Biology and treatment of cervical adenocarcinoma
Satoshi Takeuchi
Department of Obstetrics and Gynecology,School of Medicine,Iwate Medical University,Iwate Prefecture 0208505,Japan
Abstract
Uterine cervical adenocarcinoma (ADC) has been increasing in its prevalence world widely despite the decrease of squamous cell carcinoma (SCC). It comprises nearly 20—25% of the all cervical malignancy in developed countries. The worse biological behavior had been reported in patients with intermediateand high risk factors after surgery,and in advanced stage over III,radiotherapy (RT) alone and concurrent chemo-radiotherapy (CCRT) with cisplatin was not always effective. As for chemotherapy (CT),the induction CT has not established,as well. Further molecular targeted therapy (MTT) has been studied. The targets of oncogenic driver mutations were vascular endothelial growth factor (VEGF) in SCC,or tyrosine kinase (TK) of endothelial growth factor receptor 2 (EGFR2,Her2/neu)-Ras-MAPK-ERK pathway. Bevacizumab (Bev,anti-VEGF monoclonal antibody) is considered as one of key agent with paclitaxel and carboplatin in SCC,but not for ADC. This article focuses on up-to-date knowledge of biology and possible specifi c therapeutic directions to explore in the management of cervical ADC.
Biology and treatment of cervical adenocarcinoma
Satoshi Takeuchi
Department of Obstetrics and Gynecology,School of Medicine,Iwate Medical University,Iwate Prefecture 0208505,Japan
Abstract
Uterine cervical adenocarcinoma (ADC) has been increasing in its prevalence world widely despite the decrease of squamous cell carcinoma (SCC). It comprises nearly 20—25% of the all cervical malignancy in developed countries. The worse biological behavior had been reported in patients with intermediateand high risk factors after surgery,and in advanced stage over III,radiotherapy (RT) alone and concurrent chemo-radiotherapy (CCRT) with cisplatin was not always effective. As for chemotherapy (CT),the induction CT has not established,as well. Further molecular targeted therapy (MTT) has been studied. The targets of oncogenic driver mutations were vascular endothelial growth factor (VEGF) in SCC,or tyrosine kinase (TK) of endothelial growth factor receptor 2 (EGFR2,Her2/neu)-Ras-MAPK-ERK pathway. Bevacizumab (Bev,anti-VEGF monoclonal antibody) is considered as one of key agent with paclitaxel and carboplatin in SCC,but not for ADC. This article focuses on up-to-date knowledge of biology and possible specifi c therapeutic directions to explore in the management of cervical ADC.
Keywords:Cervical cancer; adenocarcinoma (ADC); atypical glandular cell (AGC); gastric type adenocarcinoma (GAS)
Submitted Jan 26,2015. Accepted for publication Apr 15,2016.
View this article at: http://dx.doi.org/10.21147/j.issn.1000-9604.2016.02.11
Introduction
From the point of treatment,for cervical squamous cell carcinoma (SCC),especially in patients with stage IB2—IVA,concurrent chemo-radiotherapy (CCRT) with cisplatin has been gold standard from 2001,in Japan. From the point of treatment,for cervical SCC,especially in advanced stage IB2—IVA,CCRT with cisplatin has been gold standard from 2001. As for CCRT in patients with cervical adenocarcinoma (ADC),there is no prospective study. Some retrospective studies revealed poorer overall survival (OS)than that of SCC (Table 1). To improve the OS in CCRT some trials that concomitant agent changed with platinum and paclitaxl,or the subsequent CT with full dose paclitaxel and carboplatin (1,2).
It is well known that ADC of cervix has radio-resistant and chemo-resistant,in general sated bellow. Bevacizumab with TP therapy is the gold standard for cervical cancer,nowadays,for SCC the response was effective significantly,but not significant for cervical ADC. As for new agents,some new molecular targets were identifi ed by full genomic analyses with new generation sequencing (NGS). In order to conquer the disease,new strategy or development of new agents must be mandatory.
This article focuses on up-to-date knowledge of biology and possible specifi c therapeutic directions to explore in the management of cervical ADC.
