Comparing overall survival between first generation EGFR-TKIs and chemotherapy in lung cancer patients with Del19/L858R
Wei Deng, Yuanyuan Lei, Siyang Liu, Jinji Yang, Haiyan Tu, Honghong Yan, Yilong Wu1Second Clinical Medial Committee, Southern Medical University, Guangzhou 510515, China;Guangdong Lung Cancer Institute,Guangdong General Hospital and Guangdong Academy of Medical Sciences, Guangzhou 510080, China*These authors contributed equally to this work.
Comparing overall survival between first generation EGFR-TKIs and chemotherapy in lung cancer patients with Del19/L858R
Wei Deng1,2*, Yuanyuan Lei1,2*, Siyang Liu2*, Jinji Yang2, Haiyan Tu2, Honghong Yan2, Yilong Wu21Second Clinical Medial Committee, Southern Medical University, Guangzhou 510515, China;2Guangdong Lung Cancer Institute,Guangdong General Hospital and Guangdong Academy of Medical Sciences, Guangzhou 510080, China
*These authors contributed equally to this work.
Correspondence to: Yilong Wu. Guangdong Lung Cancer Institute, Guangdong General Hospital, 106 Zhongshan 2nd Road, Guangzhou 510080, China. Email: syylwu@live.cn.
Abstract
Objective: Combined overall survival (OS) analysis of Lux-Lung 3 and Lux-Lung 6 demonstrated that patients with epidermal growth factor receptor (EGFR) exon 19 deletions (Del19) would benefit from first-line second generation EGFR tyrosine kinase inhibitors (TKIs) afatinib but not for those with L858R. This study was to investigate the survival difference between first-line first generation EGFR-TKIs and chemotherapy in patients with either Del19 or L858R, and to directly compare OS in these two mutation groups.
Methods: Eligibles were all prospective and retrospective studies comparing EGFR-TKIs with conventional chemotherapy or receiving single agent EGFR-TKIs and demonstrating survival analysis based on mutation types. The primary outcome was OS measured as pooled hazard ratios (HRs). All measures were pooled using randomef ects models and 95% conf dential interval (95% CI) was calculated.
Results: A total of 14 studies incorporating 1,706 patients with either Del19 or L858R were included. Enrolling patients with Del19 or L858R in randomized controlled trials (RCTs), first-line first generation EGFR-TKIs were associated with no OS benefit, compared with chemotherapy (pooled HRTKI/Chemofor Del19: 0.82, 95% CI: 0.64-1.06, P=0.14; pooled HRTKI/Chemofor L858R: 1.15, 95% CI: 0.85-1.56, P=0.38). Direct comparison of Del19 with L858R receiving with first-line first generation EGFR-TKIs demonstrated no significant survival difference (pooled HR19/21: 0.88, 95% CI: 0.67-1.16, P=0.37).
Conclusions: Among patients with advanced non-small cell lung cancer (NSCLC) harboring Del19 and L858R,first-line first generation EGFR-TKIs demonstrated no survival benefit comparing with chemotherapy. Direct comparison between Del19 and L858R revealed no significant survival difference after first-line first generation EGFR-TKIs.
Keywords:NSCLC; EGFR; Del19/L858R; f rst generation EGFR-TKIs; OS
Submitted Jun 01, 2015. Accepted for publication Dec 22, 2015.
View this article at: http://dx.doi.org/10.21147/j.issn.1000-9604.2016.03.08
Introduction
The epidermal growth factor receptor (EGFR)-dependent signaling pathway plays an indispensable role in the development and progression of non-small cell lung cancer (NSCLC) (1). Several large randomized controlled trials (RCTs) enrolling patients with EGFR mutations have demonstrated that first-line EGFR-tyrosine kinase inhibitors(TKIs) are superior to chemotherapy in terms of objective response rate (ORR) and progression-free survival (PFS) (2-8). However, post hoc analyses of overall survival (OS) in these trials showed that there was no statistical difference between EGFR-TKIs and chemotherapy (9-13). However, EGFRTKIs are still recommended as the standard f rst-line treatment for advanced NSCLC patients harboring EGFR mutations,primarily exon 19 deletions (Del19) and a point mutation in exon 21 (L858R) (14).
