Spectroscopic Properties of Energy Transfer Effect in Sm3+/Eu3+ Doped LaF3 Nanocrystals
FU Zhen-xing, LIU Bi-rui, YANG Bing-xiong
1. School of Physics and Electronic Information Engineering, Ningxia Normal University, Guyuan 756000, China
2. School of Physics Electrical Information Engineering, Ningxia University, Yinchuan 750021, China
Spectroscopic Properties of Energy Transfer Effect in Sm3+/Eu3+Doped LaF3Nanocrystals
FU Zhen-xing1, LIU Bi-rui1, YANG Bing-xiong2
1. School of Physics and Electronic Information Engineering, Ningxia Normal University, Guyuan 756000, China
2. School of Physics Electrical Information Engineering, Ningxia University, Yinchuan 750021, China
The samples of LaF3∶Sm3+, LaF3∶Eu3+and LaF3∶Sm3+/Eu3+nanocrystals with high quality mono-disperse and uniform sizes were synthesized with hydrothermal method. The crystallographic phase, surface morphology, crystalline sizes and fluorescence properties of Sm3+/Eu3+sole- and co-doped nanocrystals were characterized with X-ray powder diffraction (XRD), transmission electron microscopy (TEM) and photoluminescence (PL) spectroscopic technique, respectively. The results of XRD and TEM show that the microstructure of the nanocrystals is hexagonal, with the average size about 40 nm. Using 442 nm He-Cd continuous wave (CW) laser to pump the Sm3+ions doped in the LaF3∶Sm3+/Eu3+nanocrystals, the typical fluorescence emissions originating from the Eu3+ions were observed in the emission spectra, and that the energy transfer from Sm3+ions to Eu3+ions was completed. The mechanism and efficiency of the energy transfer from Sm3+ions to Eu3+ions were investigated and discussed systematically based on the spectroscopic method. It is shown that the energy transfer of Sm3+→Eu3+is attributed to the cross-relaxation between the excited state4G5/2of Sm3+ion and the5D1and5D0states of Eu3+ion. Meanwhile, the intensities of the characteristic fluorescence emissions of Eu3+ions become stronger and stronger with the increase of the Eu3+doping concentration, which suggest that the efficiency of energy transfer from Sm3+ions to Eu3+ions can be effectively improved by increasing the doping concentration of Eu3+acceptor.
Fluorescence spectrum; LaF3nanocrystals; Sm3+/Eu3+; Energy transfer
Introduction
Trivalent samarium (Sm3+) and europium (Eu3+) ions are two important red-light activators among Rare-earth ions[1-2]. The4G7/2,4F3/2and4G5/2states of Sm3+ion can produce excellent red/orange fluorescence emissions with high quantum efficiency, which can be widely used in LED, color display, optical apparatus and information communication, etc[3-5]. While Eu3+ion can also give off excellent red/orange fluorescence emissions in the region of 550~750 nm since its energy level structure is similar to that of Sm3+ion. Additionally, the electric-dipole transition due to5D0→7F2emission of Eu3+ion is hypersensitive to the local crystal field, which can be used as “fluorescence probe” to explore the site symmetry of the doping Eu3+ion[6-7]. Many researchers have introduced a large quantity of literature investigating the spectroscopic properties of Sm3+, Eu3+sole-doped systems. Recently, there are some reports on the energy transfer and fluorescence properties of Sm3+/Eu3+co-doped systems, and the investigation of the energy transfers of Sm3+/Eu3+in various hosts is much favorable by many researchers[8-12]. With the rising of the demand for visible laser and light, LED, optical information transfer and red phosphors, Sm3+and Eu3+ions as two representative red-light activators have played more and more important roles in the development of luminescent materials[13-15].
