An incidental case of Wellens' syndrome in a community emergency department
Deep Jaiswal, Dan BoudreauFaculty of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada Corresponding Author: Dan Boudreau, Email: boudrea6@dal.ca
An incidental case of Wellens' syndrome in a community emergency department
Deep Jaiswal, Dan Boudreau
Faculty of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada Corresponding Author: Dan Boudreau, Email: boudrea6@dal.ca
World J Emerg Med 2016;7(2):153–156
INTRODUCTION
Wellens' syndrome, also called left anterior descending (LAD) coronary T-wave syndrome, is a characteristic electrocardiographic (ECG) pattern suggestive of coronary artery disease in the preinfarction stage.[1–3]ECG changes in Wellens' syndrome may signify critical stenosis in the proximal LAD coronary artery.[1–5]Furthermore, ECG changes may occur during a pain-free period with no other evidence of ischemia or unstable angina and none to minimal elevation of cardiac enzyme levels.[3,5]Hence,recognition of the aforementioned pattern is important in preventing the development of a devastating anterior wall myocardial infarction.[2–5]
There are two patterns of ECG changes related to Wellens' syndrome. The first involves a minimally elevated or isoelectric ST segment with inverted T waves in leads V2 and V3, often in leads V1 and V4,and occasionally in leads V5 and V6.[1–3,5]This pattern accounts for approximately 75% of Wellens' syndrome cases.[3,5]The second pattern involves biphasic T waves in leads V2 and V3[1–3,5]and accounts for roughly 25% of Wellens' syndrome cases.[3,5]
CASE
The patient, a 59-year-old female, was accompanying her husband on his visit to the emergency department. She had complained to the staff nurse taking care of her husband about her left arm discomfort that developed overnight and resolved spontaneously by the following morning. With the assistance from the staff nurse,she registered at the department's triage station and underwent further assessment. On further questioning,she stated her discomfort did not radiate to the chest and she also experienced right jaw pain secondary to a "wisdom tooth extraction one week ago". She also complained of shortness of breath (SOB) on moderate exertion but indicated that the severity of her respiratory symptoms had not gotten worse recently.
She had familial hypercholesterolemia, hypertension (HTN), bilateral knee osteoarthritis, gastroesophageal reflux disease, migraine, anxiety and depression. Her father died at age of 48 from a myocardial infarction (MI), and her mother died at age of 86 and did not have any cardiovascular risk factors. She was treated with a series of antihypertensives but did not use statin for years because she had myalgia in the past.
On physical examination, her vital signs were stable. No adventitious respiratory sounds were heard. She had normal S1 and S2 heart sounds, but neither S3 nor S4 was present. No elevation in jugular venous pressure (JVP) was noted, and she had bilateral pitting edema to mid shin.
Investigations and laboratory results
Complete blood count, electrolytes, urea, creatinine and random glucose were all within normal limits. On admission to the emergency department, creatinine kinase was 29 units/L (normal=30 to 200) and high sensitivity troponin T was 15 ng/L (normal≤14). Three hours after admission, creatinine kinase level was 22 U/L,and troponin T level was 16 ng/L. Approximately 6 hours after admission, creatinine kinase level was 19 U/L, and troponin T level was 15 ng/L. In short, the patient had slightly elevated serial troponin T levels.
Posterior-anterior and lateral chest radiographs were not marked as there was no evidence of pleural effusion,pulmonary edema or acute intrathoracic pathology.
The nursing staff at triage conducted an ECG and notified the emergency physician of new ECG changes compared to one done approximately 18 months ago. T wave inversion in anterolateral leads was noted on two ECGs that were conducted approximately 90 minutes apart (Figures 1 and 2).
Subsequently, the patient was admitted to the department of internal medicine. After the pain was relieved overnight, an exercise stress test was performed at the department of internal medicine one day after she was admitted to the emergency department. She was able to attain 7.4 METs and the test was limited by left sided chest pain and left arm pain with less than 2 mm ST depression.
Treatment and follow-up
The patient was given acetylsalicylic acid (ASA),clopidogrel, fondaparinux and statin therapy. She also underwent cardiac catheterization (Figure 3) four days after admission, which demonstrated 90% proximal stenosis of the LAD branch of the left coronary artery and 80% attenuation in the left circumfl ex branch. Both critical LAD and circumflex lesions were intervened using drug eluting stents and no residual narrowing of stent was noted.
Before being discharged, echocardiogram of the patient showed normal left ventricular systolic function. Her low density lipoprotein level was 5.49 and high density lipoprotein level was 1.0. Her lipid level was determined with titration of statin. She was continuously subjected to ASA, clopidogrel, angiotensin converting enzyme inhibitor and statin therapy in addition to regular anti-HTN medication.
