改良DCF与XELOX方案治疗老年晚期胃癌的疗效及安全性分析
尹洪岩,石 燕,吴志勇,韩全利,戴广海(解放军总医院肿瘤内二科,北京 100853)
·临床评价·
改良DCF与XELOX方案治疗老年晚期胃癌的疗效及安全性分析
尹洪岩,石 燕,吴志勇,韩全利,戴广海(解放军总医院肿瘤内二科,北京 100853)
[摘要]目的:研究改良DCF(mDCF)与XELOX方案一线治疗老年晚期胃癌的疗效及药品不良反应。方法:回顾性分析2010年1月–2013年12月我院收治的确诊时已有远处转移的胃癌患者,筛选出年龄≥65岁且一线化疗使用mDCF或XELOX方案,共计60例,其中mDCF组22例,XELOX组38例,收集患者的各项临床资料,比较两组的客观有效率(ORR)、疾病控制率(DCR)、无进展生存时间(PFS)、总生存时间(OS)。结果:mDCF组近期疗效优于XELOX组,且PFS与OS均较XELOX组延长, 两组PFS间差异有统计学意义(14.2个月 vs 5.8个月,P = 0.002),但OS间差异无统计学意义(20.8个月 vs 12.0个月,P = 0.107)。Cox多因素分析显示:化疗方案是PFS的独立预后指标(HR 2.461;95%CI 1.308~4.628;P = 0.005),但未发现对OS有影响(P = 0.747);对于恶心呕吐、白细胞降低等药品不良反应的发生率mDCF方案明显高于XELOX方案,对于外周神经毒性、手足综合征等药品不良反应的发生率XELOX方案明显高于mDCF方案,但所有药品不良反应的Ⅲ、Ⅳ级发生率均较低。结论:老年患者中,应用mDCF方案及XELOX方案的总生存时间无显著性差异,药品不良反应均可耐受,但mDCF方案较XELOX方案近期效果好,无进展生存时间延长。
[关键词]老年患者;胃癌;化疗;疗效;无进展生存时间
胃癌是一种常见的消化道恶性肿瘤,其致死率居癌症死亡率的第3位[1]。大部分患者在确诊时已属局部晚期或有远处转移,因此全身化疗成为该类患者的主要治疗方式[2],随着我国老龄化的日渐加重,晚期胃癌在我国老年人的发病率逐渐上升。在一项Ⅲ期临床试验中(V325),对比应顺铂联合5-氟尿嘧啶(CF)方案的患者,应多西他赛联合顺铂及5-氟尿嘧啶(DCF)三药联合方案的患者在OS、PFS方面均有所延长[3]。但由于其药品不良反应大,限制了其在临床尤其是在老年患者中的应[4]。2011年一项关于Ⅳ期胃癌中对比顺铂及奥沙利铂的Meta分析结果显示:奥沙利铂显著提高了患者的PFS及OS[5],且有文献报道多西他赛联合奥沙利铂及5-氟尿嘧啶的mDCF方案显示了较好的安全性及疗效[6-7]。本研究旨在对比老年晚期胃癌患者中mDCF及XELOX方案的疗效及安全性问题,为临床合理优化治疗方案提供参考。
1 资料与方法
1.1研究对象及纳入标准
选取2010年1月–2013年12月我院收治的晚期胃癌患者,根据WHO制定的关于老年人的定义,筛选出年龄≥65岁的患者,共计60例。纳入标准:1)均经胃镜病理及影像学检查确诊为Ⅳ期胃癌;2)明确无手术治疗指征;3)全部病例均有可测量转移灶;4)KPS评分均须超过80分;5)骨髓造血功能正常;6)检测肝、肾功能处于正常水平;7)既往无恶性肿瘤病史,本次患病未接受任何抗肿瘤治疗;8)生存期均≥3个月;9)均签署化疗知情同意书。