Lanthanum fluoride (LaF3) crystals is a sort of excellent fluorescent matrix material and has broad applications in materials science, medicine, organic/inorganic synthesis and other fields due to its low phonon energy, high quantum efficiency, small probability of no-radiation and so on. However, to the best of our knowledge, there are no reports on the energy transfer effect of Sm3+/Eu3+ions in LaF3host. In this paper, LaF3∶Sm3+, LaF3∶Eu3+and LaF3∶Sm3+/Eu3+nanocrystals were synthesized with the hydrothermal method at low temperature. Using 442 nm CW laser to excite Sm3+ions doped in Sm3+/Eu3+co-doped LaF3nanocrystals, the strong characteristic fluorescence emissions of Eu3+ion originating from the5D0state were observed. It suggests that the energy transfer from Sm3+to Eu3+ions occurs in LaF3∶Sm3+/Eu3+nanocrystals. The mechanism and dynamical process of energy transfer were investigated systematically based on photoluminescence (PL) spectroscopic technique. The influence of the doping concentration of Eu3+acceptor on the energy transfer efficiency was also discussed in detail.
1 Experimental
1.1 Preparation of samples
The LaF3∶Sm3+, LaF3∶Eu3+and LaF3∶Sm3+/Eu3+nanocrystals were synthesized with the hydrothermal method at low temperature. Chemicals used for preparing the samples included sodium fluoride (NaF, Xi’an Chemical Reagent Factory, China, chemical pure, 98%), nitric acid (HNO3, Xi’an Sanpu Fine Chemical Reagent Factory, China, analytical reagent, 65%~68%), europium oxide (Eu2O3, Tongji Microelement Research Center in China, analytical reagent, 99.99%), samarium oxide (Sm2O3, Tongji Microelement Research Center in China, analytical reagent, 99.99%) and lanthanum nitric hexahydrate (La(NO3)3·6H2O, Sinopharm Chemical Reagent Co., Ltd, China, analytical reagent, 44%).
The Sm(NO3)3and Eu(NO3)3deionized water solution were obtained by reacting Sm2O3and Eu2O3with nitric acid, respectively. Initially, the Sm2O3and Eu2O3was dissolved in nitric acid and formed Sm(NO3)3and Eu(NO3)3aqueous solution (0.01 mol·L-1). Then the appropriate amount of La(NO3)3·6H2O was weighted to move to a beaker, and added 20 mL deionized water to this beaker. Stirring the mixture in the beaker for 5~10 min until La(NO3)3·6H2O was completely dissolved and a clear solution of La(NO3)3was obtained, the corresponding amount of Sm(NO3)3and/or Eu(NO3)3solution was added into the beaker according to the Sm3+, Eu3+sole- and/or co-doping concentration. Ensuring the mixture was homogeneous by stirring for 8~10 min, the appropriate amount of NaF was added into the beaker according to the molar ratio of La3+and F-ions. After stirring for 60 min, the mixture was transferred into a Teflon-lined autoclave and added deionized water into the autoclave to 80% of the total volume. Then the autoclave was put into an electric blast drying oven to conduct the reaction at 200 ℃ for 16 hours. After the reaction, air cooled the autoclave to room temperature. The autoclave was pulled out of the oven and the solution in the autoclave was transferred to centrifuge tubes. Centrifuging the solution at 5 000 r·m-1for several times, the white product was obtained. Finally, the nanocrystals of LaF3∶Sm3+/Eu3+, LaF3∶Sm3+, LaF3∶Eu3+were obtained by parching the white product at 60 ℃ for 12 hours.
1.2 Sample characterization
The XRD patterns of the samples was conducted with an X-ray powder diffractometer (D/Max2550VB+/PC) with Cu Kα (λ=0.154 06 nm) radiation to identify the crystal structure of the samples. The surface morphology, crystalline size and dispersity of the samples were performed by a Tecnai-10 (Philip, Holland) transmission electron microscope (TEM). Figure 1 shows the XRD patterns of Sm3+/Eu3+co-doped LaF3nanocrystals with the doping concentration of Sm3+and Eu3+ions are 0.5 mol% and 1.0 mol%. It can be seen that the diffractive peaks of the as-prepared samples are sharp and their positions are in good agreement with the JCPDS Card No.06-0281, which indicates the crystallographic phase of the as-prepared LaF3nanocrystals is hexagonal. No dopant peaks or other products are noticed, which implies that the slight amount of Sm3+/Eu3+dopant has almost no effect on the LaF3phase composition. Scherrer formula is applied to diffraction peaks (111). It is found that the mean particle sizes are about 40 nm.

