Immune formulation-assisted conventional therapy on anti-infective effectness of multidrug-resistant Mycobacterium tuberculosis infection mice
Xiu-Li Yuan, Qiang Wen, Ming-De Ni, Li-Kun Wang*Internal Medicine Department No. 3, Eastern Medical District of Linyi People's Hospital, Linyi City, Shandong Province, 76034Infection Department, Eastern Medical District of Linyi People's Hospital, Linyi City, Shandong Province, 76034
Contents lists available at ScienceDirect
Immune formulation-assisted conventional therapy on anti-infective effectness of multidrug-resistant Mycobacterium tuberculosis infection mice
Xiu-Li Yuan1, Qiang Wen1, Ming-De Ni1, Li-Kun Wang2*1
1Internal Medicine Department No. 3, Eastern Medical District of Linyi People's Hospital, Linyi City, Shandong Province, 276034
2Infection Department, Eastern Medical District of Linyi People's Hospital, Linyi City, Shandong Province, 276034
ABSTRACT
Objective: To study the eff ect of immune formulation-assisted conventional therapy on antiinfective ability of multidrug-resistant Mycobacterium tuberculosis infection mice. Methods: BALB/c mice were used as experimental animals, multidrug-resistant Mycobacterium tuberculosis infection models were built, randomly divided into model group, moxifl oxacin group, thymopentin group and combined treatment group and given corresponding drug intervention, and then colony numbers in the spleen and lung, T lymphocyte subset contents and programmed death-1 (PD-1) expression levels in peripheral blood were detected. Results: Colony numbers in lung and spleen of moxifl oxacin group and thymopentin group were signifi cantly lower than those of model group and colony numbers in lung and spleen of combined treatment group were significantly lower than those of moxifloxacin group and thymopentin group; contents of CD3+CD4+T cells, Th1 and Th17 in peripheral blood of moxifl oxacin group and thymopentin group were higher than those of model group, and contents of CD3+CD8+T cells, Th2 and Treg were lower than those of model group; contents of CD3+CD4+T cells, Th1 and Th17 in peripheral blood of combined treatment group were higher than those of moxifl oxacin group and thymopentin group, and contents of CD3+CD8+T cells, Th2 and Treg were lower than those of moxifl oxacin group and thymopentin group; PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of moxifl oxacin group and thymopentin group were lower than those of model group, and PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of combined treatment group were lower than those of moxifl oxacin group and thymopentin group. Conclusions: Immune formulation thymopentin can enhance the anti-infective ability of multidrug-resistant Mycobacterium tuberculosis infection mice, decrease bacterial load in lung and spleen, and enhance immune function.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Mycobacterium tuberculosis
Multidrug resistance
Thymopentin
1. Introduction
Tuberculosis is a respiratory infectious disease caused by Mycobacterium tuberculosis, and affected by bacterial mutation, wide use of chemotherapy drugs and other factors, the incidence of multidrug-resistant tuberculosis caused by multidrug-resistant Mycobacterium tuberculosis infection is rising[1,2]. The killing eff ect of conventional chemotherapy drugs on multidrug-resistant Mycobacterium tuberculosis is not ideal, and second-line antitubercular drugs have longer course of treatment, more adverse reactions and lower cure rate[3-5]. In recent years, more and more studies have realized that weakened immune function is associatedImmune functionwith multidrug-resistant tuberculosis infection, and targeted immune formulation adjuvant therapy has become an important part of anti-tuberculosis comprehensive treatment[6]. Thymopentin is a drug enhancing immune activity and clinical common immune formulation[7]. In the following research, the effect of immune formulation-assisted conventional therapy on anti-infective ability of multidrug-resistant Mycobacterium tuberculosis infection mice was analyzed.
2. Materials and methods
2.1. Experimental materials
Experimental animals were 48 SPF level BALB/c mice weighted (18-22) g, were randomly divided into model group, moxifl oxacin group, thymopentin group and combined treatment group, each group with 12 cases. Thymopentin was from Beijing Double-Crane Pharmaceuticals Co., Ltd., moxifloxacin was from Bayer Healthcare Co., Ltd., and fl uorescent antibodies were from Santa Cruz Company.