Recently, Yang et al. published the combined OS analysis of Lux-Lung 3 and Lux-Lung 6. In the whole patients, afatinib (second generation EGFR-TKI) significantly delayed disease progression in EGFR mutation patients but demonstrated no remarkable impact on survival. However, when only enrolling patients with Del19, both of the two trials revealed that firstline afatinib had a significantly advantage on OS than firstline chemotherapy (Lux-Lung 3: 33.3 months vs. 21.2 months,P=0.0015; Lux-Lung 6: 31.4 months vs. 18.4 months, P=0.023). By contrast, first-line afatinib did not benefit the survival of patients with L858R comparing with first-line chemotherapy (Lux-Lung 3: 27.6 months vs. 40.3 months, P=0.29; Lux-Lung 6: 19.6 months vs. 24.3 months, P=0.34). Individual patient data (IPD)-based pooled analysis of these two trials also demonstrated that the OS improvement only existed in patients with Del19 (31.7 months vs. 20.7 months, P=0.0001). For those with L858R, there was no evidence of survival benefit. What's more, first-line afatinib might be inferior to first-line chemotherapy on OS (22.1 months vs. 26.9 months, P=0.16) (15). This was the first indication that first-line EGFR-TKIs could prolong OS and that patients harboring Del19 and L858R might be two distant populations. When translating this knowledge to clinical practice, first-line afatinib should only be recommended for patients with the Del19 mutation. However, it remains unclear whether EGFR-TKIs should be administered as the first-line treatment for patients with L858R. Given these considerations, this potential survival difference in patients receiving first generation EGFR-TKIs, such as gefitinib and erlotinib, should be investigated. Pending these results, the guidelines for EGFR-TKIs administration in advanced NSCLC patients with EGFR mutations should be revised.
An analysis of a single study, such as IPASS (16) or NEJ002 (11,17) has demonstrated that patients with either Del19 or L858R treated with gefitinib had no survival advantage compared with first-line chemotherapy. However, several small studies have previously demonstrated that patients with Del19 have superior OS compared to patients with L858R (18-23). Other studies demonstrated that patients with Del19 who treated with EGFR-TKIs have no survival advantage compared to patients with L858R (24-27). Therefore, under the circumstance of lacking detailed individual patient's survival data, a pooled analysis of the current available studies, including patients with Del19 and L858R, may provide clinically useful insight into first-line first generation EGFR-TKIs treatment for patients harboring common EGFR mutations (Del19 and L858R). We performed this meta-analysis by including recent studies and scattered data to explore whether patients with Del19 and L858R demonstrated survival superiority with firstline first generation EGFR-TKIs compared to chemotherapy. In addition, we validated the survival difference between patients with these two mutation types after receiving gef tinib or erlotinib.
Materials and methods
Search and selection process
Comprehensive systematic search for all relevant articles through the PubMed, EMBASE and Cochrane databases from inception to July 31, 2014 (without language limitations)was performed by two authors (Deng and Lei) independently. A combination of key words were used to search: “EGFR”,“epidermal growth factor receptor”, “tyrosine kinase inhibitors”, “EGFR-TKI”, “TKI”, “gefitinib”, “erlotinib”,“first generation”, “mutation”, “mutated”, “non-small-cell lung cancer”, and “NSCLC”. We also retrieved the meeting abstracts, including the American Society of Clinical Oncology (ASCO) annual meetings, European Society of Medical Oncology (ESMO) congresses and World Conference on Lung Cancer (WCLC), for the last 5 years by hand.