Fig.1 XRD patterns of the LaF3∶Sm3+/Eu3+ nanocrystals
The surface morphology, crystalline size and dispersity of the as-prepared LaF3samples were observed with TEM. Figure 2 presents two TEM images of LaF3samples with different magnification ratio. It can be seen that the samples have similar surface morphology with high quality mono-disperse and uniform sizes. The mean particle sizes are about 40 nm, which is consistent with what has calculated through Scherrer formula.
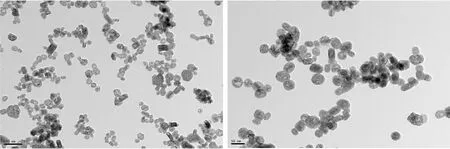
Fig.2 TEM images of LaF3∶Sm3+/Eu3+ nanocrystals
1.3 PL spectroscopic measurements
As to PL spectroscopic measurements, the fluorescence signal was collected with a spectrometer (SP2750i, focus 0.75 m) equipped with a CCD system (Princeton Instrument, PIXIS:100B). A 442 nm He-Cd laser (IK5451R-E-SP, CW, Japan) and a 532 nm Nd3+∶YAG pulsed laser (Quanta Ray Lab-170, pulse widths 2 ns, repetition rate 10 Hz) were used as the excitation source. All of the measurements were conducted at room temperature.
2 Results and discussion
2.1 Spectroscopic measurement of energy transfer
The PL spectroscopic properties of energy transfer effect from Sm3+to Eu3+are much different in different hosts. In order to investigate the spectroscopic properties of energy transfer effect from Sm3+to Eu3+in LaF3host, the LaF3∶Eu3+, LaF3∶Sm3+sole-doped and LaF3∶Sm3+/Eu3+co-doped nanocrystals were excited with the 532 and 442 nm lasers, respectively, and the emission spectra of the samples were observed and recorded. The results of the experimental measurements show that the Sm3+ions doped in LaF3host can effectively be excited with 442 nm laser, while the Eu3+ions in LaF3host can only be excited with 532 nm laser. The fluorescence emission spectra of LaF3∶Sm3+sole-doped (curvea) and LaF3∶Sm3+/Eu3+co-doped (curvec) nanocrystals are given in Fig.3. It can be clearly seen that there are four dominating emission bands located at around 558, 592, 638 and 701 nm, respectively. Combining the emission bands with the energy level structure of Sm3+ions, one can confirm that the four dominating fluorescence emissions arise from the4G5/2→6H5/2,4G5/2→6H7/2,4G5/2→6H9/2and4G5/2→6H11/2transitions, where the highest emission band is located at 592 nm corresponding to the4G5/2→6H7/2transition, which is plotted by curveain Fig.3. However, for LaF3∶Sm3+/Eu3+co-doped samples, besides the four emission bands mentioned above, there are five new emission bands occurred in the emission spectra. The central wavelengths of the five emission bands are 612, 616, 679, 688 and 690 nm, as can be seen from the curvecin Fig.3. The central wavelengths of the new emission bands are in agreement with the typical positions of Eu3+fluorescence emissions under the excitation at 532 nm. The emission bands locate at 612 nm and 616 nm due to the transition of5D0→7F2, while the 679, 688 and 690 nm fluorescence emissions originates from the transition of5D0→7F4, which is presented by curveband curvecin Fig.3. Additionally, it is noticed that little changes have been occurred in the profile of emission band that locates at 592 nm. A further research reveals that the emission band is composed of four sub-bands, where the three stronger sub-bands located at 587, 589 and 591 nm originate from the5D0→7F1transition of Eu3+ions in LaF3host, while the weak band located at 586 nm derives from the4G5/2→6H7/2transition of Sm3+ions in LaF3host. This result can be observed more clearly by the enlarged graph shown in the inset of Fig.3. Considered that the 442 nm laser can not effectively excite the Eu3+ions doped in LaF3host, it can be concluded that the characteristic Eu3+-emissions of LaF3∶Sm3+/Eu3+nanocrystals originate from the energy transfer from Sm3+to Eu3+ions. The emission spectrum of LaF3∶Eu3+sample under the excitation at 532 nm is shown by curvebin Fig.3. Combined curvebwith the curvec, it can be seen that the positions of fluorescence emissions of Eu3+sole-doped nanocrystals and Sm3+/Eu3+co-doped ones are coincident with each other very well. The experimental results mentioned above confirm that the characteristic fluorescence emissions of Eu3+ion in LaF3∶Sm3+/Eu3+samples are attributed to the energy transfer from Sm3+to Eu3+ions.