2.2. Model establishment and drug intervention methods
7H9 liquid medium containing 1×106/mL multidrug-resistant Mycobacterium tuberculosis was prepared, and multidrug-resistant Mycobacterium tuberculosis infection models of aerosol infection mice were built and given drug intervention from the 21 d after infection. Moxifl oxacin group received intragastric administration of 100 mg/kg moxifl oxacin, thymopentin group received subcutaneous infection of 1 mg/kg thymopentin, combined treatment group received intragastric administration of 100 mg/kg moxifl oxacin and subcutaneous infection of 1 mg/kg thymopentin, and model group received subcutaneous infection and intragastric administration of same doses of saline.
2.3. Detection of colony numbers in visceral organs
Four weeks, eight weeks and sixteen weeks after treatment, mice were killed and anatomized under sterile conditions to get the lung and spleen, appropriate amount of tissue was cut off , homogenized, diluted, then inoculated in 7H11 medium and continuously cultured for 4 weeks, and then colony forming unit was counted.
2.4. Detection of T lymphocyte subset contents in peripheral blood
Sixteen weeks after treatment, mice were taken and killed by decapitation to collect peripheral blood, fl uorescent antibodies of CD3, CD4 and CD8 as well as IFN-γ, IL-4, Th17 and CD25 were incubated respectively away from light, hemolysin was added for 15 min of hemolysis, then DPBS 1 000 μL was added to re-suspend cells, contents of different T cell subsets were detected in flow cytometer, and at the time of detection, excitation light was argon ion laser 488 nm.
2.5. Detection of PD-1 expression in lymphocytes in peripheral blood
Sixteen weeks after treatment, mice were taken and killed by decapitation to collect peripheral blood, fl uorescent antibodies of CD3, CD19 and CD14 as well as programmed death-1 (PD-1) were incubated away from light respectively, hemolysin was added for 15min of hemolysis, then DPBS 1 000 μL was added to re-suspend cells, contents of different T cell subsets were detected in flow cytometer, and at the time of detection, excitation light was argon ion laser 488 nm.
2.6. Statistical process methods
SPSS19.0 software was used to input and process data, comparison among groups was by variance analysis, pair wise comparison was by LSD-t method, and P < 0.05 was the standard of statistical signifi cance in diff erences.
3. Results
3.1. Colony numbers in lung and spleen
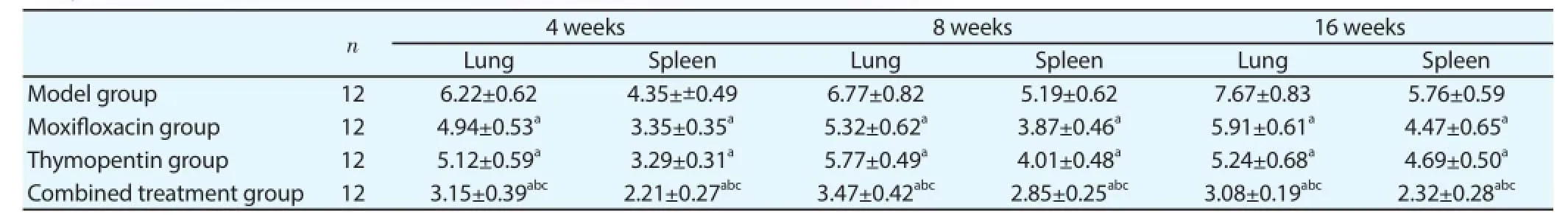
Table 1Comparison of colony numbers in lung and spleen (lg colony forming unit).
Four weeks, eight weeks and sixteen weeks after treatment,analysis of colony numbers in lung and spleen was as follows: (1) variance analysis showed that colony numbers in lung and spleen of four groups were different. (2) Pair wise comparison showed that colony numbers in lung and spleen of moxifl oxacin group and thymopentin group were signifi cantly lower than those of model group and colony numbers in lung and spleen of combined treatment group were signifi cantly lower than those of moxifl oxacin group and thymopentin group (Table 1).