Eligibility criteria
All included prospective and retrospective studies satisf ed the following eligibility criteria: 1) patients were diagnosed with local advanced (stage IIIB) or metastatic or recurrent disease (stage IV); 2) patients harbored the EGFR mutation (Del19 or L858R) and received first generation EGFR-TKIs (gefitinib or erlotinib) for monotherapy, f rst-line therapy or otherwise (with a detailed number of patients with each EGFR mutation type available); and 3) special hazard ratios (HRs) or survival curves of EGFR-TKIs compared to conventional chemotherapy for OS in patients harboring Del19 or L858R and definitive HRs or survival curves of Del19 compared to L858R for OS after EGFR-TKI treatment were available. All studies failing to meet the eligibility criteria were excluded, including reviews and in vitro and animal experiments; the number of patients harboring Del19 or L858R was not available; EGFR-TKIs were administered for maintenance treatment; or EGFR-TKIs were combined with chemotherapy. If the data were unavailable in the abstracts, we used the data in the posters and presentation slides from the ASCO, ESMO and WCLC meetings.
Data extraction and quality assessment
The data were extracted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (datanot shown) (28). The RCTs were assessed with the Jadad scale,and the other studies were assessed with the Newcastle-Ottawa Scale (NOS). The following items were also extracted from the included studies: author, publication time, research name and type, therapeutic regimens, line of EGFR-TKI treatments,and number of patients harboring Del19 or L858R in each subgroup. The OS data were extracted as the HR and 95% conf dence interval (95% CI). If the data could not be extracted directly, we soft-extracted the data from the survival curves and calculated the HR with the validated method (29). During the extraction process, we assumed that there was no significant dif erence in the chemotherapy ef cacy for patients with Del19 and L858R and calculated the adjusted indirect comparison as previously described. Briefly, the log hazard ratio (logHR) of the adjusted indirect comparison for intervention A vs. B was estimated by logHRAB=logHRAC—logHRBCand its standard error for the logHR was(30),where logHRACpresents the logHR for the direct comparison of EGFR-TKIs vs. chemotherapy in patients with Del19; the logHRBCindicates the logHR for the direct comparison of EGFR-TKIs vs. chemotherapy in patients with L858R; and SE(logHRAB) is the standard error of the logHR for the direct comparison between patients harboring Del19 and patients harboring L858R who received EGFR-TKIs. Two authors (Deng and Lei) conducted the assessments independently to avoid evaluation deviations. The data were discussed among the three authors (Deng, Lei and Liu) to resolve all discrepancies in the extraction.
Statistical analysis
As there are no two identical studies, each of them is dif erent. For this reason, we recommend random effects model in general for calculating the pooled HRs for OS with 95% CIs. The statistical heterogeneity between studies was tested with the Cochran Q test and was quantified using I2and the respective 95% CIs (31). All analyses were performed in R3.1.2. All P values are two-sided, and P<0.05 was considered statistically signif cant. The publication bias was tested with the Egger funnel plot.
Results
Flow of studies screening
The study screening process is illustrated in Figure 1. A total of 6,645 potential records were identified in our initial search. After duplication and eligibility screening of all the titles and relevant abstracts, 276 promising articles were remained. After screening these articles by reading the full articles and abstracts in detail, 15 studies were included. In the post hoc analysis, one retrospective study used survival curve f tting to determine the HR value, but we excluded this study due to inaccuracy. Finally,14 studies were included into this meta-analysis.
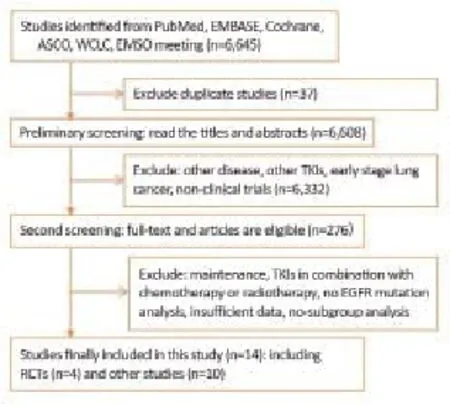
Figure 1 Flow of study screening. RCTs, randomized controlled trials; other studies included retrospective and prospective without randomized controlled.