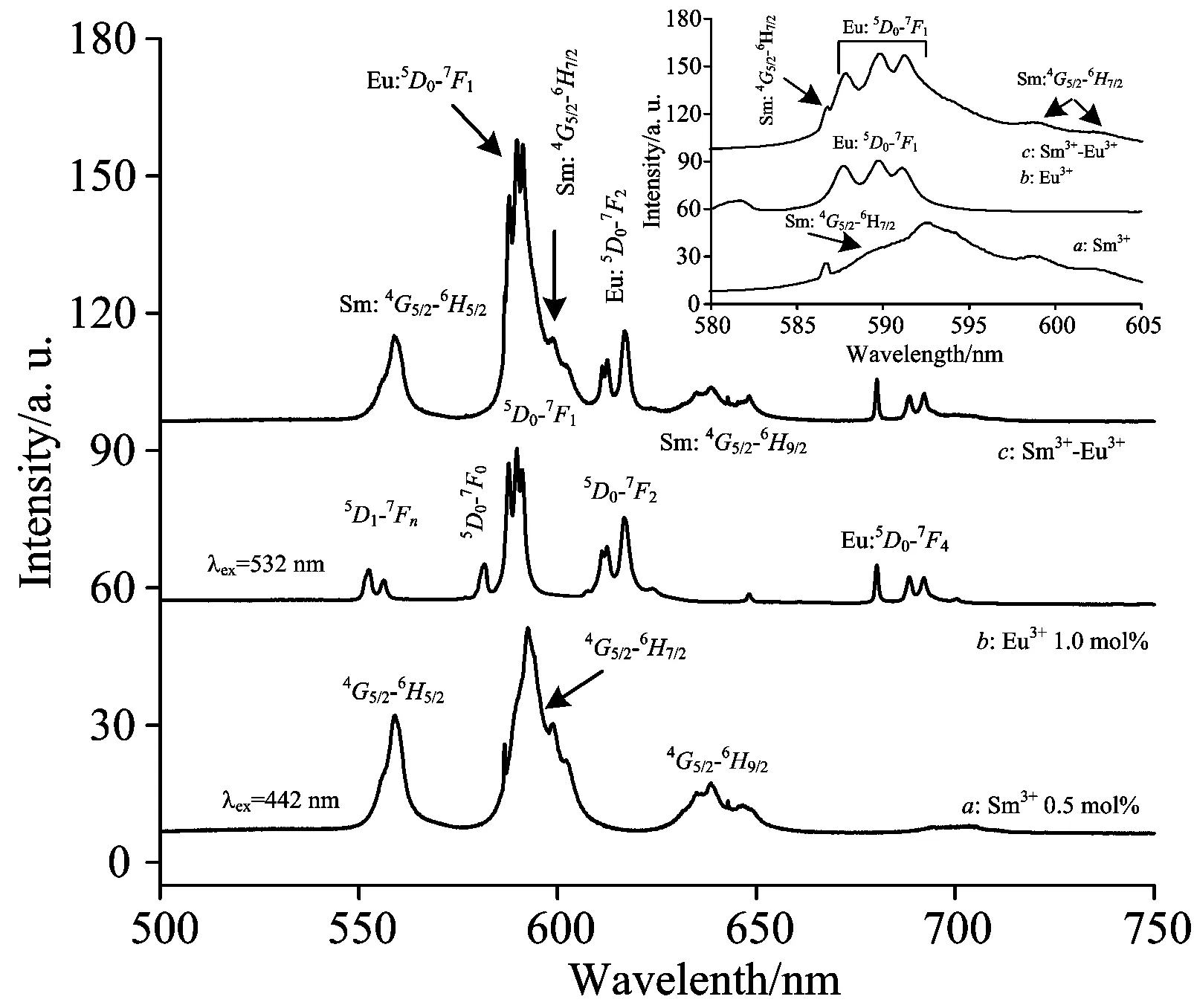
Fig.3 Emission spectra of Sm3+/Eu3+
2.2 Analysis on energy transfer mechanism
The new fluorescence bands in the emission spectrum of LaF3∶Sm3+/Eu3+nanocrystals under the excitation at 442 nm is attributed to the transition from the excited state5D0to the ground state7FJ(J=0~6). The mechanism of the energy transfer and related relaxation process can be obtained through the analysis on the specific energy level structures and the transition processes of Sm3+and Eu3+ions. Initially, the Sm3+ions populated at the ground state are pumped to the excited4G9/2state when the LaF3∶Sm3+/Eu3+nanocrystals are excited by the 442 nm CW laser. The Sm3+ions at the4G9/2state will relax quickly to populate at the lower level4G5/2through a nonradiative way due to the small energy space between4F3/2state and other adjacent ones. Then part of ions at4G5/2state will relax to the6HJ(J=5/2, 7/2, 9/2, 11/2, 13/2, 15/2) lower states to give off the fluorescence emissions of Sm3+ion. Four fluorescence emission bands of Sm3+ions were measured and recorded in the experimental measurements. The central wavelengths of the four bands are located at 558, 592, 638 and 701 nm corresponding to the transition