3.2. Contents of T lymphocyte subsets in peripheral blood
Contents of CD3+CD4+T cells in peripheral blood of moxifl oxacin group and thymopentin group were higher than that of model group, and contents of CD3+CD8+T cells were lower than that of model group; content of CD3+CD4+T cells in peripheral blood of combined treatment group was higher than those of moxifl oxacin group and thymopentin group, and content of CD3+CD8+T cells was lower than those of moxifl oxacin group and thymopentin group (Table 2).
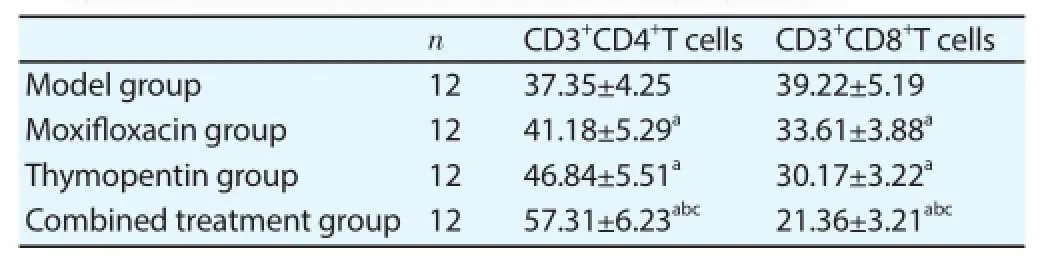
Table 2Comparison of T lymphocyte subset contents in peripheral blood.
3.3. Contents of different CD4+T lymphocyte subsets in peripheral blood
Contents of Th1 and Th17 cells in peripheral blood of moxifl oxacin group and thymopentin group were higher than those of model group, and contents of Th2 and Treg cells were lower than those of model group; contents of Th1 and Th17 cells in peripheral blood of combined treatment group were higher than those of moxifl oxacin group and thymopentin group, and contents of Th2 and Treg cells were lower than those of moxifl oxacin group and thymopentin group (Table 3).
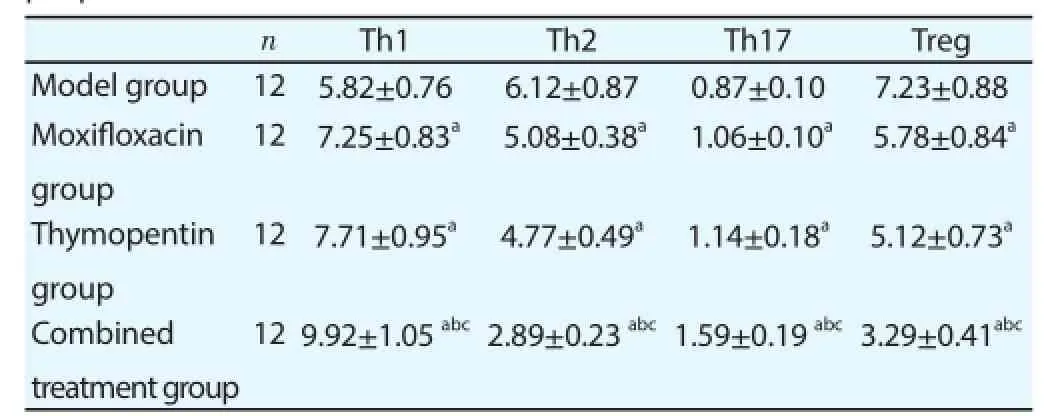
Table 3Comparison of the contents of different CD4+T lymphocyte subsets in peripheral blood.
3.4. PD-1 expression levels in lymphocytes and monocytes in peripheral blood
PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of moxifl oxacin group and thymopentin group were lower than those of model group, and PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of combined treatment group were lower than those of moxifl oxacin group and thymopentin group (Table 4).
