An association between the location of white matter changes and the behavioral and psychological symptoms of dementia in Alzheimer’s disease patients
Tzuchou Lin, Yihui Lin, Linli Kao, Yihui Kao, Yuanhan Yang,3, Pingsong Chou,3, Mengni Wu,4(✉)
An association between the location of white matter changes and the behavioral and psychological symptoms of dementia in Alzheimer’s disease patients
Tzuchou Lin1, Yihui Lin1, Linli Kao1, Yihui Kao2, Yuanhan Yang1,3, Pingsong Chou1,3, Mengni Wu1,4(✉)
1Department of Neurology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, China
2Department of Neurology, “National Taiwan University Hospital Yun‐Lin Branch”, Kaohsiung, Taiwan, China
3Department of Neurology, Kaohsiung Municipal Ta‐Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, China4Department of Master’s Program in Neurology, Faculty of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, China
ARTICLE INFO
Received: 12 January 2016
Revised: 18 February 2016
Accepted: 19 February 2016
© The authors 2016. This article is published with open access at www.TNCjournal.com
KEYWORDS
Alzheimer’s diseas;
white matter changes;
leukoaraiosis;
behavioral and psychological symptoms of dementia
ABSTRACT
Objective: The frontal lobe may be involved in circuits associated with depression, apathy, aggression, and other psychiatric symptoms. Although white matter changes (WMC) are related to the severity of behavioral and psychological symptoms of dementia (BPSD) in patients with Alzheimer’s disease (AD), it is unclear which part of the WMC may play the most important role in BPSD. This study was designed to investigate the relationship between the location of WMC and the severity of BPSD in AD patients.
Methods: Among patients diagnosed with Alzheimer’s disease between 2009 and 2014, 387 patients were retrospectively reviewed after those with pre‐existing organic brain syndrome, psychiatric diseases, or toxic‐metabolic encephalopathy were excluded. Patients’demographic and laboratory data, WMC measured with brain computed tomography and scored using the age‐related white matter changes (ARWMC) scale, and neuropsychological tests, including the cognitive abilities screening instrument (CASI), the Mini‐Mental State Examination (MMSE), the clinical dementia rating scale with sum‐box (CDR‐SB), and the neuropsychiatric inventory (NPI) were analyzed.
Results: There was no significant difference in the NPI between patients with and without a history of stroke, hypertension, and diabetes. No significant difference in the NPI was identified between different sexes or different Apolipoprotein E (APOE) alleles. The NPI score was significantly correlated with the duration of education (r = –0.4515, P = 0.0172), CASI (r = –0.2915, P < 0.0001), MMSE (r = –0.8476, P < 0.0001), and CDR‐SB (r = 2.2839, P < 0.0001). WMC in the right frontal lobe showed a significant difference in NPI in comparison to those without WMC (P = 0.0255). After adjusting for age, duration of education, and CASI, WMC in the right frontal lobe remained significantly associated with the NPI score (β = 3.8934, P = 0.042).
Conclusions: WMC involving the right frontal lobe may play an important role in the BPSD in AD patients during their dementia diagnosis. Further studies are necessary to confirm whether controlling the risk factors of WMC can slow the progression of BPSD.
Citation Lin TC, Lin YH, Kao LL, Kao YH, Yang YH, Chou PS, Wu MN. An association between the location of white matter changes and the behavioral and psychological symptoms of dementia in Alzheimer’s disease patients. Transl. Neurosci. Clin. 2016, 2(1): 8–16.
✉ Corresponding author: Mengni Wu, E-mail: berkeley0701@gmail.com
1 Introduction
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases, and the prevalence of AD increases with age[1]. The main symptoms of AD are memory impairment and other cognitive dysfunctions. However, behavioral and psychological symptoms may occur in approximately 66%–88% of AD patients[2]. Of these behavioral and psychological symptoms of dementia (BPSD), the most common is apathy with 49% of patients experiencing those symptoms, followed by depression (42% of patients), aggression (40% of patients), anxiety (39% of patients), and sleep disorders (39% of patients)[3]. The BPSD not only worsens a patient’s disability, but also affects both the quality of life for patients and caregivers[4–6]. Therefore, patients with BPSD are prone to institutionalization or an increasing cost of care[7, 8]. Although the impact of BPSD is remarkable, the pathophysiology remains unclear and the therapeutic strategy only leads to a conservative relief of symptoms.
