DTPA)2-trastuzumab-(IRDye 800)minto SKBR-3 cells, the NIR fluorescence signals associated with internalized IRDye 800 were detected on 12-well plates. No f uorescence was observed in the case of SKBR-3 cells pretreated with111In-(DTPA)2-trastuzumab. The three-dimensional surface plot showed that the total fluorescence intensity of111In-(DTPA)2-trastuzumab-(IRDye 800)5was higher than that of the other two. However,the percentages of internalization corresponding to “m” values of 1, 3 and 5 after the normalization of the total fluorescence intensities of dual-labeled conjugates by the dye-to-protein ratio were 24.88%±2.10%, 19.99%±0.59% and 17.47%±1.26% at 4 h, respectively.
The intensity of internalized radioactivity corresponding to “m” values of 0, 1, 3 and 5 was comparable to the results presented above, i.e., 26.34%±0.03%, 23.87%±0.02%,21.07%±0.01% and 20.45%±0.01% at 4 h, respectively.
Biodistribution study
The biodistribution of111In-(DTPA)2-trastuzumab-(IRDye 800)min SKBR-3 tumor-bearing nude mice was assessed at 48 h, and the data are summarized in Table 1 as %ID/g tissue. Variable tumor uptake was observed with respect to the different “m” values. Tumor accumulation of111In-(DTPA)2-trastuzumab-(IRDye 800)5(6.77±1.73 %ID/g) was much less than that of111In-(DTPA)2-trastuzumab (15.76±2.61 %ID/g) and also less than the other two conjugates with lower “m” values,i.e., 9.96±1.05 %ID/g for111In-(DTPA)2-trastuzumab-(IRDye 800)3and 8.84±1.85 %ID/g for111In-(DTPA)2-trastuzumab (IRDye 800)2, respectively. The uptake was specific to HER2(+)tumors, which was demonstrated by the lack of tumor retention in mice pre-injected with unlabeled trastuzumab.

Figure 6 Internalization of111In-(DTPA)2-trastuzumab-(IRDye 800)m, m=1, 3 and 5, into SKBR-3 cells after incubation for 4 h at 37 °C. (A) SKBR-3 cells in wells were scanned with LI-COR Odyssey near-infrared imaging system; (B) Fluorescence intensity surface plot of SKBR-3 cells with radioconjugate uptake at 4 h.

Table 1 Biodistribution of111In-labeled trastuzumab-based agents in SKBR-3 tumor-bearing nude mice at 48 h after injection
The excretion of dual-labeled conjugates was largely via the kidney and liver, particularly the former. The renal uptake of111In-(DTPA)2-trastuzumab-(IRDye 800)5was 28.37±4.55 %ID/g, which is much higher than that of111In-(DTPA)2-trastuzumab (5.43±2.42 %ID/g), and the liver uptake of both conjugates was comparable (approximately 8 %ID/g at 48 h post-injection).
Discussion
Dual-labeling of targeting moieties with nuclear andfluorescent entities provides a non-invasive modality for the localization of lesions with the use of highly penetrative, shortlived radionuclides and then uses the fluorescence signals from optical molecular probes allowing for disease monitoring over prolonged periods of time to guide surgical resection (5,13). Furthermore, the use of dual-labeled targeting tracer effectively avoids issues related to the use of agents with differing pharmacokinetic and pharmacodynamic properties that may otherwise affect image fusion and its interpretation (17). However, the development of dual-labeled targeting pharmaceuticals is a multistep and technically more challenging technique that requires complex design considerations such as careful selection of nuclear and optical tracers to minimize physical-chemical interference between various molecular components. Thus, the modification of the moiety to the maximum extent possibly whilst ensuring that the modif cation does not compromise bioactivity is of considerable interest (15,18). In our studies, we have used trastuzumab as a targeting moiety that is covalently linked to IRDye 800CW, a NIR fluorescent dye, and DTPA, a chelator of111In, to develop a dual-labeled imaging agent to trace HER-positive primary breast lesions and metastases.