of4G5/2→6H5/2,4G5/2→6H7/2,4G5/2→6H9/2and4G5/2→6H11/2, respectively. Meanwhile, the effect of energy transfer will occur in LaF3∶Sm3+/Eu3+co-doped nano-system. Because the4G5/2state of Sm3+ion is very closed to the5D1,5D0states of Eu3+ion, the other Sm3+ions will be induced to transfer energy from Sm3+ion4G5/2state to Eu3+ion5D1and5D0states. Then the Eu3+ions at5D0states relax to the lower states7FJ(J=0~6) to produce 581, 589, 616, 648, 680 and 705 nm fluorescence emissions due to the transitions of5D0→7F0,5D0→7F1,5D0→7F2,5D0→7F3,5D0→7F4and5D0→7F5, respectively. It is noticed that the strongest fluorescence emission band is around 589 nm arising from the transition of5D0→7F1of Eu3+ions. Two stronger fluorescence emission bands locate at around 612 and 680 nm due to the transitions of5D0→7F2and5D0→7F4, respectively. The fluorescence emission bands of 581 nm (5D0→7F0) measured in Eu3+sole-doped LaF3nanocrystals can not be observed in the emission spectrum of LaF3∶Sm3+/Eu3+nanocrystals, which are plotted by curveband curvecin Fig.3. This phenomenon suggests that the transition of5D0→7F0of Eu3+ion is restrained by introducing the co-doping Sm3+ion. The mechanism of energy transfer and related transitions in LaF3∶Sm3+/Eu3+co-doped system is shown in Fig.4.