White matter changes (WMC), also known asleu‐koaraiosis, are frequently identified during routine brain imaging and are present in 27% of the non‐AD population over 65 years old[9]. Using brain magnetic resonance imaging (MRI), WMC are defined as hyper‐signals on T2 weighted and fluid‐attenuated inversion recovery (FLAIR) images, and hypo‐signals on T1‐weighted images. With brain computed tomography (CT), WMC are identified as ill‐defined lesions on subcortical areas[10]. The leukoaraiosis and disability study (LADIS) has revealed that the severity of WMC is a predictor of the transition from an independent to dependent status in three years[11]. WMC are also associated with cognitive impairment, depression, an unsteady gait, and urinary problems in the normal aging population[11]. Conversely, patients with estab‐lished psychiatric diseases had significant white matter disruption, especially in the frontal area[12]. Among patients with moderate to severe AD, WMC are inde‐pendently related with BPSD[13]. Although the WMC in the frontal lobe are associated with a comorbid depressive disorder in AD patients[14], no study has evaluated the relationship between the location of WMC and the severity of whole BPSD. This study aimed to investigate the relationship between the area involved in WMC and the severity of BPSD on the initial diagnosis of AD in patients.
2 Methods
Between January 2009 and December 2014, patients with memory and cognitive impairments who were referred to Kaohsiung Medical University Hospital were retrospectively analyzed. The hospital’s Institutional Review Board approved this study (KMUH‐IRB‐20140063).
2.1 Patients
All patients received a brain CT scan, a laboratory survey, and complete neuropsychiatric tests, including the Chinese version of the cognitive abilities screening instrument (CASI), the Mini‐Mental State Examination (MMSE), the clinical dementia rating scale with sum‐box (CDR‐SB), and the neuropsychiatric inventory (NPI)[15]. Patients who fulfilled the following inclusion criteria were included: age ≥ 20 years; AD diagnosis based on the criteria established by the “National Insti‐tute of Neurological Disorders and Stroke–Alzheimer Disease and Related Disorders (NINCDS–ADRDA)”; and undergoing examination of the APOE gene.
Patients were excluded if they had any of the following conditions: history of epilepsy; recorded history of severe brain injury that resulted in hos‐pitalization; diagnosis of a psychiatric disease or major depression 10 years before the onset of memory and cognitive impairment; concurrent metabolic, toxic or septic encephalopathy; a current drug abuser; or already receiving an inhibitor of acetyl cholinesterase.
According to review of chart and brain images, patients with a history of stroke were defined as those with a self‐reported history of stroke, or a well‐defined hypodensity more than 2 mm on the brain CT image[10]. Patients were defined as hypertensive if they were using anti‐hypertensive medication, had a systolic blood pressure of more than 140 mm Hg, or had a diastolic blood pressure of more than 90 mm Hg on the first visit. Patients were defined as diabetic if they had a self‐reported history of diabetes, had a fasting sugar level of ≥ 126 mg/dL, or had an HbA1c > 6.7%. On the basis of examination of the APOE gene, positive APOEε4 was considered as those with at least one APOEε4 allele.
2.2 Neuropsychological tests
All patients completed the Chinese version of CASI, MMSE, CDR‐SB, and NPI[15]. The CASI comprises of ten cognitive domains, including long‐term memory, short‐term memory, orientation, attention, concentra‐tion, category fluency, language, reasoning, abstraction and judgment, and visual construction. A higher CASI indicates better cognitive function[16].
The MMSE is comprised of seven items, including registration, attention and calculation, recall, language, ability to follow simple commands, and orientation. The total score is 30 points, and the higher score indicates better cognitive function[17].
The CDR consists of six domains of cognition‐related functional performances, including memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Two kinds of CDR scores are used clinically to investigate the functional impairment in patients with dementia. The global CDR scale is interpreted from the scores in each of the six categories (“box scores”), whereas the CDR‐SB is the summation of total scores in six domains. In this study, CDR‐SB, treated as continuous variables, was used to estimate the severity of functional impairment[18].
The NPI, designed to assess the behavioral and psychological symptoms of dementia (BPSD), consists of 12 items, including delusions, hallucinations, agita‐tion and aggression, depression and dysphoria, anxiety, elation and euphoria, apathy and indifference, disinhi‐bition, irritability and libility, aberrant motor behavior, sleep and night‐time behaviors, appetite and eating changes. A higher total NPI score indicates more severe BPSD[19].
2.3 Score of white matter changes
All patients underwent multi‐director brain com‐puted tomography (CT) (GE Healthcare, Milwaukee, WI, USA). All brain CT data were scored by the author, Yi‐Hui Kao, who was blinded to the results of the neuropsychological tests, using the age‐related white matter changes (ARWMC) scale[10]. The ill‐defined hypodensities on the brain CT were scored as white matter changes if the diameter of the hypodensity was more than 5 mm. The scores of white matter changes were established in five different areas, including the frontal area (ARWMC‐F), the parieto‐occipital area (ARWMC‐PO), the temporal area (ARWMC‐T), the infratentorial area (ARWMC‐Inf) and the basal gang‐lion (ARWMC‐BG). Among these areas, the ARWMC scale was rated on both side. Therefore, there were a total of 10 scales available: (1) ARWMC‐right frontal area (ARWMC‐RF); (2) ARWMC‐left frontal area (ARWMC‐LF); (3) ARWMC‐right parieto‐occipital area (ARWMC‐R‐PO); (4) ARWMC‐left parieto‐occipital area (ARWMC‐L‐PO); (5) ARWMC‐right temporal area (ARWMC‐RT); (6) ARWMC‐left temporal area (ARWMC‐LT); (7) ARWMC‐right basal ganglion (ARWMC‐RBG); (8) ARWMC‐left basal ganglion (ARWMC‐LBG); (9) ARWMC‐right infratentorial area (ARWMC‐R‐Inf); and (10) ARWMC‐left infratentorial area (ARWMC‐L‐Inf).