Previous studies have demonstrated dimer and polymer formation with an increase in the DTPA anhydride to antibody ratio, which resulted in a decreased biological affinity of the conjugate relative to the unlabeled antibody (15). However, Forrer et al. found no significant difference in the immunoreactivity between (DOTA)4-rituximab immunoconjugate and a commercially available rituximab on lymphoma cell line LVB1 (19). Our FACS results showed no signif cant dif erence in immunoreactivity between trastuzumab and the singly labeled DTPA conjugates up to a chelator-toprotein ratio of 5:1. Furthermore, a 1:1 molar ratio of DTPA to protein was not suf cient for prompt labeling with the needed amount of111In (e.g., approximately 370-740 mBq per mg of antibody). Based on radiolabeling yields, specific radioactivity,solubility and immunoreactivity, we employed a molar DTPA-to-trastuzumab coupling ratio of 2:1 in this study.
As with radionuclide conjugation, dye-to-protein ratio is also liable to impact in vivo signal levels. A low dye-to-antibody ratio will reduce f uorescence intensity, while over-conjugation on non-specific sites of the protein may cause self-quenching of the dye as well as the loss of biological activity. Gee et al. synthesized (Cy5.5)m-trastuzumab with molar ratios of the dye to protein ranging from 0.5 to 2.0 (20). A molecular dye to antibody ratio of 1.1 was found to be optimal based on the binding affinity. The use of higher dye-to-antibody ratios was found to decrease immunoreactivity, while a probe containing a lower dye-to-antibody ratio (0.5) was associated with reduced f uorescence intensity.
We synthesized (DTPA)2-trastuzumab, (DTPA)2-trastuzumab-(IRDye 800)1, (DTPA)2-trastuzumab-(IRDye 800)3and (DTPA)2-trastuzumab-(IRDye 800)5to investigate the influence of dye-to-protein ratios on the affinity, stability and the in vivo targeting potential for near-infrared fluorescence imaging. The binding affinity of the immunoconjugates was tested in HER2-overexpressing SKBR-3 tumor cells and immunoreactivity assessed using FACS. The representative histogram shows a decrease in MFI corresponding to an increase in the dye-to-protein ratios. The successive percentage of immunoreactivity fell from 100% (without any dye on the antibody) to 83%, at a molar dye-toprotein ratio of 1:1, which demonstrates a high af nity of the duallabeled conjugate for binding to HER2 on breast cancer cells. At molar dye-to-protein ratios of 5:1, a 42% loss of immunoreactivity was observed. Furthermore, the formation of large proteins (>150 kD) was observed with all three dual-labeled conjugates after incubation with human serum for 24 h. The larger the dye-toprotein ratio, the greater the amount of >150 kD proteins found in the conjugate solution.
To the best of our knowledge, the relationship between the dye-to-protein ratio and specific binding affinity has only been investigated in vitro, because quantitative measurement of tissue fluorescence is liable to be affected by variability in tissue absorption properties and scattering of f uorescent signals. Li et al. compared the biodistribution of111In-labeled c (KRGDf) peptide and dual-labeled c (KRGDf) peptide, both of which targeted ανβ3 integrin in melanoma xenografts, with a 1:1 molar ratio of chelator and f uorophore, using tissue radioactivity measurements (21). We evaluated the relationship of the dye-to-protein ratio with specif c targeting using nuclear medicine techniques.
As compared to the biodistribution of111In-(DTPA)2-trastuzumab, the percentage of HER2(+) tumor accumulation also decreased significantly (P<0.05) with increasing dyeto-protein molar ratios, which is consistent with our in vitro findings. Tumor uptake decreased from 15.76±2.61 %ID/g for111In-(DTPA)2-trastuzumab to 6.77±1.73 %ID/g for111In-(DTPA)2-trastuzumab-(IRDye 800)5, which represents an approximate 57% reduction. Furthermore, the renal uptake increased from 5.43±2.42 %ID/g for111In-(DTPA)2-trastuzumab to 28.37±4.55 %ID/g for111In-(DTPA)2-trastuzumab-(IRDye 800)5, which is partly attributable to the decreased conjugate stability.
Conclusions
Dual-labeled trastuzumab conjugates were successfully coupledwith DTPA and IRDye 800CW. Increasing dye-to-protein ratios decreased immunoreactivity and serum stability, and lowered tumor accumulation.
Acknowledgements
Funding: This work was supported by Beijing Natural Science Foundation (No. 7132037) and the National Cancer Institute Network for Translational Research U54 CA136404-01.
Footnote
Conf icts of Interest: The authors have no conf icts of interest to declare.
References
1. Lu YF, Goldstein DB, Angrist M, et al. Personalized medicine and human genetic diversity. Cold Spring Harb Perspect Med 2014;4:a008581.