Baseline characteristics of eligible studies
A total of 14 studies with 1,706 patients harboring the EGFR exon 19 deletion and L858R mutations were included. The baseline characteristics of all RCTs and non-RCTs included in this meta-analysis are summarized in Table 1 and Table 2,respectively. Three RCTs (EURTAC, IPASS, and NEJ002)with 639 patients provided the HR for OS comparing first-line EGFR-TKIs with chemotherapy based on Del19 and L858R,respectively. Four RCTs (EURTAC, IPASS, NEJ002, and WJTOG3405) with 409 NSCLC patients were treated with first-line gefitinib or erlotinib. From the data provided, we performed a direct survival comparison between patients with Del19 and L858R receiving first-line EGFR-TKIs. Ten non-RCT studies enrolled 895 patients. Among them 4 studies with 422 patients received first-line gefitinib or erlotinib. The remaining 6 studies included 473 patients did not describe the treatment line of EGFR-TKIs.

Table 1 Characteristics of included studies of RCTs
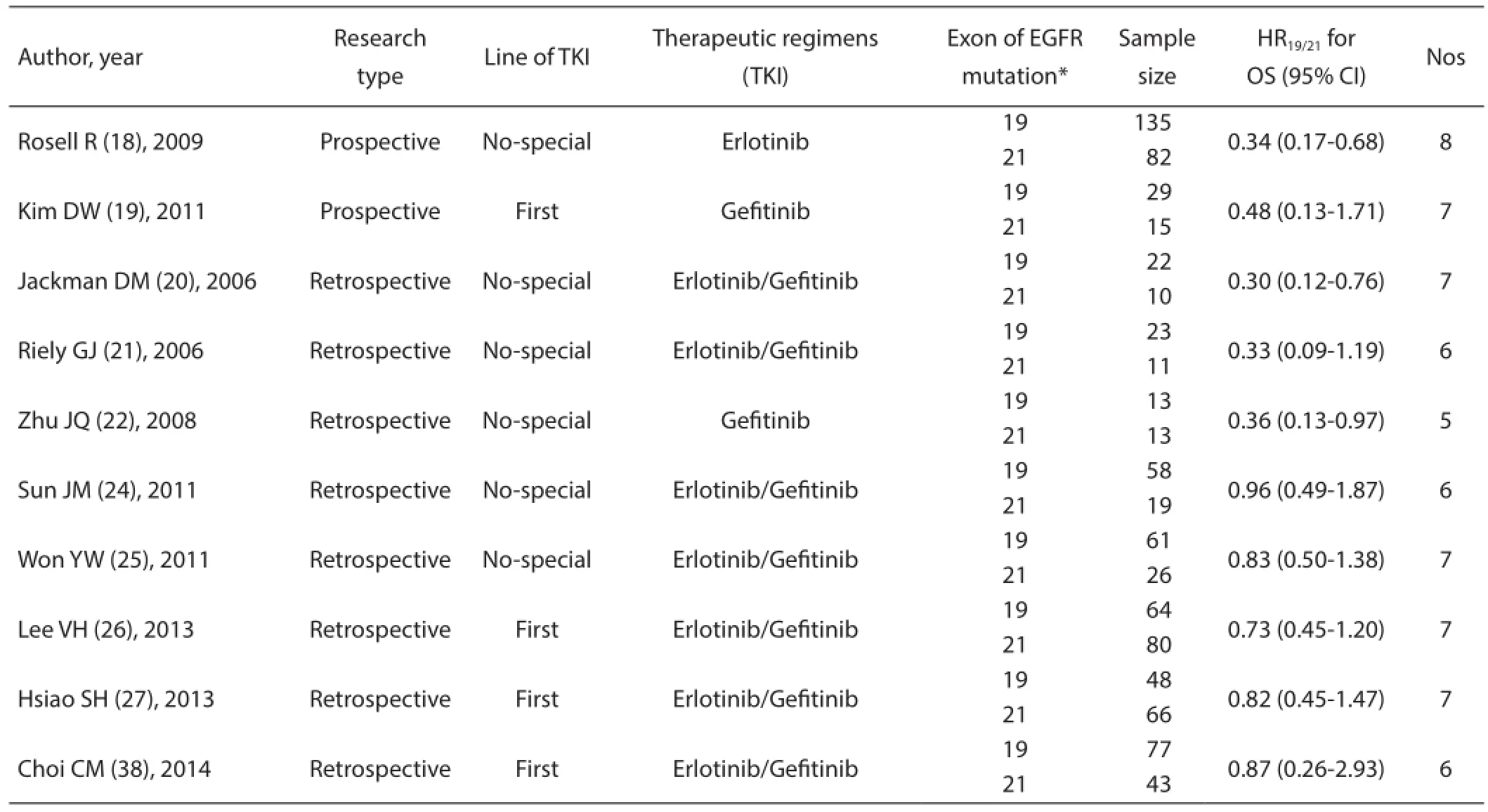
Table 2 Characteristics of included studies of non-RCTs (prospective and retrospective)
Association of first generation EGFR-TKIs vs. chemotherapy in the first-line setting in NSCLC patients with Del19 or L858R in terms of OS
Among the four randomized clinical trials we could obtain the data of hazard ratio from only three trials (EURTAC, IPASS,and NEJ002) for the direct comparison of EGFR-TKIs vs. chemotherapy in patients with Del19 or L858R. From the WJTOG3405, we could only acquire the data of HR for the direct comparison of patients with Del19 vs. patients with L858R under EGFR-TKIs therapy. So, three trials (EURTAC,IPASS, and NEJ002) were included into pooled analysis in this part. The pooled HRTKI/Chemoof EGFR-TKIs vs. chemotherapy for NSCLC patients with Del19 was 0.82 (95% CI: 0.64-1.06, P=0.14). The pooled HRTKI/Chemoof EGFR-TKIs vs. chemotherapy for patients with L858R was 1.15 (95% CI: 0.85-1.56, P=0.38). Figure 2 presents association of first generation EGFR-TKIs vs. chemotherapy in the first-line setting in NSCLC patients with Del19 (Figure 2A) or L858R (Figure 2B)in terms of OS. No significant heterogeneity existed in this part analysis. As the results indicate, there was no dif erence in first-line EGFR-TKIs vs. conventional platinum-based doublet chemotherapy regarding OS for patients with Del19 or L858R.