1.2治疗方案
1.3疗效及毒性评价
两种方案均至少完成2周期,进行疗效及药品不良反应的评价。根据RECIST评价方法,将疗效分为完全缓解(complete remission,CR)、部分缓解(partial remission,PR)、稳定(stable disease,SD)、进展(progressive disease,PD),以CR及PR之和得出客观有效率(objective response rate,ORR),以CR、PR及SD之和得出疾病控制率(disease control rate,DCR)。随访时间于2015年3月1日截止,无进展生存时间(progression-free survival,PFS)定义为治疗开始至病变进展或患者死亡的时间,总生存时间(overall survival,OS)定义为从治疗开始至患者死亡或随访截止的时间。化疗前、化疗期间及间歇期均复查血常规、血生化,根据WHO制定的评价抗肿瘤药品不良反应的经典标准将不良反应分为0~Ⅳ级。
1.4统计学方法
2 结果
2.1一般资料
共纳入60例老年患者,男性49例(81.7%),女性11例(18.3%);中位年龄70岁(65~78岁);肝转移29例(48.3%);肺转移7例(11.7%)。两组间特征比较无统计学差异,详见表1。
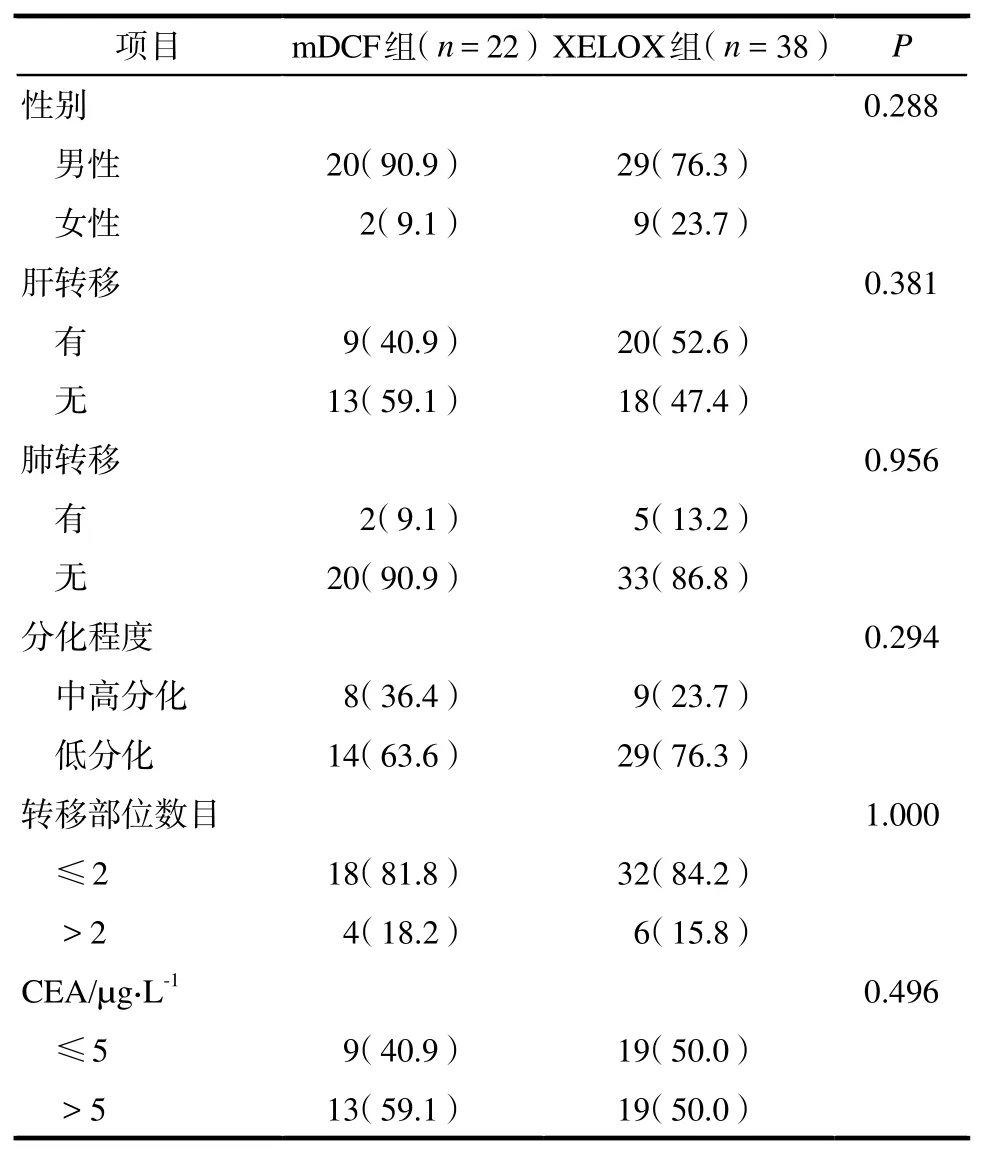
表1 改良DCF方案组与XELOX方案组患者基本特征. 例数(%)Tab 1 Baseline characteristics of patients in mDCF group and XELOX group. case(%)
2.2疗效评价
2.2.1近期疗效 化疗2周期后进行影像学评估,mDCF组ORR为63.6%,DCR为90.9%(SD 6例,PR 14例,PD 2例);XELOX组ORR为39.5%,DCR为68.4%(SD 11例,PR 15例,PD 12例)。经统计学分析,mDCF组的DCR显著高于XELOX组,P = 0.047;但ORR间差异不具有统计学意义,P = 0.071。
2.2.2生存分析 mDCF组患者的mPFS与mOS均较XELOX组延长,两组mPFS间差异有统计学意义(14.2个月vs 5.8个月,P = 0.002),但mOS间差异无统计学意义(20.8个月 vs 12.0个月,P = 0.107)。详见图1~2。
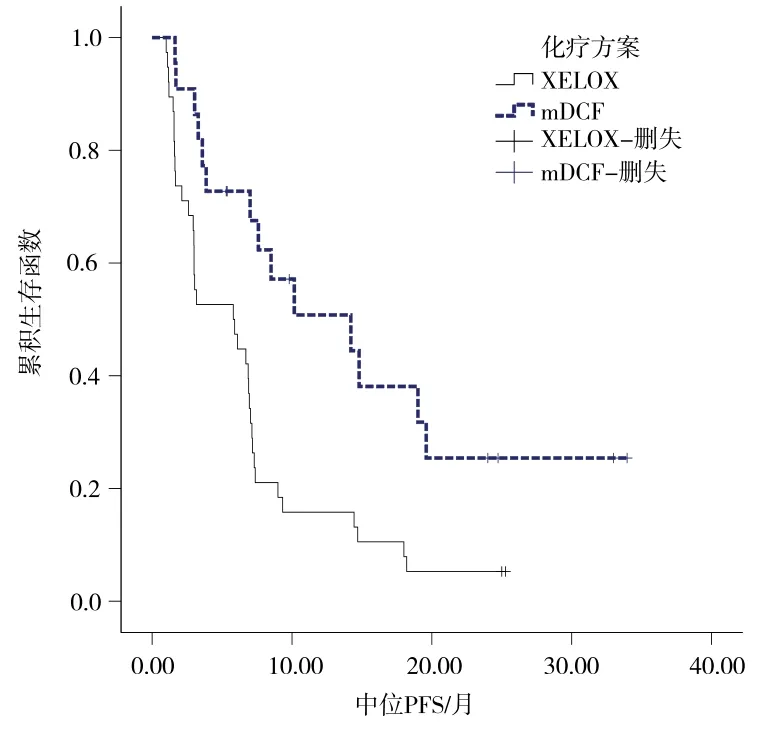
图1 两组患者中位PFS的差异比较Fig 1 Comparison of median PFS between the two groups
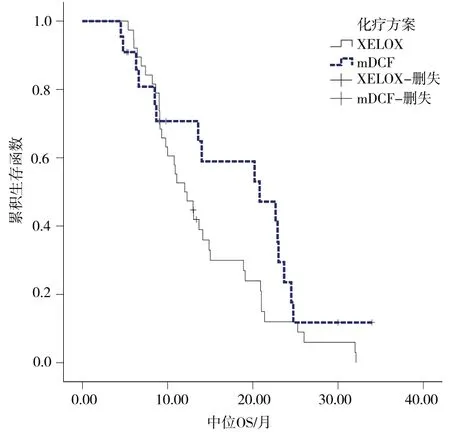
图2 两组患者中位OS的差异比较Fig 2 Comparison of median OS between the two groups