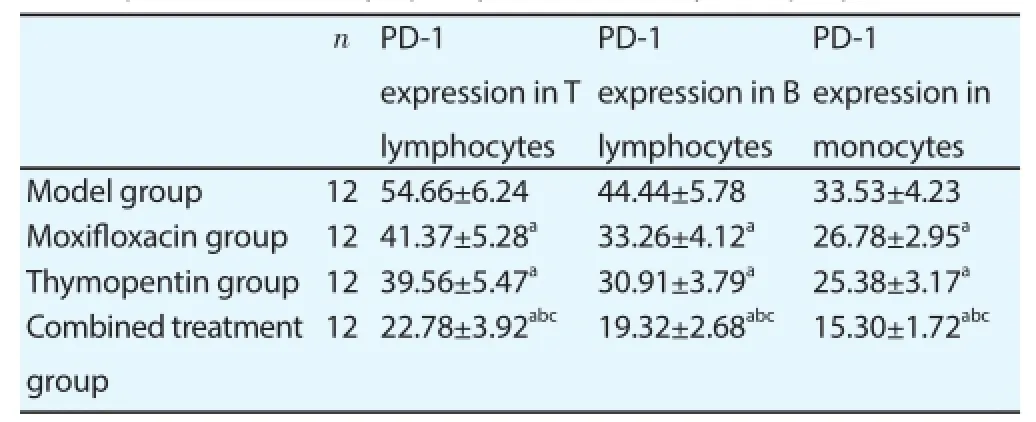
Table 4PD-1 expression levels in lymphocytes and monocytes in peripheral blood.
4. Discussion
Multidrug-resistant tuberculosis caused by multidrug-resistant Mycobacterium tuberculosis infection is the diffi culty and emphasis of clinical treatment. The main mechanism of Mycobacterium tuberculosis resistance is inhibiting body’s cellular immune response[8]. Related study confirms that normal Mycobacterium tuberculosis H37Rv strain doesn’t have inhibitory effect on the activation and maturation of Th1 and Th2 cells, and after bacterial infection, the body can induce Th1 type immune response through autoimmune response and kill Mycobacterium tuberculosis[9]; multidrug-resistant Mycobacterium tuberculosis strains can inhibit the diff erentiation and maturation of CD4+T lymphocytes, inhibit the maturation of Th1 cells, promote the maturation of Th2 cells, and reduce the generation of cytokines such as IL-2, TNF-α and IFN-γ as well as the killing eff ect of above cytokines on Mycobacterium tuberculosis, and it is difficult for the body to kill Mycobacterium tuberculosis through autoimmune mechanism[10,11]. Multidrugresistant tuberculosis is mostly insensitive to fi rst-line chemotherapy drugs, second-line chemotherapy drugs are with higher price, more adverse reactions as well as longer drug administration time and course of treatment, and the cure rate is not ideal[12,13].
Broad-spectrum antibacterial drug moxifl oxacin is the essential drug of clinical treatment of multidrug-resistant tuberculosis at present[14]. The drug has high affinity to quinolone resistancedetermining area of DNA helicase A subunit in multidrug-resistant strains, and it can infect bacteria DNA replication and damagethe structure of the bacteria[15,16]. However, the curative eff ect of moxifl oxacin alone is not very ideal. Based on the inhibitory eff ect of multidrug-resistant Mycobacterium tuberculosis on immune function, more and more scholars advocate use of immune formulation to treat multidrug-resistant tuberculosis[17]. Thymopentin is a kind of bioactive peptide extracted from newborn calf thymus tissue, and it has the function of stimulating T lymphocyte diff erentiation, maturation and proliferation as well as releasing a variety of cytokines. Related clinical studies prove that thymopentin has promoting eff ect on cellular immune function, which is specifi cally manifested as regulating the contents and function of T lymphocyte subsets[18,19].