Figure 2 Forest plot of HRTKI/Chemofor EGFR-TKIs vs. chemotherapy in NSCLC patients with EGFR Del19 or L858R in terms of OS. TE, lnHR; SeTE, SelnHR; CI, confidence interval; W,weight; HR, (A) HRTKI/Chemomeans hazard ratio for the direct comparison of EGFR-TKIs vs. chemotherapy in patients with Del19;(B) HRTKI/Chemomeans hazard ratio for the direct comparison of EGFR-TKIs vs. chemotherapy in patients with L858R.
Association of NSCLC patients with Del19 or L858R receiving f rst generation EGFR-TKIs in terms of OS
All studies were divided into RCT and non-RCT studies. The pooled HR19/21of patients with Del19 vs. L858R after first-line gefitinib or erlotinib was 0.88 (95% CI: 0.67-1.16,P=0.37) in the four RCTs (Figure 3). For other studies,the pooled HR19/21of patients with Del19 vs. L858R after EGFR-TKIs was 0.62 (95% CI: 0.47-0.81, P=0.006) (Figure 4). No significant heterogeneity was noted in this analysis (I2=24.4%, P=0.22). We performed an influential analysis reflecting consistent results. It means that if we eliminate any of the studies, the pooled analysis results of the rest studies had no obvious change in all non-RCTs. Moreover,we conducted subgroup analyses according to the type of EGFR-TKIs. The pooled HR19/21of Del19 vs. L858R for patients receiving first-line EGFR-TKIs therapy was 0.75 (95% CI: 0.53-1.06) with no significance. The pooled HR19/21of Del19 vs. L858R for patients with non-special lines (no-special line of EGFR-TKIs means the treatment line of patients with Del19 or L858R received EGFR-TKIs did not describe specifically in the studies, maybe first-line or second-line or third-line and so on) of EGFR-TKIs was 0.51 (95% CI: 0.33-0.81) and was significant. There was no significant survival difference between patients with Del19 and L858R receiving first-line EGFR-TKIs. However, when non-special lines of EGFR-TKIs were used, patients with Del19 had superior OS compared to patients with L858R.