2.3 Concentration effect of spectroscopic properties
It is known that the efficiency of energy transfer base on donor and acceptor is more dependent on the doping concentration of the acceptor ion. Therefore, the influence of Eu3+doping concentration on the energy transfer effect is only investigated in this section because it has much greater advantage than that of Sm3+ion in LaF3∶Sm3+/Eu3+nanocrystals. Keeping the doping concentration of Sm3+-donor fixed at 0.5 mol%, the fluorescence emission spectra of LaF3∶Sm3+/Eu3+nanocrystals with various Eu3+doping concentration were observed and recorded, where the doping concentrations of Eu3+-acceptor are 1.0 mol%, 1.5 mol% and 2.0 mol%, respectively. The fluorescence emission spectra of LaF3∶Sm3+/Eu3+nanocrystals with different Eu3+doping concentrations under the excitation at 442 nm laser is shown in Fig.5. The fluorescence emission bands located at 589, 616 and 680 nm arising from the transitions of5D0→7F1,5D0→7F2and5D0→7F4were selected to investigate, as plotted by curvea,bandcin Fig.5. It can be seen that the intensities of the fluorescence emissions at 589, 616 and 680 nm become more stronger with the increase of the Eu3+doping concentration. Therefore, the efficiency of the energy transfer can be effectively improved by increasing the doping concentration of Eu3+acceptor. The reason may be that the amount of the Eu3+ions around Sm3+ion increases with the increase of Eu3+doping concentration, which enhances the possibility of energy transfer from Sm3+to Eu3+because there are more Eu3+ions that can accept the energy from Sm3+donor in LaF3∶Sm3+/Eu3+sample. Some literatures have reports that the quenching concentration of Eu3+ion in LaF3host is more than 2.0 mol%[16-17]. Consequently, the enhancement of the energy transfer possibility will lead to the increase of the intensity of Eu3+fluorescence emission when the Eu3+doping concentration is less than its quenching concentration. Additionally, for observing more clearly, the enlarged graph of the 589 nm emission bands are shown in the inset of Fig.5.