In the basal ganglion, the severity of the ARWMC scale was defined as 0 for no lesion; 1 for one focal lesion ≥ 5 mm; 2 for more than one focal lesion; and 3 for a confluent lesion. In other areas, the severity of the ARWMC scale was defined as 0 for no lesion; 1 for focal lesions; 2 for beginning confluence of lesions; and 3 for lesions involving the entire region. In this study, we considered a patient as having a white matter change in each area if the severity of the ARWMC scale was more than 0.
2.4 Statistical analysis
Statistical analyses were performed using JMP Soft‐ware, Version 9 (SAS Institute Inc, Cary, NC, USA). Continuous data were analyzed using the Student’s t test and simple linear regression. All variables with p < 0.1 in a univariate analysis were entered into the forced entry model of the multiple linear regression analysis. Diagnosis of collinearity was performed, and no collinearity was identified. The entered variables are presented with their standardized coefficient (β), 95% confidence interval (95% CI), and p value after adjustment for other confounding variables. A p value < 0.05 was considered statistically significant.
3 Results
Of 870 patients with Alzheimer’s disease, 420 patients fulfilling the inclusion criteria were enrolled in thisstudy. Based on a chart review, 387 patients were eligible for further analysis.
3.1 Baseline data
Table 1 shows the baseline demographic characteristics and the ARWMC scales of all AD patients. The 387 AD patients included 115 men (29.72%) and 272 women (70.28%). The meanage (± SD) was 77.18 ± 7.58 years. The mean CASI, MMSE, CDR‐SB, and NPI (± SD) were 57.75 ± 19.36, 16.61 ± 5.97, 5.84 ± 3.02, and 17.38 ± 18.91, respectively. There were 69 (17.83%) patients with a history of stroke, 236 (60.98%) patients with a history of hypertension, and 118 (30.49%) patients with a history of diabetes.
3.2 Univariate analysis
The relationship among demographic data, ARWMC scales, duration of education, and neuropsychologi‐cal tests are presented in Tables 2 and 3. The NPI was not significantly different between men and women (mean: 17.52 ± 18.22 vs. mean: 17.32 ± 19.22, P = 0.9251). There was no significant difference of NPI between patients with and without a history of stroke, hy‐pertension, and diabetes. The NPI of patients with positive APOE ε4 was not significantly different from those with negative APOE ε4.
There was a significant difference of NPI between patients with white matter changes in the right frontal lobe and those without white matter changes in the right frontal lobe (mean: 19.10 ± 20.13 vs. 14.70 ± 16.52, P = 0.0255). However, there was no significant differ‐ence of NPI between those with white matter changes in the parieto‐occipital area, the temporal area, the infratentorial area, or the basal ganglion on either side and those without white matter changes.
There were significantly negative correlations bet‐ween NPI and the duration of education (r = –0.4515, P = 0.0172), MMSE (r = –0.8476, P < 0.0001), and CASI (r = –0.2915, P < 0.0001). Conversely, a significantly positive correlation between NPI and CDR‐SB was identified (r = 2.2839, P < 0.0001). There was no signi‐ficant correlation between age and NPI.