Figure 3 Forest plot of HR19/21for patients with Del19 vs. patients with L858R under EGFR-TKIs therapy in the four RCTs. TE,lnHR; SeTE, SelnHR; CI, confidence interval; W, weight; HR,HR19/21means hazard ratio for the direct comparison of for patients with Del19 vs. patients with L858R under EGFR-TKIs therapy in the RCTs; RCTs, randomized controlled trials.
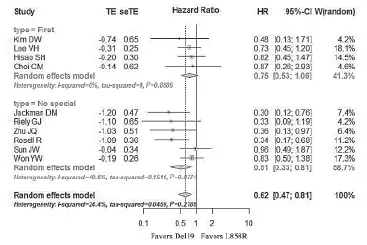
Figure 4 Forest plot of HR19/21for patients with Del19 vs. patients with L858R under EGFR-TKIs therapy in non-RCTs. TE, lnHR;SeTE, SelnHR; CI, confidence interval; W, weight; HR, HR19/21means hazard ratio for the direct comparison of for patients with Del19 vs. patients with L858R under first-line EGFR-TKIs; firstline means patients received EGFR-TKIs in the first-line setting;no-special means patients received EGFR-TKIs in any line; other studies included retrospective and prospective without randomized controlled.
Publication bias
The publication bias was analyzed for non-RCTs. When P values were greater than 0.05, it means that there was no publication bias for the outcome measures. The Egger funnel plot analysis presented a symmetrical appearance, and the P value was 0.08 (Figure 5).

Figure 5 Funnel plot by Egger's test.
Discussion
This study focuses on the survival difference between firstline first generation EGFR-TKIs and chemotherapy based on EGFR mutation types. A newly published meta-analysis revealed that patients with Del19 demonstrated superior PFS after receiving first-line EGFR-TKIs compared to patients with L858R (32). Furthermore, the findings from the two Lux-Lung trials also indicate that only patients with Del19 can benefit from first-line afatinib. If Del19 and L858R are two distinct mutation types, we should reconsider the treatment strategy for patients with L858R. It is very important to understand whether first generation EGFR-TKIs, such as erlotinib and gefitinib, have different efficacies on patients with Del19 or L858R.
Our results indicate that neither patients with Del19 nor L858R have significant overall survival benefits from first-line,first generation EGFR-TKIs compared to chemotherapy. Our results agreed with the primary results from the individual f rstline, first generation RCT analyses, such as EURTAC (10) and IPASS (16). Based on this analysis, we anticipate that patients with common EGFR mutations (Del19/L858R) share the same OS benefit when receiving first-line, first generation EGFRTKIs.
Our findings regarding first generation EGFR-TKIs are inconsistent with the afatinib trials. As we know, patients with EGFR common mutations could achieve survival benefits from first-line afatinib. However, in our meta-analysis, in patients with Del19 or L858R, first-line first generation EGFR-TKIs demonstrated no superiority over first-line chemotherapy in terms of OS, but, there was a trend that patients with Del19 received EGFR-TKIs therapy had longer OS. The obvious discrepancy between first and second generation EGFR-TKIs encouraged us to explore the potential factors that lead to the survival benef t of afatinib.