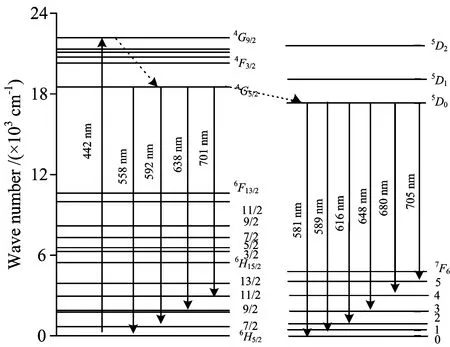
Fig.4 Mechanism of energy transfer from Sm3+ to Eu3+ ions
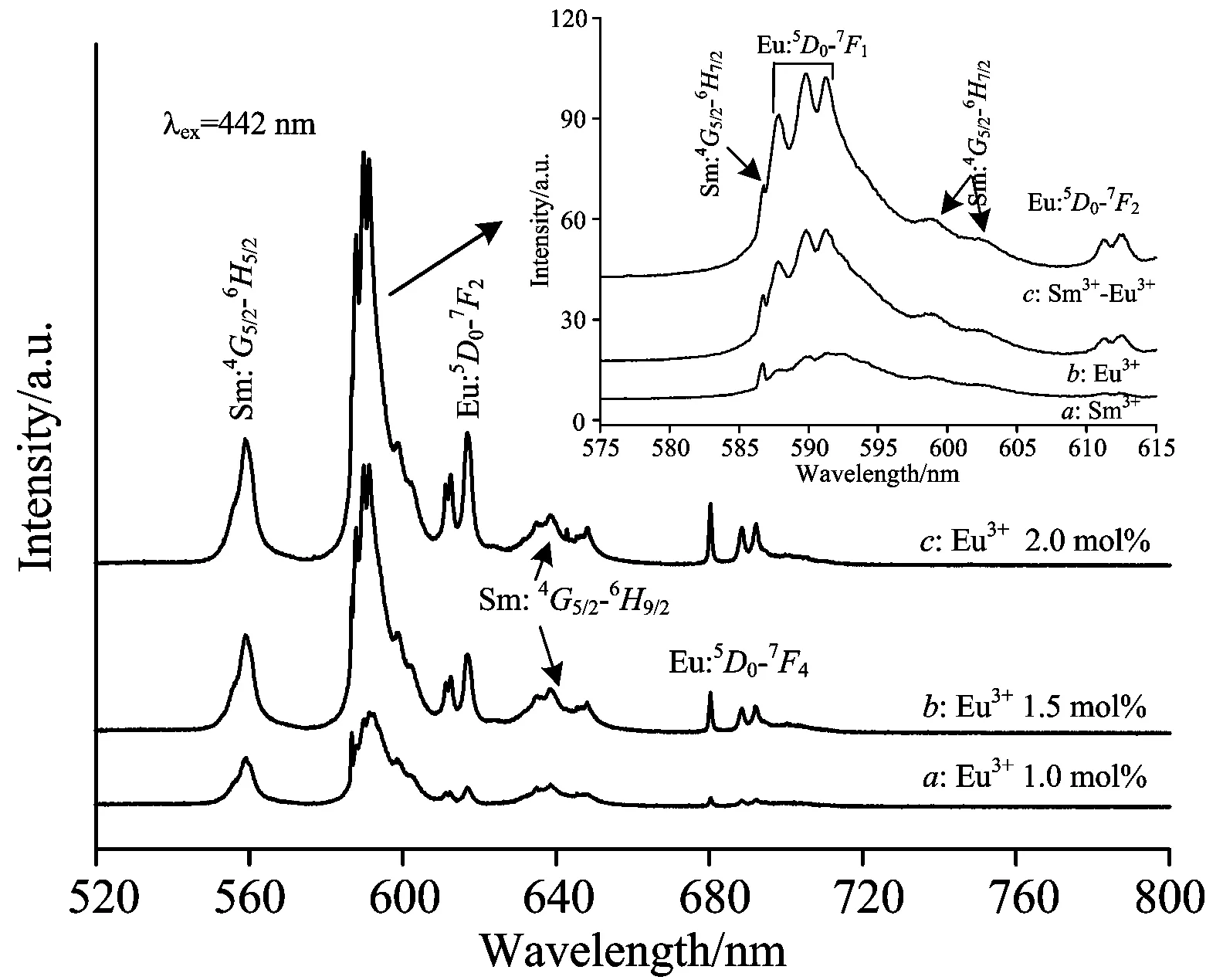
Fig.5 Relation of fluorescence spectra
3 Conclusions
Sm3+, Eu3+sole- and co-doped LaF3nanocrystal samples has been achieved with the hydrothermal method and their crystallographic phase, surface morphology and spectroscopic properties have been characterized by XRD, TEM and PL techniques. With a 442 nm CW laser as the excitation source to pump the Sm3+ions doped in LaF3∶Sm3+/Eu3+nanocrystals, the characteristic fluorescence emissions of Eu3+ions was observed, which is the results of the energy transfer from Sm3+to Eu3+ions. The mechanism and dynamical process of the energy transfer in LaF3∶Sm3+/Eu3+nanocrystals are investigated and analyzed. The results show that the observed energy transfer from Sm3+to Eu3+ions in LaF3∶Sm3+/Eu3+nano-system is attributed to the cross-relaxation between the4G5/2state of Sm3+ions and the5D1and5D0states of Eu3+ions. The further spectroscopic research reveals that the intensities of the characteristic fluorescence emissions originating from the energy transfer effect increase with the increase of the doping concentration of Eu3+acceptor.
[1] Yang Fu, Liu Yufeng, Tian Xiaodong, et al. Journal of Solid State Chemistry, 2015, 225: 19.
[2] Xie Mubiao, Zeng Lihua, Zhou Xiaoping, et al. Solid State Sciences, 2015, 39: 6.
[3] Sun Jiayue, Di Qiumei, Cui Dianpeng. Materials Research Bulletin, 2014, 60: 201.
[4] Zhu Daoyun, Mu Zhongfei. Displays, 2014, 35(5): 261.
[5] Ratnam B V, Jayasimhadri M, Jang Kiwan. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 132(11): 563.
[6] Tong Yiping, Yan Jiayan. Spectroscopy and Spectral Analysis, 2014, 34(12): 3210.
[7] Zhang Xinguo, Chen Yibo, Zeng Suiwen, et al. Ceramics International Part A, 2014, 40(9): 14537.
[8] Yan Fan, Yihua Hu, Li Chen, et al. Physica B: Condensed Matter, 2014, 450: 99.
[9] Naresh V, Rudramadevi B H, Buddhudu S. Journal of Alloys and Compounds, 2015, 632.
[10] Zhang Feng, Zhang Weifeng, Zhang Zhiya, et al. Journal of Luminescence, 2014, 152: 160.