2.2.3老年晚期胃癌患者生存期的单因素与Cox多因素分析 单因素及Cox多因素分析结果显示:性别、肝转移、肺转移、转移部位数目、CEA水平不是PFS、OS的独立影响因素,而分化程度、化疗方案是PFS独立影响因素,仅分化程度是OS的独立影响因素。详见表2。
2.3药品不良反应
两组患者的药品不良反应主要为胃肠道反应、骨髓抑制、外周神经毒性、手足综合征,对于恶性呕吐、白细胞降低的发生率mDCF组明显高于XELOX组,外周神经毒性、手足综合征的发生率XELOX方案明显高于mDCF方案,余不良反应两组发生率相似,所有不良反应的Ⅲ、Ⅳ级发生率均较低,且两组间均无明显差异。详见表3。
3 讨论
尽管胃癌化疗疗效局限,且维持时间短[8],但以化疗为主的综合治疗仍是晚期胃癌的最佳治疗方式,与最佳支持治疗相比,化疗不仅可延长患者的OS,还可改善患者的生活质量[9]。晚期胃癌的化疗临床未有标准的治疗方案,我国主要的治疗方案有DCF、mDCF、ECF、mECF、FOLFIRI、XELOX方案等,V325研究证实DCF方案较CF方案延长了患者的TTP、OS、PFS[3],因此被视为是体能状态良好的晚期胃癌患者的首选治疗方案,但由于该方案药品不良反应较多,故各种改良方案不断被尝试[10]。Chi等[11]报道了mDCF方案治疗14例进展期胃癌的效果。Cunningham 等[12]报道了在胃食管结合部肿瘤中,将奥沙利铂取代顺铂的改良方案较原方案获得较长的OS,且Ⅲ、Ⅳ级毒副反应低,Kim等、Montagnani等研究结果也表明,含奥沙利铂的方案疗效不亚于含顺铂的方案[5,13]。上述研究均提示,mDCF方案的疗效及安全性更高。但老年患者各器官功能均有不同程度下降,加之胃部病变及转移灶的影响,对化疗药物的耐受性更差,其是否适合应三药联合化疗有待研究,本文首次对比分析了年龄≥65岁晚期胃癌患者应mDCF方案及XELOX方案的疗效及安全性。
老年人是一个特殊的群体,对于老年患者化疗方案的选择,不仅要考虑化疗的有效性,还要考虑化疗药物的安全性。因为老年患者的耐受性差,药品不良反应往往会加重[16],有些不良反应甚至是致死性的。因此对于老年患者化疗方案的选择更要谨慎。
本研究结果显示:老年晚期胃癌患者中,mDCF方案近期疗效优于XELOX方案,且PFS较XELOX方案组明显延长,但两组OS间差异无统计学意义。所以对于一般情况良好的老年晚期胃癌患者,可以尝试行mDCF方案化疗,争取化疗后的手术机会,但应严密观察药品不良反应,根据患者情况调整剂量。在药品不良反应方面,两组主要表现在胃肠道副反应、骨髓抑制、手足综合征、外周神经毒性,两组的Ⅲ~Ⅳ度不良反应均较少,对于恶性呕吐、白细胞降低等不良反应的发生率mDCF方案明显高于XELOX方案,对于外周神经毒性、手足综合征等副反应的发生率XELOX方案明显高于mDCF方案,但给予对症处理后均可耐受。
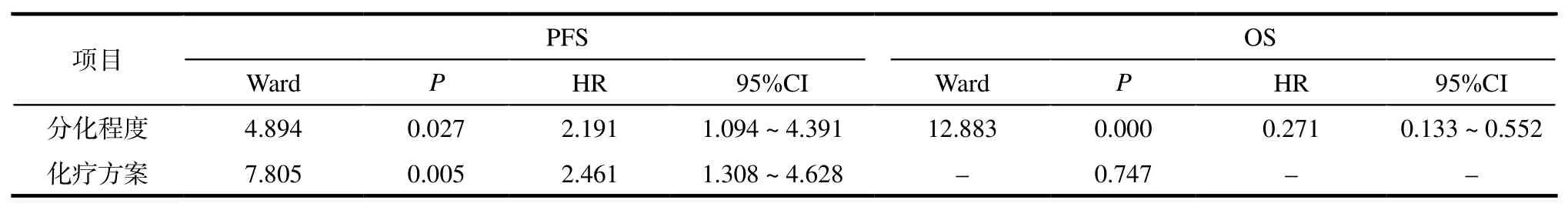