3.3 Multivariate analysis
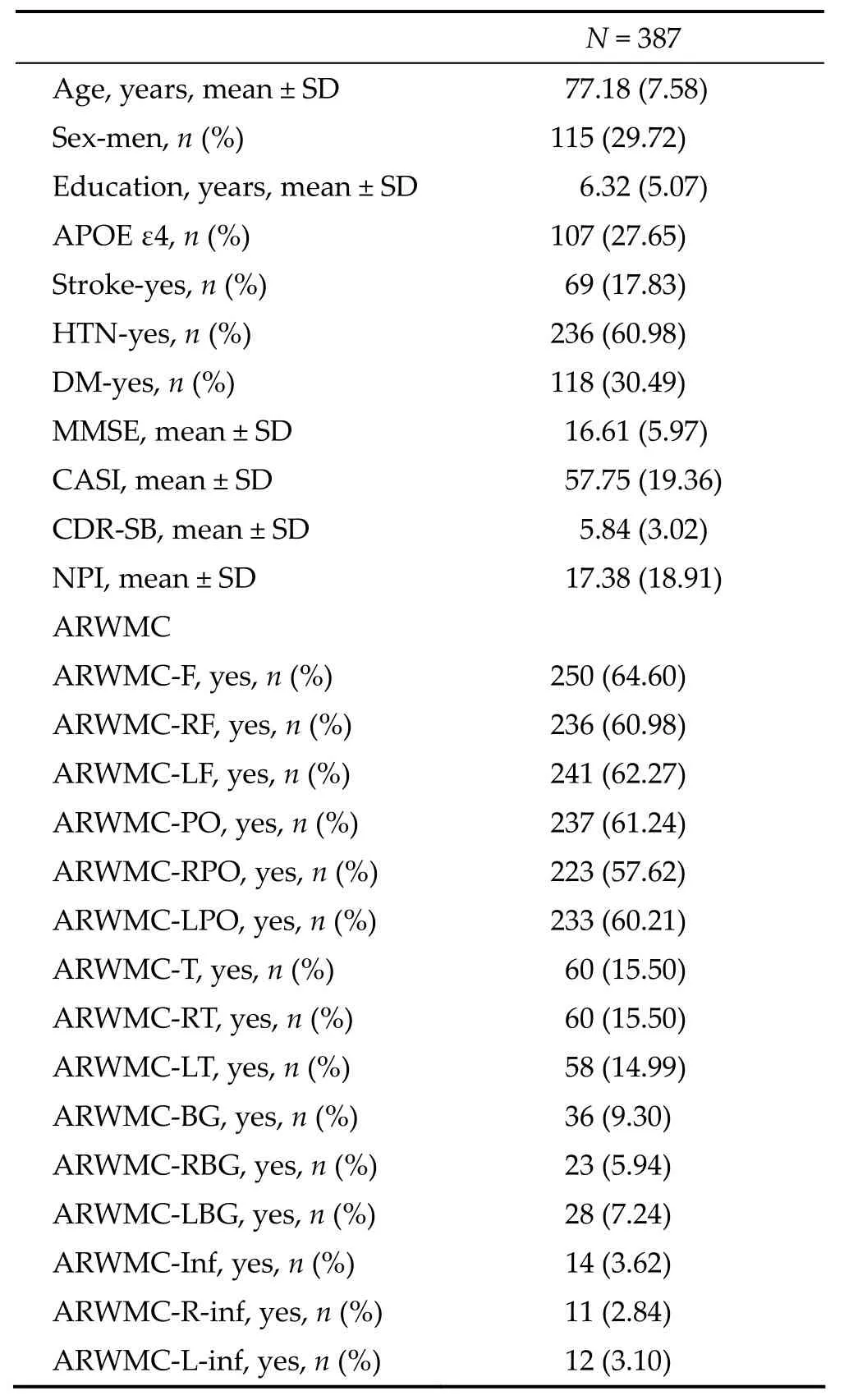
Table 1 Demographic data and ARWMC scales of patients (N = 387)
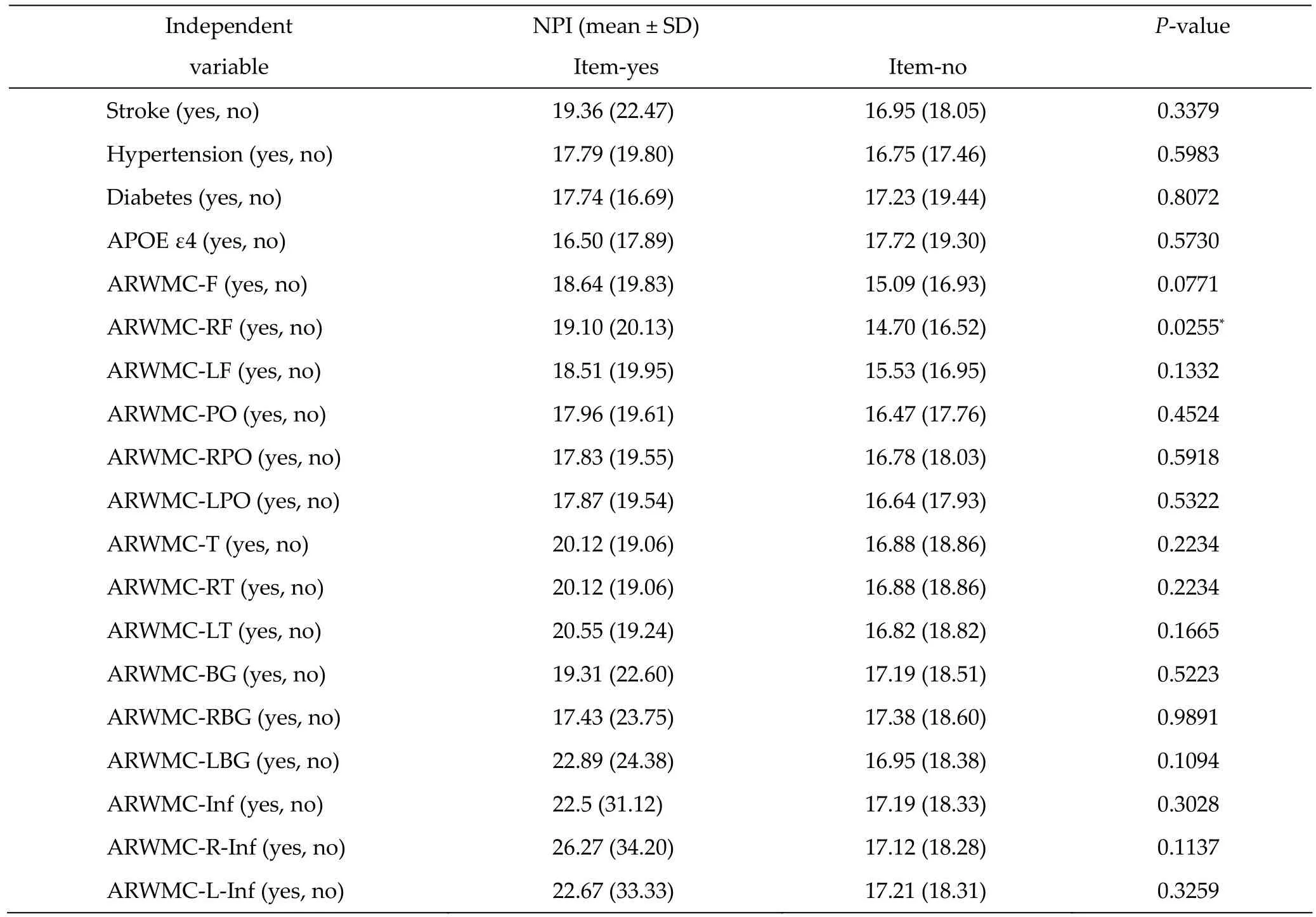
Table 2 Relationship between demographic data, ARWMC scales, and NPI
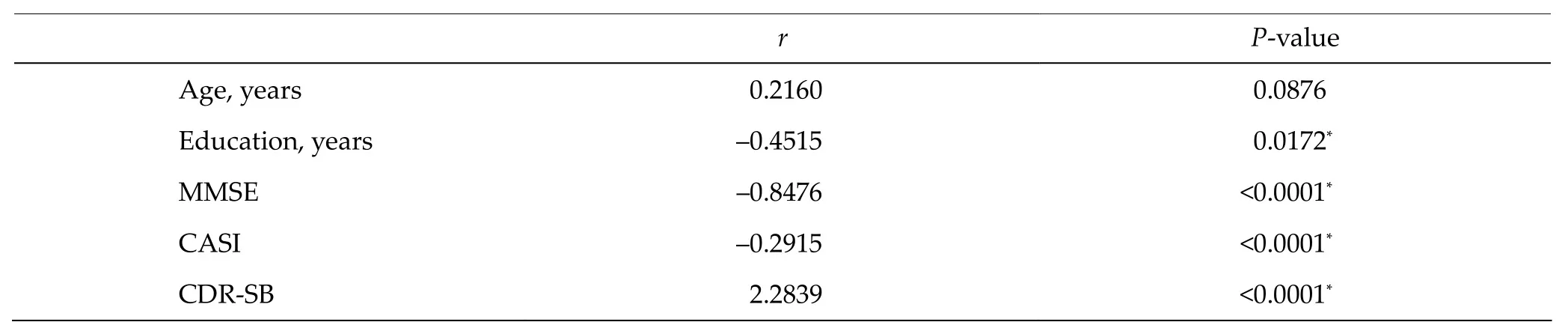
Table 3 Simple linear regression of NPI and age, duration of education, and other neuropsychological test
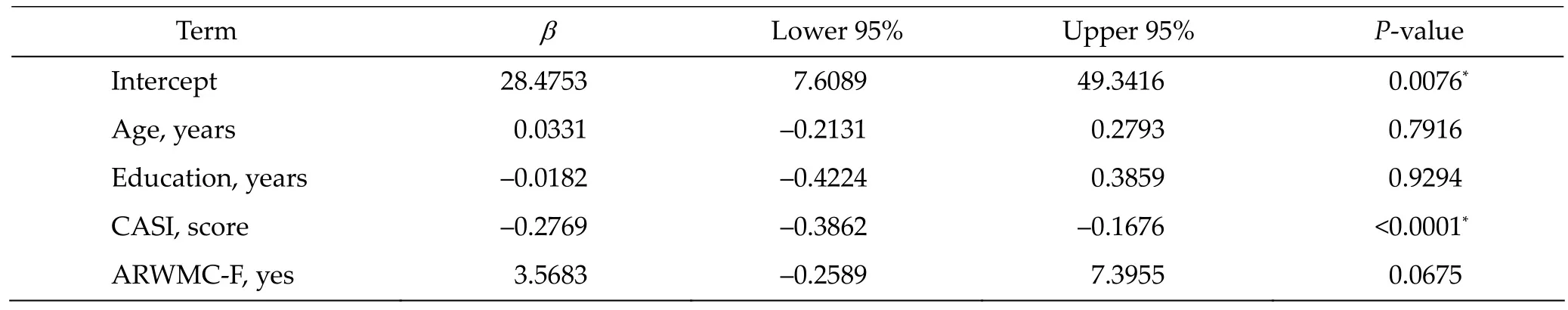
Table 4 Multivariate analysis of NPI and adjustment of age, education, CASI, ARWMC‐F

Table 5 Multivariate analysis of NPI and adjustment of age, education, CASI, ARWMC‐RF
The results of the multivariate analyses are demonstrated in Tables 4 and 5. There was trend between NPI and ARWMC‐F (β = 3.5683, 95% CI: –0.2589 to 7.3955, P = 0.0675) after adjusting for age, duration of education, and CASI. Although no significant associa‐tion between ARWMC‐LF and NPI was identified, ARWMC‐RF was significantly associated with NPI (β = 3.8934, 95% CI: 0.1408 to 7.6460, P = 0.0420) after adjusting for age, duration of education, and CASI.