In the research, based on routine moxifloxacin anti-infection treatment, immune formulation thymopentin was used for adjuvant therapy, aiming to exert the regulating effect of thymopentin on immune function. In order to clarify the effect of immune formulation-assisted conventional therapy on anti-infective ability of multidrug-resistant Mycobacterium tuberculosis infection mice, colony numbers in lung and spleen of diff erent treatment groups were compared. After Mycobacterium tuberculosis infection, the use of antibacterial drugs and body’s autoimmune mechanism can kill pathogenic bacteria or inhibit the growth of pathogenic bacteria, and reduce the number of colonies in the visceral organs. Analysis of colony numbers in lung and spleen showed that colony numbers in lung and spleen of moxifl oxacin group and thymopentin group were signifi cantly lower than those of model group and colony numbers in lung and spleen of combined treatment group were signifi cantly lower than those of moxifl oxacin group and thymopentin group. This indicated that both moxifloxacin and thymopentin had inhibitory eff ect on the growth of multidrug-resistant Mycobacterium tuberculosis, and combined use of the two drugs had synergistic eff ect and enhanced the anti-infective ability of multidrug-resistant Mycobacterium tuberculosis infection mice together.
Cellular immunity is the main immune mechanism of the body to kill Mycobacterium tuberculosis, and T lymphocytes are the main cells to execute cellular immune response[20]. T lymphocyte maturation process experiences positive and negative selection, two types of mature T cell that are generated include CD3+CD4+T cell and CD3+CD8+T cell, the former is an important helper cell and the latter is an important suppressor cell[21,22]. In the body’s anti-tuberculosis immune response, CD3+CD4+T cell content and CD4+/CD8+ratio increase, and CD3+CD8+T cell content decreases. In the research, the proportions of T lymphocyte subsets in peripheral blood were compared and analyzed after thymopentin treatment, and results showed that the contents of CD3+CD4+T cells in peripheral blood of moxifl oxacin group and thymopentin group were higher than that of model group, and contents of CD3+CD8+T cells were lower than that of model group; content of CD3+CD4+T cells in peripheral blood of combined treatment group was higher than those of moxifl oxacin group and thymopentin group, and content of CD3+CD8+T cells was lower than those of moxifl oxacin group and thymopentin group. It indicated that after moxifl oxacin and thymopentin monotherapy, the immune function of multidrug-resistant Mycobacterium tuberculosis infection mice was enhanced, and the eff ect of combined use of the two drugs on enhancing cellular immune function of mice was more signifi cant.
In the process of exerting immune function, CD4+CD8-T lymphocytes can be activated into different subsets that exert diff erent functions, specifi cally including Th1, Th2, Th17 and Treg cells[23]. Th1 and Th2 is a pair of cells earliest discovered in CD4+T cell subsets, and the former mainly secretes IL-2 and IFN-γ, and can induce cellular immune response and kill pathogenic microorganism; the latter mainly secretes IL-4 and IL-10, and can induce B cells to generate antibodies and inhibit the killing eff ect of macrophages on pathogenic microorganism to a certain extent[24,25]. Th17 and Treg are newly discovered members of CD4+T cell subsets, Th17 can secrete IL-17 and exert pathogen-killing eff ect similar to that of cytokines such as IL-2 and IFN-γ; Treg can inhibit the activation of Th17 and the generation of IL-17, and it has immunosuppressive effect[26,27]. In the research, further analysis of CD4+CD8-T lymphocyte subset contents in peripheral blood of four groups showed that both moxifl oxacin and thymopentin monotherapy could increase Th1 and Th17 contents and decrease Th2 and Treg contents, and the modulating eff ect of combined use of the two drugs on cellular immune function was more signifi cant.
Studies about the body’s anti-tuberculosis immune response in recent years believe that PD-1 and its ligand PD-L1, as negative costimulatory molecules, are involved in the regulation of body’s immune response and have negative regulatory eff ect on lymphocyte activation. There is PD-1 expression on T lymphocyte, B lymphocyte and monocyte surface, PD-1 and its ligand PD-L1 can input suppressor signal through immunoreceptor tyrosine-based inhibitory motif of cytoplasm end sequence, and fi nally realize the inhibitory effect on immune response. Multidrug-resistant Mycobacterium tuberculosis infection can activate PD-1 and inhibit body’s immune response. In the research, analysis of PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface showed that PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of moxifl oxacin group and thymopentin group were lower than those of model group, and PD-1 expression levels on T lymphocyte, B lymphocyte and monocyte surface in peripheral blood of combined treatment group were lower than those of moxifl oxacin group and thymopentin group.