First, in our article, the sample was limited. Three RCTs (EURTAC, IPASS, and NEJ002) with 639 patients provided the HR for OS comparing first-line EGFR-TKIs with chemotherapy based on Del19 and L858R, respectively. But, in afatinib trials, 709 cases were included. Second, this prolonged OS could be attributed to the low crossover rate to EGFR-TKIs after the chemotherapy arm in Lux-Lung 3 and Lux-Lung 6. Compared to RCTs investigating first-line, first generation EGFR-TKIs, the pooled crossover rate was only 62% in the two afatinib trials (15). In contrast, the crossover rate of IPASS (9), NEJ002 (11) and WJTOG3405 (12) were 64.3%, 98.0%, and 91.0%, respectively. The improved OS in patients receiving f rst-line afatinib may be partly related to therelatively lower frequency of patients receiving EGFR-TKIs in the chemotherapy arm. According to the OPTIMAL (13)trial for EGFR mutations in NSCLC patients, patients will benefit more from the sequential combination of TKIs and chemotherapy than either treatment alone. Third, the survival benefit resulting from first-line afatinib may correlate with the different mechanisms of afatinib and gefitnib or erlotinib. Afatinib, an irreversible tyrosine kinase inhibitor, blocks the entire ErbB family, which includes the kinase domains of EGFR, human epidermal growth factor receptor 2 (HER2) and HER4. Afatinib also inhibits transphosphorylation of HER3 (33, 34). However, gefitinib and erlotinib only inhibit the tyrosine kinase activity of EGFR (35,36). Moreover, afatinib combined with various agents has been investigated as a strategy to overcome EGFR-TKI acquired resistance mediated by the EGFR T790M mutation after gefitnib or erlotinib exposure (37). The relative blocking advantage of afatinib can partially explain the superior OS after receiving f rst-line afatinib.
This study had several limitations. First, until now, seven large RCTs have performed head-to-head comparisons between first generation EGFR-TKIs and chemotherapy. This analysis only enrolled three RCTs comparing firstline EGFR-TKIs with conventional chemotherapy based on EGFR mutation types. For the direct comparison of Del19 and L858R, we acquired data from only four trials. The results would be stronger if we could include all seven trials. Therefore,we strongly recommend that investigators collaborate to include individual patient's survival data in those seven trials for analysis. Second, during the direct survival comparison of Del19 and L858R receiving first line EGFR-TKIs, we assumed that patients with Del19 and L858R had no difference in the ef cacy of f rst-line chemotherapy. Few studies focused on the prognostic value of different EGFR mutation in patients with advanced NSCLC with chemotherapy, and as a consequence,our hypothesis is in need of confirmation by more convincing evidence (39). In addition, there may be a deviation from the actual results because the survival data of NEJ002 was extracted from the survival curves.
Conclusions
For patients with Del19 or L858R, first-line, first generation EGFR-TKIs demonstrated no survival benefit compared with platinum-based chemotherapy. Additionally, no significant survival differences were found between Del19 and L858R after receiving gefitinib or erlotinib. We have no evidence to support the differential treatment of patients with Del19 and L858R. The NEJ002 trial reported that the response rate of EGFR-TKIs in the second-line setting was 58.5%, which was lower than it used in first-line (73.3%) (7). Besides, EGFR mutated patients had a risk of losing their EGFR mutation after chemotherapy (40). Considering the assurance of EGFR-TKIs,better tolerance, improved quality of life and prolonged PFS,first-line EGFR-TKIs are still the optimal choices for patients with these two common mutations.
Acknowledgements
None.
Footnote
Conf icts of Interest: The authors have no conf icts of interest to declare.
References
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80.
2. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label,randomised phase 3 trial. Lancet Oncol 2014;15:213-22.
3. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre,open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
4. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-smallcell lung cancer (EURTAC): a multicentre, open-label,randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
5. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34.
6. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
7. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
8. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
9. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III,randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74.
10. Khozin S, Blumenthal GM, Jiang X, et al. U.S. Food and Drug Administration approval summary: Erlotinib for the f rst-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist 2014;19:774-9.
11. Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gef tinib with carboplatin-paclitaxel for chemonaïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9.
12. Yoshioka H, Mitsudomi T, Morita S, et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2014;32:5s (suppl; abstr 8117).
13. Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83.
14. Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53.
15. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51.
16. Yang J, Wu YL, Saijo N, et al. Efficacy Outcomes in Firstline Treatment of Advanced NSCLC With Gefitinib (G) vs Carboplatin/paclitaxel (C/P) by Epidermal GrowthFactor Receptor (EGFR) Gene-copy Number Score and by Most Common EGFR Mutation Subtypes - Exploratory Data From IPASS. Eur J Cancer 2011;47(suppl 1):S633.