4 Discussion
This study demonstrated that the presence of WMC in the frontal lobe is associated with the NPI score. After adjusting for age, duration of education, and CASI, WMC in the right frontal area remained significantly associated with the total NPI score, i.e., the subcortical circuits in the right frontal area may play a role in the severity of BPSD in patients during the diagnosis of AD.
Because the NPI consists of 12 items associated with BPSD, our findings may show the intersection of all of the BPSD. In patients with AD and Down’s syndrome, apathy, disinhibition, and executive dysfunc‐tion are associated with multiple subcortical circuits in the frontal lobe, whereas apathy in schizophrenia is associated with a frontal subcortical abnormality[20, 21]. Additionally, a reduced volume of WMC in the frontal lobe increases the risk of concurrent depression in AD[14]. MRI studies reveal that major depressive disorder may involve pathological changes of the temporal and frontal lobes, including the superior temporal gyrus, the hippocampus, the amygdala, the anterior cingulate gyrus, and the orbitofrontal area[22]. Involve‐ment of the frontal lobe and infarcts on frontal subcortical circuits are also an independent predictor of the development of post‐stroke depression[23–25]. Medial pre‐frontal networks that are connected to the medial pre‐frontal cortex and the medial as well as the caudo‐lateral orbital cortex are involved in the neural networks related to depressive episodes[26]. Patients with dementia and concurrent aggression show significantly reduced activity in the left anterior temporal cortex, the bilateral dorso‐frontal cortex, and the right parietal cortex[27]. In patients with schizophrenia, the impaired executive function and violent behavior are associated with frontal and inferior parietal inactivity[28, 29]. Therefore, we assumed that the frontal lobe, commonly involved in apathy, depression, and aggression, is significantly associated with the severity of BPSD in this study because these three symptoms comprise the majority of BPSD.
In the early stages of ALS, the severity of apathy is associated with the degeneration of the righthemisphere, especially in the anterior cingulate tract and right temporal white matter[30]. Additionally, social behavioral abnormalities, including apathy, disinhibition, eating disorders and aberrant motor behaviors, are correlated with tissue loss of the right hemisphere in AD patients[31]. Right hemisphere dysfunction, structural lesions, or hypo‐metabolism could cause delusions, and impaired ego boundaries, self‐monitoring and familiarity to stimuli[32–34], whereas dysfunction on the left hemisphere may cause unchecked false explanations to outer stimuli[35]. Furthermore, comprehension and expression are mainly operated by the right hemisphere[30], which may explain why right hemisphere dysfunction might induce poor impulse control, childish behavior and poor modulated affect[31]. Therefore, we assumed that the right hemisphere, instead of left hemisphere, may play an important role in the BPSD.
This study also supported the finding that the BPSD is significantly negatively associated with the duration of education, and the severity of dementia, as presented by the MMSE and CASI scores[36, 37]. It had also been reported that the CDR scores had positive correlations with the number of BPSD symptoms in patients with either vascular dementia or AD[38, 39]. Comparatively, age is not significantly associated with BPSD severity[36]. Therefore, we emphasized that physicians and caregivers should pay attention to the signs of BPSD in patients with AD, especially those with low education level and severe cognitive impairment.
The WMC progression is related to hypertension, diabetes, and hyperglycemic status, whereas lacunas are related to hypertension, high body mass index, hyper‐lipoproteinemia and hypertriglyceridemia[11, 40]. Because of the association between WMC and the severity of BPSD, it is reasonable to assume that there is a relationship between the risks of WMC and the severity of BPSD. Further studies should be conducted to clarify whether the intensive control of WMC risks is beneficial to slow the progression of the BPSD.