Etiology and prevalence
In China,cervical cancer mortality rate was reported as 9.2 per 100.000 in 2007—2008 (3). In Japan,cervical cancer mortality was 4.1 per 100,000 in 2012—2013 (4). In the world,cervical cancer is responsible for 10—15% of cancer-related deaths in women worldwide (data for cervical cancer are available for 602,225 women. A total of 192 registries in 51 countries provided data for1995—1999,244 registries in 58 countries contributed data for 2000—2004,and 244 registries in 61 countries provided data for 2005—2009) (5). The percentage of cervical ADC wasincreasing in many countries especially in younger women than those in patient with SCC. In developed countries it accounts for 20—25% in all cervical cancer and it accounts for 22% in 2012 in Japan as well (6,7).
In China,even in patients with early stages (stage IB—IIB),a retrospective study of 255 patients (including 36 patients of non-squamous histology) revealed a poor prognosis of ADC.
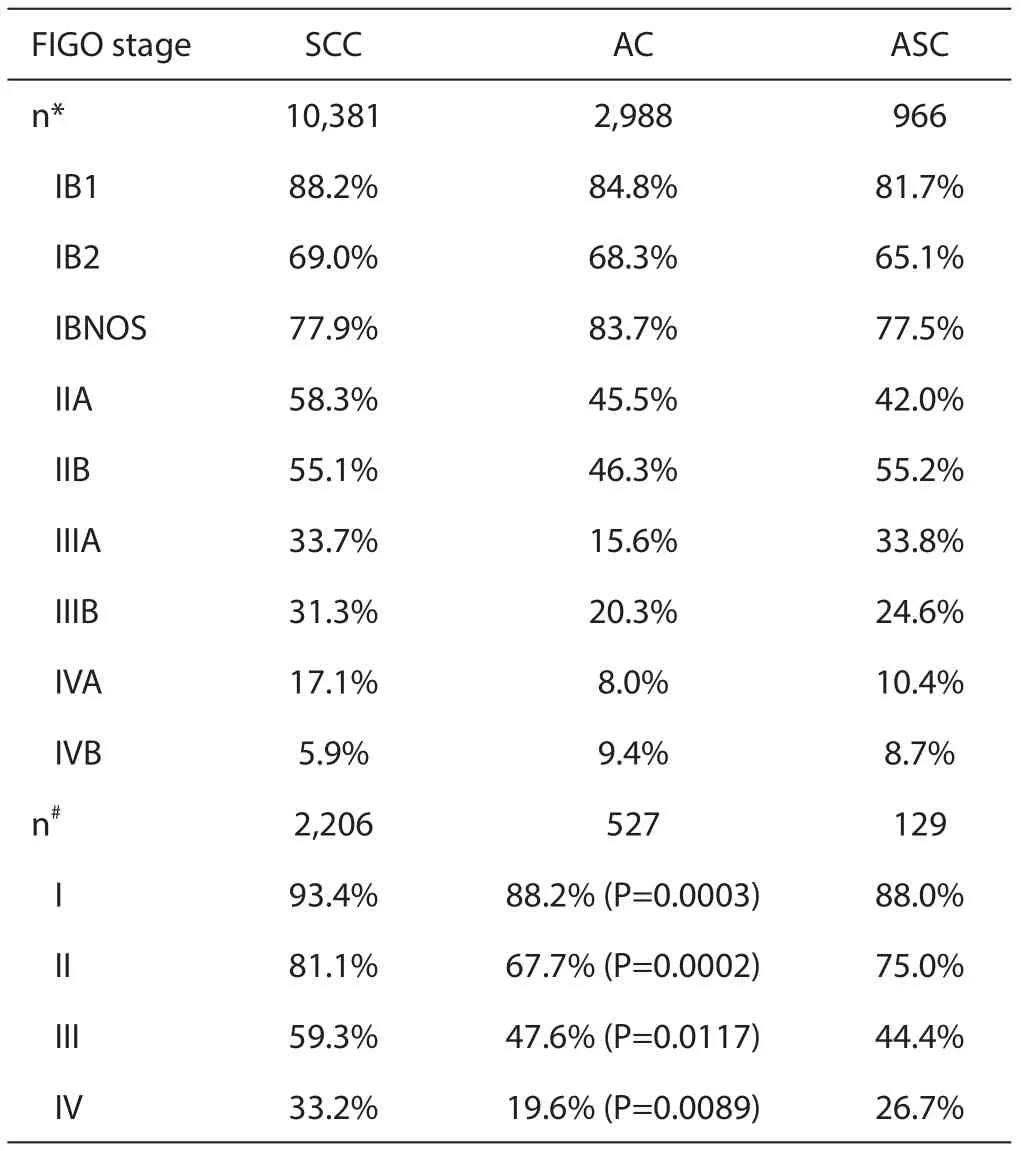
Table 1 5-year survival in cervical cancer between subtypes,such as squamous,adenocarcinoma,and adenosquamous type
Biology of cervical adenocarcinoma (ADC)
Recently,genomic alteration of cervical carcinoma (SCC and ADC) was demonstrated by whole exon sequence in 79 SCC and 24 ADC including 79 transcriptome sequence and 14 whole genome sequence. The somatic mutations were detected in PIK3CA,PTEN,TP53,STK11,ERBB2 and KRAS in SCC,ELF3,PIK3CA,ERBB2,STK11,and CBFB were detected in ADC (8). Further precise evaluation will be necessary about the co-relation between the gene mutations for targeting therapy.