17. Inoue A, Kobayashi K, Maemondo M, et al. Final overall survival results of NEJ002, a phase III trial comparing gefitinib to carboplatin (CBDCA) plus paclitaxel (TXL) as the first-line treatment for advanced non-small cell lung cancer (NSCLC) with EGFR mutations. J Clin Oncol 2011;29 (suppl; abstr 7519).
18. Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67.
19. Kim DW, Lee SH, Lee JS, et al. A multicenter phase II study to evaluate the efficacy and safety of gefitinib as first-line treatment for Korean patients with advanced pulmonary adenocarcinoma harboring EGFR mutations. Lung Cancer 2011;71:65-9.
20. Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gef tinib or erlotinib. Clin Cancer Res 2006;12:3908-14.
21. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gef tinib or erlotinib. Clin Cancer Res 2006;12:839-44.
22. Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinibtreated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 2008;265:307-17.
23. Li J, Qu L, Wei X, et al. Clinical observation of EGFR-TKI as a f rst-line therapy on advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi (in Chinese) 2012;15:299-304.
24. Sun JM, Won YW, Kim ST, et al. The different efficacy of gef tinib or erlotinib according to epidermal growth factor receptor exon 19 and exon 21 mutations in Korean nonsmall cell lung cancer patients. J Cancer Res Clin Oncol 2011;137:687-94.
25. Won YW, Han JY, Lee GK, et al. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol 2011;64:947-52.
26. Lee VH, Tin VP, Choy TS, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic nonsmall-cell lung cancer. J Thorac Oncol 2013;8:1148-55.
27. Hsiao SH, Liu HE, Lee HL, et al. Distinct clinical outcomes of non-small cell lung cancer patients with epidermal growth factor receptor (EGFR) mutationstreated with EGFR tyrosine kinase inhibitors: nonresponders versus responders. PLoS One 2013;8:e83266.
28. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94.
29. Wang Y, Zeng T. Response to: Practical methods for incorporating summary time-to-event data into metaanalysis. Trials 2013;14:391.
30. Yang Q, Wei Y, Chen YX, et al. Indirect comparison showed survival benef t from adjuvant chemoradiotherapy in completely resected gastric cancer with d2 lymphadenectomy. Gastroenterol Res Pract 2013;2013:634929.
31. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
32. Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One 2014;9:e107161.
33. Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11.
34. Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342-50.
35. Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 2002;62:5749-54.
36. Perez-Soler R: The role of erlotinib (Tarceva, OSI 774) in the treatment of non-small cell lung cancer. Clin Cancer Res 2004;10:4238s-40s.
37. Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitorresistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45.
38. Choi CM, Kim MY, Lee JC, et al. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology 2014;270:574-82.
39. Yamashita F, Azuma K, Yoshida T, et al. Prognostic value of EGFR mutation and ERCC1 in patients with non-small cell lung cancer undergoing platinum-based chemotherapy. PLoS One 2013;8:e71356.
40. Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-smallcell lung cancer. J Clin Oncol 2012;30:3077-83.
Cite this article as: Deng W, Lei Y, Liu S, Yang J, Tu H, Yan H, Wu Y. Comparing overall survival between first generation EGFR-TKIs and chemotherapy in lung cancer patients with Del19/L858R. Chin J Cancer Res 2016:28(3):339-347. doi:10.21147/j.issn.1000-9604.2016.03.08
doi:10.21147/j.issn.1000-9604.2016.03.08
 Chinese Journal of Cancer Research2016年3期
Chinese Journal of Cancer Research2016年3期
- Chinese Journal of Cancer Research的其它文章
- Cancer incidence and mortality in Shandong province, 2012
- Cancer incidence and mortality in Henan province, 2012
- Estimated cancer incidence and mortality in Hebei province, 2012
- Cancer incidence and mortality in Gansu province, 2012
- Cancer incidence and mortality in Guangdong province, 2012
- Incidence, mortality and survival of female breast cancer during 2003-2011 in Jiangsu province, China