There are some limitations in this study. First, the design of cross‐sectional studies cannot establish the cause‐effect relationship between the structural changes of WMC and the presentation of BPSD. Second, the ARWMC scale, a semi‐quantitative visual rating scale, was used to investigate the presence of WMC rather than lesion volume analysis. However, the ARWMC scale is well‐established and widely used in other studies, such as the LADIS study, and the inter‐rater reliability is accep table[10, 11, 40]. Furthermore, one author, who was blinded to the results of the neuropsychological tests, was assigned to complete all brain imaging analyses in this study.
5 Conclusions
In conclusion, the presence of WMC in the right frontal area is associated with the severity of BPSD during the diagnosis of AD. Our findings support that the circuits connecting the frontal lobe may involve apathy, depression, and aggression that are the most common BPSD, whereas the right hemisphere is related to comprehension and expression of emotion and social behavior. Because the risks of WMC were well‐established, it is important to clarify the role of the risks of WMC on the mechanism of development of the BPSD in AD patients. Future studies should be conducted to clarify whether intensive control of risks related to WMC can slow or reverse the progression of BPSD in AD patients.
Conflict of interests
The authors have no financial interest to disclose regarding the article.
References
[1] Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons. JAMA 1989, 262(18): 2551–2556.
[2] Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol 2002, 15(4): 445–450.
[3] Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Xu W, Li JQ, Wang J, Lai TJ, Yu JT. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 2016, 190: 264–271.
[4] Rapoport MJ, van Reekum R, Freedman M, Streiner D, Simard M, Clarke D, Cohen T, Conn D. Relationship of psychosis to aggression, apathy and function in dementia. Int J Geriatr Psychiatry 2001, 16(2): 123–130.
[5] Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry 2005, 13(6): 469–474.
[6] Berger G, Bernhardt T, Weimer E, Peters J, Kratzsch T, Frolich L. Longitudinal study on the relationship between symptomatology of dementia and levels of subjective burden and depression among family caregivers in memory clinic patients. J Geriatr Psychiatry Neurol 2005, 18(3): 119–128.
[7] Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry 1990, 147(8): 1049–1051.
[8] Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002, 17(5): 403–408.
[9] Breteler MMB, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, Hofman A. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology 1994, 44(7): 1246–1252.
[10] Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for agerelated white matter changes applicable to MRI and CT. Stroke 2001, 32(6): 1318–1322.
[11] The LADIS Study Group. 2001–2011: A decade of the LADIS (leukoaraiosis and disability) study: What have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis 2011, 32(6): 577–588.
[12] Lagopoulos J, Hermens DF, Hatton SN, Battisti RA, Tobias-Webb J, White D, Naismith SL, Scott EM, Ryder WJ, Bennett MR, Hickie IB. Microstructural white matter changes are correlated with the stage of psychiatric illness. Transl Psychiatry 2013, 3(4): e248.
[13] Kandiah N, Chander R, Zhang A, Yee CC. Cerebral white matter disease is independently associated with BPSD in Alzheimer’s disease. J Neurol Sci 2014, 337(1–2): 162–166.
[14] Lee JJ, Lee EY, Lee SB, Park JH, Kim TH, Jeong HG, Kim JH, Han JW, Kim KW. Impact of white matter lesions on depression in the patients with Alzheimer’s disease. Psychiatry Investig 2015, 12(4): 516–522.
[15] Li CH, Liu CK, Yang YH, Chou MC, Chen CH, Lai CL. Adjunct effect of music therapy on cognition in Alzheimer’s disease in Taiwan: A pilot study. Neuropsychiatr Dis Treat 2015, 11: 291–296.
[16] Lin KN, Wang PN, Liu CY, Chen WT, Lee YC, Liu HC. Cutoff scores of the cognitive abilities screening instrument, Chinese version in screening of dementia. Dement Geriatr Cogn Disord 2002, 14(4): 176–182.
[17] Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975, 12(3): 189–198.
[18] Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology 1993, 43(11): 2412–2414.
[19] Cummings JL. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48(5 Suppl 6): S10–S16.
[20] Ball SL, Holland AJ, Watson PC, Huppert FA. Theoretical exploration of the neural bases of behavioural disinhibition, apathy and executive dysfunction in preclinical Alzheimer’s disease in people with Down’s syndrome: Potential involvement of multiple frontal-subcortical neuronal circuits. J Intellect Disabil Res 2010, 54(4): 320–336.
[21] Roth RM, Flashman LA, Saykin AJ, McAllister TW, Vidaver R. Apathy in schizophrenia: Reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry 2004, 161(1): 157–159.