[11] Hachani S, Moine B, El-akrmi A, et al. Journal of Luminescence, 2010, 130(10): 1774.
[12] Min Xin, Fang Minghao, Huang Zhaohui, et al. Optical Materials, 2014, 37: 110.
[13] Fu Zhenxing, Ma Lun, Sahi Sunil, et al. Journal of Luminescence, 2013, 143: 657.
[14] Wang G Q, Gong X H, Lin Y F, et al. Optical Materials, 2014, 37: 229.
[15] Wang Zhijun, Li Panlai, Yang Zhiping, et al. Journal of Luminescence, 2014, 151: 170.
[16] Pi Daibo, Wang Feng, Fan Xianping, et al. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2005, 61(11-12): 2455.
[17] Meng Jianxin, Zhang Maofeng, Liu Yingliang, et al. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2007, 66(1): 81.
O482.3
A
LaF3纳米晶体中Sm3+/Eu3+能量传递效应的光谱学特性
伏振兴1, 刘碧蕊1, 杨秉雄2
1. 宁夏师范学院物理与电子信息工程学院, 宁夏 固原 756000
2. 宁夏大学物理电气信息学院, 宁夏 银川 750021
采用水热合成法, 在较低的温度下制备了分散性, 均匀性良好的LaF3∶Sm3+, LaF3:Eu3+和LaF3∶Sm3+/Eu3+纳米晶体样品。 通过X射线衍射(XRD), 透射电子显微镜(TEM)和光致发光(PL)等手段, 分别对Sm3+/Eu3+单掺和共掺LaF3纳米晶体的物相, 表面形貌, 晶粒尺寸和荧光特性进行了表征。 XRD和TEM检测结果显示, 所制备的LaF3纳米晶体呈六方晶体相, 平均粒径在40 nm左右。 当采用波长为442 nm的He-Cd连续激光器激发Sm3+/Eu3+共掺LaF3样品中的Sm3+时, 在样品发射光谱中观测到了Eu3+的特征荧光发射谱线, 实现了Sm3+向Eu3+的能量传递。 采用光谱学研究方法讨论了能量传递的机理和效率。 结果表明, 能量传递过程是Sm3+的4G5/2激发态与Eu3+的5D1和5D0激发态之间的交叉驰豫所致, 并且随着Eu3+的掺杂浓度的增大, 共掺LaF3∶Sm3+/Eu3+样品的发射谱中的Eu3+的特征荧光发射强度也随之增强, 这说明增加受主Eu3+的掺杂浓度能够有效地提高Sm3+→Eu3+能量传递的效率。
荧光光谱; LaF3纳米晶体; Sm3+/Eu3+; 能量传递
2015-03-06,
2015-07-19)
2015-03-06; accepted: 2015-07-19
National Natural Science Foundation of China (11174190), Natural Science Foundation of Ningxia Province (NZ13208), Scientific Research in C&U (NGY2013112) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars (2014-486-4) of Ningxia Province, and the Project of Scientific Research of Ningxia Normal University (NXSFZD1513)
10.3964/j.issn.1000-0593(2016)08-2686-06
Biography: FU Zhen-xing, (1972—), Professor, Ph. D., Ningxia Normal University e-mail: zxfuo@sohu.com
——《光谱学与光谱分析》已全文上网
——《光谱学与光谱分析》已全文上网
——《光谱学与光谱分析》已全文上网
- 光谱学与光谱分析的其它文章
- Natural Bond Orbitals (NBO), Natural Population Analysis,Mulliken Analysis of Atomic Charges of L-Alaninium Oxalate
- A Novel Method to Monitor Coal Fires Based on Multi-Spectral Landsat Images
- Study on Spatial Distribution and Seasonal Variations of Trace Metal Contamination in Sediments from the Three Adjacent Areas of the Yellow River Using HR-ICP-MS
- Study on the Detection of Rice Seed Germination Rate Based on Infrared Thermal Imaging Technology Combined with Generalized Regression Neural Network
- 小型球形脉冲氙灯光谱分析与实验测定
- 基于深对流云目标的风云二号可见光通道辐射定标