On follow-up, about one month after admission to the emergency department, the patient was asymptomatic and denied chest pain or worsening of SOB. Vital signs were within normal limits and JVP was not elevated. ECG after a 6-month follow-up showed resolution of her initial ECG changes (Figure 4).

Figure 1. Initial ECG with inverted T waves in anterolateral leads especially precordial leads V2 and V3.
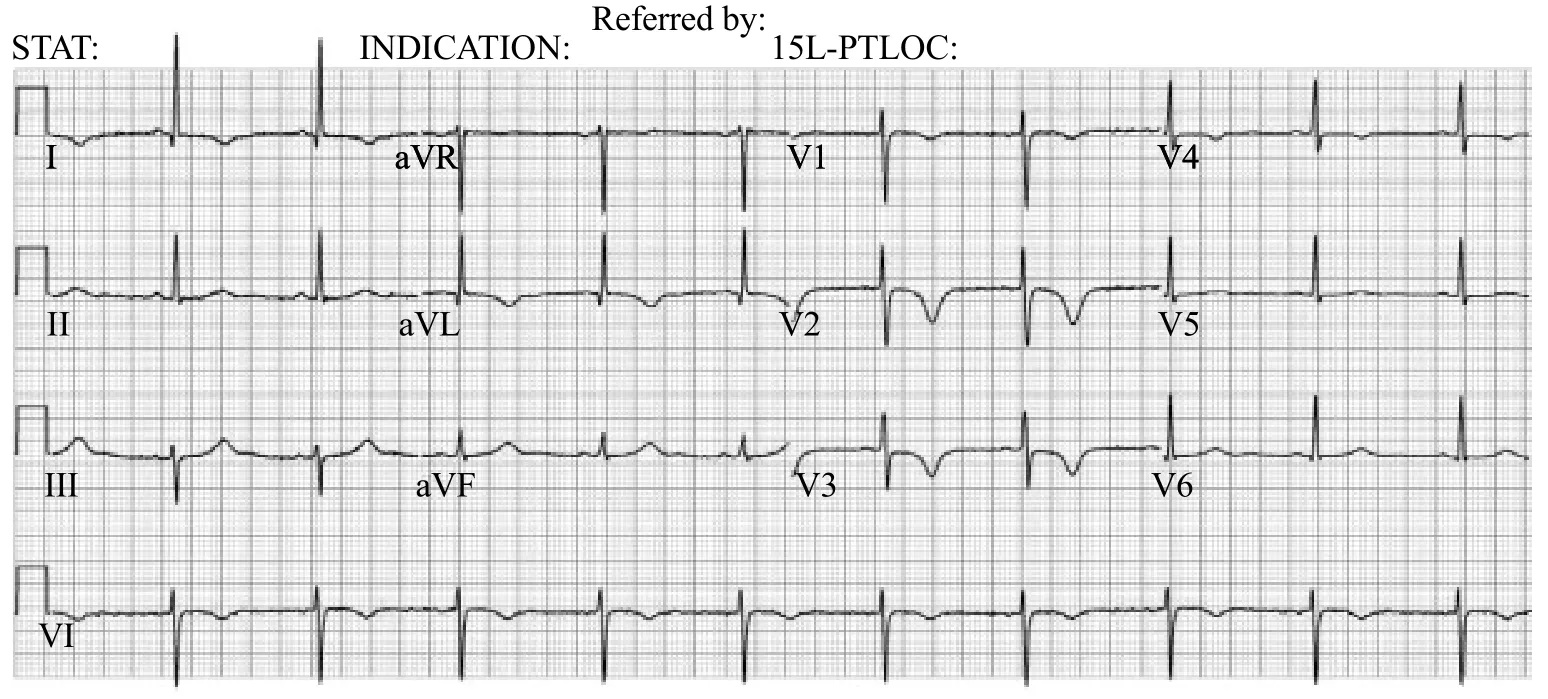
Figure 2. Second ECG 90 minutes later with inverted T waves in anterolateral leads especially precordial leads V2 and V3.