Pathogenesis and classifi cation
The oncogenesis of ADC has not been elucidated in precise. In speculation,HPV 18 and 16 and their proteomes thought to be responsible for oncogenesis with estrogens which stimulate the endometrioid type’s and endocervical type’s proliferation,but recently new entity of gastric type adenocarcinoma (GAS) was detected and its prognosis is worst among subtypes of ADC (9). GAS,which driver gene has not been elucidated,had somatic gene mutations such as ERBB2 (Her2/neu2),k-Ras,PIK3CA,STKII,ELF3,and CBFB (8).
On the other hand,Harvard group,Wright et al. (10)reported the comparison of the spectrum of cancer-related gene mutations in the two main subtypes of cervical cancer ADC and SCC.
They found that 31 percent of the samples had PIK3CA mutations; 17.5 percent of the ADC (and non-SCC) had KRAS mutations; and 7.5 percent of the SCC (but none of the ADC) had a rare mutation in the gene EGFR. Patients with the ADC subtype of cervical cancer may benefit from targeted agents known as MEK inhibitors,which have shown some success in clinical trials (9). In one case of ADC,Ojesina et al. revealed EGFR mutation but,Wright et al. reported no case of EGFR mutation in their data set. There might unknown heterogeneity of EGFRs’dimerizations.
Among them,STK11 was suspected as a responsible gene mutation of GAS.
Germ line mutations in this gene have been associated with Peutz-Jeghers syndrome (PJS). Patients with PJS have a cancer risk in cervix,such as lobular endocervical glandular hyperplasia (LEGH),or minimal deviation adenocarcinoma (MDA) and mutinous ADC,which depends on STK11 mutation (11).
STK11 (LKB1) is a member of serine/threonine kinase family and regulates cell polarity and suppresses tumor genesis via adenine monophosphate-activated protein kinase (AMPK) which is member of PI3K-mTOR pathway. Activation of AMPK by SKT11 suppresses tumor growth and proliferation when energy and nutrient levels are scarce. Activation of AMPK-related kinases by LKB1 plays vital roles maintaining cell polarity thereby inhibiting inappropriate expansion of tumor cells.
WHO 2014 classification has involved GAS as new subtype of ADC which had SKT11 mutation (WHO 2014) (12).
As for cytology,the expression of atypical glandular cells (AGC) by PAP smear test such as AGC is unstable and the subsequent re-examination or endocervical curettage reveals often negative,and further precise absolute examination must be excisional conization or hysterectomy.
Clinical risks
It is controversy whether subtype of histology such as ADC had poor prognosis in some randomized clinical trial (RCT) in some setting of trials (1). In general point of view,there were few RCTs in this field of disease. From some retrospective trials the prognostic factors had been extracted and clinical risks were selected as intermediate-high risk or high risk (Table 2).