2. Maeda A, Bu J, Chen J, et al. Dual in vivo photoacoustic and fluorescence imaging of HER2 expression in breast tumors for diagnosis, margin assessment, and surgical guidance. Mol Imaging 2014;13.
3. Sevick-Muraca EM, Rasmussen JC. Molecular imaging with optics: primer and case for near-infrared f uorescence techniques in personalized medicine. J Biomed Opt 2008;13:041303.
4. Zanzonico P. Principles of nuclear medicine imaging: planar, SPECT, PET, multi-modality, and autoradiography systems. Radiat Res 2012;177:349-64.
5. Houston JP, Ke S, Wang W, et al. Quality analysis of in vivo near-infrared fluorescence and conventional gamma images acquired using a dual-labeled tumor-targeting probe. J Biomed Opt 2005;10:054010.
6. Puthenveetil S, Musto S, Loganzo F, et al. Development of solid-phase site-specific conjugation and its application toward generation of dual labeled antibody and fab drug conjugates. Bioconjug Chem 2016;27:1030-9.
7. Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992;89:4285-9.
8. Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51.
9. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12.
10. Ignatov T, Eggemann H, Burger E, et al. Hormone receptor status does not alter the effect of trastuzumab in breast cancer. Endocr Relat Cancer 2016 Mar 23. pii:ERC-16-0084. [Epub ahead of print]
11. Wang X, Aldrich MB, Marshall MV, et al. Preclinical characterization and validation of a dual-labeled trastuzumab-based imaging agent for diagnosing breast cancer. Chin J Cancer Res 2015;27:74-82.
12. Sampath L, Kwon S, Hall MA, et al. Detection of cancer metastases with a dual-labeled near-infrared/positron emission tomography imaging agent. Transl Oncol 2010;3:307-217.
13. Sampath L, Kwon S, Ke S, et al. Dual-labeled trastuzumabbased imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med 2007;48:1501-10.
14. Paulus A, Desai P, Carney B, et al. Development of a clickable bimodal fluorescent/PET probe for in vivo imaging. EJNMMI Res 2015;5:120.
15. Hnatowich DJ, Childs RL, Lanteigne D, et al. The preparation of DTPA-coupled antibodies radiolabeled with metallic radionuclides: an improved method. J Immunol Methods 1983;65:147-57.
16. Lub-de Hooge MN, Kosterink JG, Perik PJ, et al. Preclinical characterisation of111In-DTPA-trastuzumab. Br J Pharmacol 2004;143:99-106.
17. Sun L, Ding J, Xing W, et al. Novel Strategy for Preparing dual-modality optical/PET imaging probes via photo-click chemistry. Bioconjug Chem 2016;27:1200-4.
18. Schellenberger EA, Sosnovik D, Weissleder R, et al. Magneto/optical annexin V, a multimodal protein. Bioconjug Chem 2004;15:1062-7.
19. Forrer F, Chen J, Fani M, et al. In vitro characterization of177Lu-radiolabelled chimeric anti-CD20 monoclonal antibody and a preliminary dosimetry study. Eur J Nucl Med Mol Imaging 2009;36:1443-52.
20. Gee MS, Upadhyay R, Bergquist H, et al. Human breast cancer tumor models: molecular imaging of drug susceptibility and dosing during HER2/neu-targeted therapy. Radiology 2008;248:925-35.
21. Li C, Wang W, Wu Q, et al. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol 2006;33:349-58.
Cite this article as: Wang X, Aldrich M, Yang Z, Zhou N,Xie Q, Liu C, Sevick-Muraca E. Influence of chelator and near-infrared dye labeling on biocharacteristics of duallabeled trastuzumab-based imaging agents. Chin J Cancer Res 2016;28(3):362-369. doi: 10.21147/j.issn.1000-9604.2016.03.11
doi:10.21147/j.issn.1000-9604.2016.03.11
 Chinese Journal of Cancer Research2016年3期
Chinese Journal of Cancer Research2016年3期
- Chinese Journal of Cancer Research的其它文章
- Cancer incidence and mortality in Shandong province, 2012
- Cancer incidence and mortality in Henan province, 2012
- Estimated cancer incidence and mortality in Hebei province, 2012
- Cancer incidence and mortality in Gansu province, 2012
- Cancer incidence and mortality in Guangdong province, 2012
- Incidence, mortality and survival of female breast cancer during 2003-2011 in Jiangsu province, China