[22] Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord 2009, 117(1–2): 1–17.
[23] Shi YZ, Xiang YT, Wu SL, Zhang N, Zhou J, Bai Y, Wang S, Wang YL, Zhao XQ, Ungvari GS, Chiu HFK, Wang YJ, Wang CX. The relationship between frontal lobe lesions, course of post-stroke depression, and 1-year prognosis in patients with first-ever ischemic stroke. PloS one 2014, 9(7): e100456.
[24] Singh A, Black SE, Herrmann N, Leibovitch FS, Ebert PL, Lawrence J, Szalai JP. Functional and neuroanatomic correlations in poststroke depression: The sunny brook stroke study. Stroke 2000, 31(3): 637–644.
[25] Tang WK, Lu JY, Chen YK, Chu WC, Mok V, Ungvari GS, Wong KS. Association of frontal subcortical circuits infarcts in poststroke depression: A magnetic resonance imaging study of 591 Chinese patients with ischemic stroke. J Geriatr Psychiatry Neurol 2011, 24(1): 44–49.
[26] Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct 2008, 213(1-2): 93–118.
[27] Hirono N, Mega MS, Dinov ID, Mishkin F, Cummings JL. Left frontotemporal hypoperfusion is associated with aggression in patients with dementia. Arch Neurol 2000, 57(6): 861–866.
[28] Kumari V, Aasen I, Taylor P, Ffytche DH, Das M, Barkataki I, Goswami S, O’Connell P, Howlett M, Williams SCR, Sharma T. Neural dysfunction and violence in schizophrenia: An fMRI investigation. Schizophr Res 2006, 84(1): 144–164.
[29] Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull 2011, 37(5): 913–920.
[30] Tsujimoto M, Senda J, Ishihara T, Niimi Y, Kawai Y, Atsuta N, Watanabe H, Tanaka F, Naganawa S, Sobue G. Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J Neurol Sci 2011, 307(1–2): 34–40.
[31] Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain 2005, 128(Pt 11): 2612–2625.
[32] Lee K, Shinbo M, Kanai H, Nagumo Y. Reduplicative paramnesia after a right frontal lesion. Cogn Behav Neurol 2011, 24(1): 35–39.
[33] Likitcharoen Y, Phanthumchinda K. Environmental reduplication in a patient with right middle cerebral artery occlusion. J Med Assoc Thai 2004, 87(12): 1526–1529.
[34] Perneczky R, Drzezga A, Boecker H, Wagenpfeil S, Förstl H, Kurz A, Häussermann P. Right prefrontal hypometabolism predicts delusions in dementia with Lewy bodies. Neurobiol Aging 2009, 30(9): 1420–1429.
[35] Devinsky O. Delusional misidentifications and duplications: Right brain lesions, left brain delusions. Neurology 2009, 72(1): 80–87.
[36] Xue HB, Xiao SF, Ng TP, Chen C, Meng GR, Lu XJ, Bu SM, Fang WL, Lv J, Zhang MY, McCabe MP. Prevalence and severity of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Chinese: Findings from the Shanghai three districts study. Aging Ment Health 2013, 17(6): 748–752
[37] Ito T, Meguro K, Akanuma K, Meguro M, Lee E, Kasuya M, Ishii H, Mori E. Behavioral and psychological symptoms assessed with the BEHAVE-AD-FW are differentially associated with cognitive dysfunction in Alzheimer’s disease. J Clin Neurosci 2007, 14(9): 850-855.
[38] Bandyopadhyay TK, Biswas A, Roy A, Guin DS, Gangopadhyay G, Sarkhel S, Ghoshal MK, Senapati AK. Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. Ann Indian Acad Neurol 2014, 17(3): 325–330.
[39] Gupta M, Dasgupta A, Khwaja GA, Chowdhury D, Patidar Y, Batra A. The profile of behavioral and psychological symptoms in vascular cognitive impairment with and without dementia. Ann Indian Acad Neurol 2013, 16(4): 599–602.
[40] Teodorczuk A, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, Wallin A, Wahlund LO, Scheltens P, Waldemar G, Schrotter G, Ferro JM, Chabriat H, Bazner H, Visser M, Inzitari D, O’Brien JT. Relationship between baseline whitematter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med 2010, 40(4): 603–610.
 Translational Neuroscience and Clinics2016年1期
Translational Neuroscience and Clinics2016年1期
- Translational Neuroscience and Clinics的其它文章
- Malignant transformation and treatment of cystic mixed germ cell tumor
- Surgical complications secondary to decompressive craniectomy for patients with severe head trauma
- A new drainage tube device
- Effects of aging on working memory performance and prefrontal cortex activity: A time‐resolved spectroscopy study
- Effects of regional cerebral blood flow perfusion on learning and memory function and its molecular mechanism in rats
- Repairing skull defects in children with nano-hap/collagen composites: A clinical report of thirteen cases