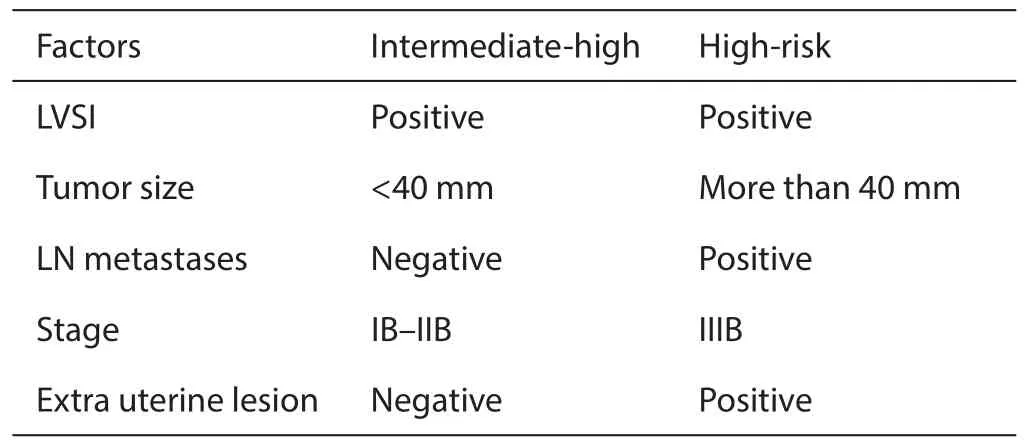
Table 2 Risk factors of adenocarcinoma of cervix
Intermediate-high risks thought to be lymph vascular space invasion (LVSI) (13,14),tumor size no less than 40 mm (13,14),cervical muscle-layer invasion less than 1/3 (14).
High risk had lymph-nodes metastases (13-15),positive of surgical margin (13),distal metastases (13),vaginal metastases (14).
Issues of diagnosis
Cytology and histology
The issue of diagnosis depends on the location of foci. If the foci located near squamous-columnar junction (SCJ),it is easy to biopsy,but most of the cases the abnormal lesion located deep in glands along with narrow cervical canal. The macroscopic observation is often impossible even if endoscopy. From this issue,even if blush-taken cytology,or endocervical curettage,it is very difficult to diagnose AIS,or micro invasive ADC. Only frank invasive ADC serves easily the specimen diagnosed malignant.
Minimal deviation ADC (adenoma malignum: MDA)and its related endocervical tumors such as lobular glandular endocervical hyperplasia (LEGH),atypical LEGH,and ADC in situ including endocervical dysplasia had been classified in 2014 WHO classification. The differential diagnosis by cytology or endocervical curettage was very difficult because of its location of disease exists deep in endocervical gland generally. Only conization (excisional conization) or extirpation of uterus revealed by negative for HPV18 analysis,HIK1038 staining,p16 ink4 immunoblotting,mib-1 (Ki-67)staining,MUC6,and carbonic anhydrase type IX (16-19).
Issues of imaging
Magnetic resonance imaging (MRI) is a high-resolution diagnostic modality of tissue (water concentration)-dependent quality in tumors. Comparing the T1,T2 (conventional images),weighted images,the feature of the tumor (both cystic or solid) was depicted specific signal intensities as low to high deserved serous to mucinous (according to viscosity: free degree of proton spins). Furthermore,using enhancer of A gadolinium with diethylenetriaminepentaacetate (Gd-DTP) the nature of blood flow (arterial phase),blood dissemination (capillary phase) and blood drainage (venous return phase) was depicted as an angiography,the consume rate of blood in the foci and around the micro environment of the tumor would be elucidated (dynamic MRI).
Diffusion weighted image (DWI) was depended on proton diffusion in magnetic gradient. DWI positive images were often obtained in malignancy.
The images of minimal deviation of cervical ADC and its related tumors was depicted accumulation of small to large sized high signal intensity on T2,and low on T1,lesions at deep cervical stroma. It is called cosmos flower sign (20).
Issues of treatment
Surgery (radical hysterectomy)
Up to stage IB (FIGO stage),Korea group reported aretrospective study as a difference between ADC and ADSQ,in factors of clinical risk. ADC and ADSQ showed that mean size of tumor and percentage of LVSI were 2.3 vs. 2.7 mm (P=0.019) and 14.7% vs. 29.2% (P=0.008). Both recurrent disease and death of the disease were 15.3% vs. 12.5% (P=0.47),and 18.1% and 14.7% (P=0.56),respectively. Five-year recurrent free disease was 85% vs. 89%,respectively,and it was no significant difference (21).