表2 Cox多因素分析各临床指标与PFS、OS的关系Tab 2 Relationship between clinical index and PFS, OS by Cox multivariate analysis

表3 改良DCF方案组与XELOX方案组的药品不良反应比较Tab 3 Comparison of adverse drug reaction between mDCF group and XELOX group
[参考文献]
[1] Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359-E386.
[2] Buzzoni R, Bajetta E, Di Bartolomeo M, et al. Pathological features as predictors of recurrence after radical resection of gastric cancer[J]. Br J Surg, 2006, 93(2): 205-209.
[3] Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase Ⅲstudy of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fuorouracil as frst-line therapy for advanced gastric cancer: a report of the V325 Study Group[J]. J Clin Oncol, 2006, 24(31): 4991-4997.
[4] Wang J, Xu R, Li J, et al. Randomized multicenter phaseⅢstudy of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fuorouracil as frst-line therapy for advanced or locally recurrent gastric cancer[J]. Gastric Cancer, 2016, 19(1): 234-244.
[5] Montagnani F, Turrisi G, Marinozzi C, et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and metaanalysis[J]. Gastric Cancer, 2011, 14(1): 50-55.
[6] Yao Z, Guo H, Yuan Y, et al. Retrospective analysis of docetaxel, oxaliplatin plus fuorouracil compared with epirubicin,cisplatin and fluorouracil as first-line therapy for advanced gastric cancer[J]. J Chemother, 2014, 26(2): 117-121.