DISCUSSION
Since the patient was asymptomatic on admission and had almost normal biomarkers, Wellens' syndrome could be diagnosed on the basis of ECG changes. ECG changes with inverted T waves can occur in a variety of contexts other than Wellens' syndrome including previous myocardial infarction, persistent juvenile T wave pattern, acute coronary ischemia (non-Wellenoid ischemia and non-ST segment elevation acute myocardial infarction), bundle branch block, left ventricular hypertrophy, acute myocarditis, pre-excitation syndromes, acute pulmonary embolism, later stages of pericarditis, cerebrovascular accident, and digitalis effect.[3]Consequently, the criteria listed below can be used to differentiate Wellens' syndrome from other causes of inverted T wave changes on ECG:[3,5]prior history of chest pain; normal or slightly elevated cardiac enzymes; absence of precordial Q waves; patterns in a pain free state; isoelectric or minimally elevated ST segment; biphasic T waves in leads V2 and V3 or deeply inverted T waves in leads V2 and V3.

Figure 3. Coronary angiogram showing lesions (arrows) in the LAD and left circumfl ex branches of the left coronary artery.

Figure 4. ECG at a 6-month follow-up showing resolution of initial ECG changes.
Although the presented patient did not experience typical chest pain, she did complain of left arm discomfort suggestive of an atypical cardiac manifestation.[6–7]Moreover, her serial troponin T levels were slightly elevated and ECG changes were noted while she was pain free. ECG changes included inverted T waves in V2 and V3. No precordial Q waves were noted and ST segment was isoelectric. In turn, the patient met the discussed criteria.
Current guidelines lack specifi c recommendations for the management of Wellens' syndrome. In our patient, the consulting service employed the conservative strategy for revascularization. Conservatively, patients with unstable angina or non-ST segment elevation MI (NSTEMI)should receive pharmacological therapy and then undergo revascularization if they have recurrent symptoms of inducible ischemia upon non-invasive testing.[8]Alternatively, a routine invasive strategy is suitable for all patients with unstable angina or NSTEMI who undergo coronary angiography followed by revascularization based on angiography results.[8]Benefi ts of the routine invasive strategy include a significant reduction in mortality and MI from hospital discharge to the end of follow-up.[8]However, the routine invasive strategy is associated with a higher early mortality.[8]
Our patient underwent an exercise stress test, such a procedure should not be performed in patients with Wellens' syndrome as increased cardiac demand along with a critically stenosed LAD branch can trigger a MI.[5,9]Thus, we recommend the routine invasive strategy for patients with Wellens' syndrome. It is very important for health care providers to identify patients with ECG findings that are characteristic of Wellens'syndrome. Furthermore, as in our case, ECG changes can normalize completely in 90% of cases after appropriate intervention.[1]
Funding: None.
Ethical approval: Not needed.
Confl icts of interest: The authors declare that there is no confl ict of interest.
Contributors: Jaiswal D proposed the study and wrote the report. All authors contributed to the design and interpretation of the study and approved the fi nal version.
REFERENCES
1 de Zwaan C, Bär FW, Janssen JH, Cheriex EC, Dassen WR,Brugada P, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117: 657–665.
2 de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103: 730–736.
3 Rhinehardt J, Brady WJ, Perron AD, Mattu A. Electrocardiographic manifestations of Wellens' syndrome. Am J Emerg Med 2002; 20: 638–643.
4 Parikh KS, Agarwal R, Mehrotra AK, Swamy RS. Wellens syndrome: A life saving diagnosis. Am J Emerg Med 2012; 30: 255e3–255e5.
5 Tandy TK, Bottomy DP, Lewis JG. Wellens' syndrome. Ann Emerg Med 1999; 33: 347–351.
6 Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G,Pepine CJ. Symptom of presentation of women with acute coronary syndrome: Myth vs reality. Arch Intern Med 2007; 167: 2405–2413.
7 Eslick GD. Usefulness of chest pain character and location as diagnostic indicators of an acute coronary syndrome. Am J Card 2005; 95: 1228–1231.
8 Mehta SR, Cannon CP, Fox KAA, Wallentin L, Boden WE,Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: A collaborative metaanalysis of randomized trials. JAMA 2005; 293: 2908–2917.
9 Mead NE, O'Keefe KP. Wellen's syndrome: An ominous EKG pattern. J Emerg Trauma Shock 2009; 2: 206–208.
Received September 15, 2015
Accepted after revision February 22, 2016
DOI:10.5847/wjem.j.1920–8642.2016.02.012
 World journal of emergency medicine2016年2期
World journal of emergency medicine2016年2期
- World journal of emergency medicine的其它文章
- Evaluation of preventable trauma death in emergency department of Imam Reza hospital
- Application of scoring systems with point-of-care ultrasonography for bedside diagnosis of appendicitis
- A scoring system for assessing the severity of acute diarrhea of adult patients
- Circulating microRNAs as potential biomarkers for diagnosis of congenital heart defects
- Emergency medicine providers' opioid prescribing practices stratifi ed by gender, age, and years in practice
- Instructions for Authors