Based on above discussion, it can be concluded that immune formulation thymopentin can enhance anti-infective ability of multidrug-resistant Mycobacterium tuberculosis infection mice, decrease bacterial load in lung and spleen, and enhance immune function.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Chang KC, Yew WW, Sotgiu G. Clinical research in the treatment of tuberculosis: current status and future prospects. Int J Tuberc Lung Dis 2015; 19(12): 1417-1427.
[2] Taneja R, Garcia-Prats AJ, Furin J, Maheshwari HK. Paediatric formulations of second-line anti-tuberculosis medications: challenges and considerations. Int J Tuberc Lung Dis 2015; 19(Suppl 1): 61-68.
[3] Singhal R, Singla N, Myneedu VP, Singh N, Sarin R. Multidrug-resistant tuberculosis among diff erent types of suspected cases: Study from New Delhi. Indian J Tuberc 2015; 62(3): 183-186.
[4] Stagg HR, Brown J, Ibraim E, Riekstiņa V, Viiklepp P, Cīrule A, et al. Drug susceptibility patterns in MDR-TB patients: challenges for future regimen design. A cross-sectional study. PLoS One 2015; 10(11): e0142425.
[5] Senbayrak S, Ozkutuk N, Erdem H, Johansen IS, Civljak R, Inal AS, et al. Antituberculosis drug resistance patterns in adults with tuberculous meningitis: results of haydarpasa-iv study. Ann Clin Microbiol Antimicrob 2015; 4(14): 47.
[6] Shin SS, Modongo C, Ncube R, Sepako E, Klausner JD, Zetola NM. Advanced immune suppression is associated with increased prevalence of mixed-strain Mycobacterium tuberculosis infections among persons at high risk for drug-resistant tuberculosis in Botswana. J Infect Dis 2015; 211(3): 347-351.
[7] Wang Y, Ke XY, Khara JS, Bahety P, Liu S, Seow SV, et al. Synthetic modifi cations of the immunomodulating peptide thymopentin to confer anti-mycobacterial activity. Biomaterials 2014; 35(9): 3102-3109.
[8] Hsu DC, Kerr SJ, Thongpaeng P, Iampornsin T, Pett SL, Zaunders JJ, et al. Incomplete restoration of Mycobacterium tuberculosis-specifi c-CD4 T cell responses despite antiretroviral therapy. J Infect 2014; 68(4): 344-354.
[9] Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 2014; 22(5): 180.
[10] Tan Q, Xie WP, Min R, Dai GQ, Xu CC, Pan HQ, et al. Characterization of Th1- and Th2-type immune response in human multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 2012; 31(6): 1233-1242.
[11] Yang E, Wang F, Xu Y, Wang H, Hu Y, Shen H, et al. A lentiviral vectorbased therapeutic vaccine encoding Ag85B-Rv3425 potently increases resistance to acute tuberculosis infection in mice. Acta Biochim Biophys Sin 2015; 47(8): 588-596.
[12] Elmi OS, Hasan H, Abdullah S, Mat Jeab MZ, Bin Alwi Z, Naing NN. Multidrug-resistant tuberculosis and risk factors associated with its development: a retrospective study. J Infect Dev Ctries 2015; 9(10): 1076-1085.
[13] Acosta CD, Dadu A, Ramsay A, Dara M. Drug-resistant tuberculosis in Eastern Europe: challenges and ways forward. Public Health Action 2014; 4(Suppl 2): 3-12.
[14] Gupta UD, Vemuri N, Gupta P, Kumar V, Tanushree P, Khuller GK. Effi cacy of moxifl oxacin & econazole against multidrug resistant (MDR) Mycobacterium tuberculosis in murine model. Indian J Med Res 2015; 142(3): 323-329.