[7] Ma J, Yao S, Li XS, et al. Neoadjuvant therapy of DOF regimen plus bevacizumab can increase surgical resection ratein locally advanced gastric cancer: a randomized, vontrolled study[J]. Medicine (Baltimore), 2015, 94(42): e1489.
[8] 戴广海,郭晓川.胃癌靶向治疗药物新进展[J].中国药物应与监测,2014,11(5):259-262.
[9] Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data[J]. J Clin Oncol, 2006, 24(18): 2903-2909.
[10] 张昉,陈雅敏,荆超,等.改良DCF方案与FOLFOX 4方案治疗晚期胃癌的临床疗效[J].临床肿瘤学杂志,2014,19(3):231-234.
[11] Chi Y, Ren JH, Yang L, et al. PhaseⅡclinical study on the modified DCF regimen for treatment of advanced gastric carcinoma[J]. Chin Med J (Engl), 2011, 124(19): 2997-3002.
[12] Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer[J]. N Engl JMed, 2008, 358(1): 36-46.
[13] Kim YS, Sym SJ, Park SH, et al. A randomized phaseⅡstudy of weekly docetaxel/cisplatin versus weekly docetaxel/oxaliplatin as frst-line therapy for patients with advanced gastric cancer[J]. Cancer Chemother Pharmacol, 2014, 73(1): 163-169.
[14] 王轶霖.卡培他滨联合奥沙利铂治疗晚期胃癌118例疗效观察[J].中外医疗,2011,(34):122,124.
[15] Park YH, Kim BS, Ryoo BY, et al. A phaseⅡstudy of capecitabine plus 3-weekly oxaliplatin as first-line therapy for patients with advanced gastric cancer[J]. Br J Cancer, 2006, 94(7): 959-963.
[16] Bando H, Yamada Y, Tanabe S, et al. Effcacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer[J]. Gastric Cancer, 2015. [Epub ahead of print].
The efficacy and safety of modified DCF regimen and XELOX regimen in elderly patients with advanced gastric cancer
YIN Hong-yan, SHI Yan, WU Zhi-yong, HAN Quan-li, DAI Guang-hai(Medical Oncology Department Ⅱ of PLA General Hospital, Beijing 100853, China)
[ABSTRACT]Objective: To investigate the effcacy and adverse drug reaction of modifed DCF (mDCF) regimen and XELOX regimen as frst-line chemotherapy in elderly patients with advanced gastric cancer. Methods: From January 2010 to December 2013,60 patients diagnosed with metastases and treated with frst-line chemotherapy of mDCF or XELOX regimen in our hospital were analyzed retrospectively. The ages of the patients were all greater than 65, mDCF group contained 22 cases, while XELOX group included 38 cases. The clinical data of these 60 patients were collected. The objective response rates (ORR), disease control rates (DCR), progression-free survival (PFS) and overall survival (OS) were compared between the two groups. Results: The shortterm effect of mDCF regimen was better than that of XELOX regimen, and either PFS or OS was longer than XELOX group. There was statistically signifcant difference in PFS between the two groups (14.2 vs 5.8 months,P = 0.002), but the difference of OS was not statistically signifcant (20.8 vs 12.0 months,P = 0.107). Multivariate Cox analysis showed that chemotherapy was a independent prognostic indicator of PFS (HR 2.461; 95%CI 1.308–4.628; P = 0.005), but no significant association was found between chemotherapy and OS (P = 0.747). The incidence of nausea, vomit and leukopenia were higher in mDCF regimen group than that of XELOX group, while the incidence of peripheral neurotoxicity and hand-foot syndrome was lower in mDCF group. But the toxicities were rarely in grade Ⅲ–Ⅳ. Conclusion: In elderly patients, both mDCF regimen and XELOX regimen have similar overall survival time,and toxicities are all tolerated. However,mDCF regimen has better short-term effcacy and prolonged progression-free survival time.
[KEY WORDS]Elderly patients; Gastric cancer; Chemotherapy; Effcacy;Progression-free survival
[中图分类号]R969.4
[文献标识码]A
[文章编号]1672–8157(2016)02–0069–05
[基金项目]中国胃肠肿瘤临床研究协作组胃癌研究基金项目(20130101005);北京市自然科学基金面上项目(7152140);国家自然科学基金资助项目(81402016);北京市科技新星计划(xx2015B098)
[通信作者]戴广海,男,主任医师,教授,博士生导师,研究方向:消化系统肿瘤的综合治疗。E-mail:daigh60@sohu.com
[作者简介]尹洪岩,女,在读硕士研究生,研究方向:消化系统肿瘤。E-mail:18801213977@163.com
收稿日期:(2015-12-12 修回日期:2016-02-01)