[15] Heysell SK, Moore JL, Peloquin CA, Ashkin D, Houpt ER. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in virginia, 2009-2014. Tuberc Respir Dis 2015; 78(2): 78-84.
[16] Wang Q, Zhang C, Guo J, Huang J, Xi X, Zhang L, et al. Super-compact treatment with a high dose of moxifl oxacin in patients with drug-resistant tuberculosis and its resistance mechanisms. Exp Ther Med 2015; 9(4): 1314-1318.
[17] Zumla A, Chakaya J, Centis R, D’Ambrosio L, Mwaba P, Bates M, et al. Tuberculosis treatment and management-an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 2015; 3(3): 220-234.
[18] Gao J, Ding X, Chu C, Lu L, Zhang Y, Chen Y, et al. Dry powder inhalations containing thymopentin and its immunomodulating eff ects in Wistar rats. Eur J Pharm Sci 2009; 36(4-5): 572-579.
[19] Wang Y, Cao Y, Meng Y, You Z, Liu X, Liu Z. The novel role of thymopentin in induction of maturation of bone marrow dendritic cells (BMDCs). Int Immunopharmacol 2014; 21(2): 255-260.
[20] Geffner L, Basile JI, Yokobori N, Kviatcovsky D, Sabio y García C, Ritacco V, et al. Mycobacterium tuberculosis multidrug resistant strain M induces an altered activation of cytotoxic CD8+T cells. PLoS One 2014; 9(5): e97837.
[21] Yan RQ, Fang Y, Zhao JJ, Luo JM, Luo JB, Liu XY. Preliminary study on the expression of CD4+and CD8+memory T cells subgroups and the levels of IL-17/IL-27 from patients’ peripheral blood with pulmonary tuberculosis. Chinese J Immunol 2012; 28(10): 930-935.
[22] Li W, Gao Y, Pappas D. A complementary method to CD4 counting: measurement of CD4+/CD8+T lymphocyte ratio in a tandem affinity microfl uidic system. Biomed Microdevices 2015; 17(6): 113.
[23] Méndez-Samperio P. Modulation of tuberculosis-related immune responses by helminths. J Egypt Soc Parasitol 2014; 44(1): 141-144.
[24] Rahman MA, Sobia P, Dwivedi VP, Bhawsar A, Singh DK, Sharma P, et al. Mycobacterium tuberculosis TlyA Protein Negatively Regulates T Helper (Th) 1 and Th17 Differentiation and Promotes Tuberculosis Pathogenesis. J Biol Chem 2015; 290(23): 14407-144017.
[25] Bhattacharya D, Dwivedi VP, Kumar S, Reddy MC, Van Kaer L, Moodley P, et al. Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances Bacillus Calmette-Guerin vaccine effi cacy against tuberculosis. J Biol Chem 2014; 289(48): 33404-33411.
[26] Hu LA, Li DR, Luo YA, Yang XM, Huang XC. Peripheral blood CD4+CD25+FoxP3+regulatory T cells in active pulmonary tuberculosis patients. Acta Academiae Medicinae Militaris Tertiae 2011; 33(20): 2124-2127.
[27] Ostadkarampour M, Eklund A, Moller D, Glader P, Olgart Höglund C, Lindén A, et al. Higher levels of interleukin IL-17 and antigen-specifi c IL-17 responses in pulmonary sarcoidosis patients with Löfgren’s syndrome. Clin Exp Immunol 2014; 178(2): 342-352.
Document heading 10.1016/j.apjtm.2016.01.031
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Li-Kun Wang, Professor, Eastern Medical District of Linyi People’s Hospital, Fenghuang Street, Hedong District, Linyi City, Shandong Province Tel: 13705491856
E-mail: lkwang999@126.com
Fundation project: Science and Technology Development Program of Linyi City (No: 201113018).
 Asian Pacific Journal of Tropical Medicine2016年3期
Asian Pacific Journal of Tropical Medicine2016年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of dimethyl fumarate on rats with chronic pancreatitis
- Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Effect of miR-467b on atherosclerosis of rats
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