Yamauchi et al. reported a retrospective study for early stage of cervical ADC,as well. Ninety one ADC and 364 SCC were enrolled and the prognosis of ADC was poorer than that of SCC significantly (P=0.001). As for prognostic factors,LVSI (P=0.008),stromal invasion (P=0.024) and adnexal metastasis (P=0.032) were the shorter survival by multivariate analyses in ADC (22).
Pelvic exenteration
Pelvic exenteration has an important role in the treatment of advanced or recurrent cervical cancer to obtain a complete cure or longer survival. Pelvic exenteration comprises of three types that is anterior exenteration,posterior exenteration and total exenteration. Reconstruction is necessary,i.e.,urinary tracts re construction for anterior exenteration,rectal anastomosis for posterior exenteration,and both for total exenteration (23-26).
Laterally extended endopelvic resection (LEER)
Hockel (27) and Caceres et al. (24) reported the efficacy of LEER. To demonstrate the therapeutic potential of the (LEER),phase 2 study was conducted especially for patients with recurrent cervical carcinomas involving the side wall of an irradiated pelvis. These patients,suffering from the most common situation of local failure,have so far no longer been considered for curative therapy. The procedures in general were as follows: extending the lateral resection plane of pelvic exenteration to the medial aspects of the lumbosacral plexus,sacrospinous ligament,acetabulum,and obturator membrane enables the complete removal of a subset of locally advanced and recurrent tumors of the lower female genital tract fi xed to the pelvic wall with free margins (R0).
Thirty-six patients with recurrent (n=29) or primary advanced (n=7) gynecologic malignancies involving the side wall of the lesser pelvis underwent LEER from July 1996 until October 2002. The majority of the patients suffered from cervical carcinoma (n=29) and had received previous pelvic irradiation (n=24). Tumor-free (R0) lateral margins were obtained in 34 patients. Severe postoperative complications occurred in 14 patients with one treatmentrelated death (TRD). Five-year survival probability is 49% for the whole group and 46% for those patients considered only for palliation with current treatment options. Most patients without evidence of disease at least 1 year after LEER achieved good quality of life (24). Fourteen treatment derived severe adverse events (including one TRD) deserves nearly 40% of the cases. The comparisons of survivals were shown in Table 3. It might be reduced under 5.0% by using new energy devices or MRI-guided operation in near future.
Surgery and adjuvant radiotherapy (RT)
A retrospective study of stage IB—IIA cervical cancer with adjuvant RT showed 83.7%,66.5% and 79.6%,in 5-year survival of SCC,AD,ADSQ,respectively. ADC was signifi cantly poor prognosis compared to SCC (P<0.0001) (14).
From Japanese study,Shimada et al. reported a retrospective study adjuvant RT after radical hysterectomy,comparing the efficacy of RT in different histology. Eight hundred and twenty patients were enrolled (280ADC and 540SCC). Among them 139 ADC and 327 SCC patients were received adjuvant RT.
The histological type did not affect the outcome for patients with stage I disease. However,in stage II disease,ADC was significantly worse prognosis than SCC. Patient with SCC exhibited significantly higher lymph node involvement in stage IB,but not in IB2 and II. Among patients with lymph node involvement patients with ADC exhibited a significantly worse 5-year survival rate compared to those with SCC (46.4% vs. 72.3%,respectively,P=0.0005). Among the patients receiving the adjuvant RT,those with ADC showed higher recurrence rate of central recurrence (pelvic and stump) than those with SCC (24.6% vs. 10.5%,P=0.0022). As for distant recurrence and paraaortic recurrence there was no diff erence between histological subtypes (2).
Okazawa et al. reported a retrospective study in patients with stage IB1—IIB for efficacy of CCRT vs. RT as an adjuvant therapy for intermediate-risk or high-risk. Both PFS and OS of CCRT were superior to those of RT.
Issues of chemotherapy (CT)
Bevacizumab with TP therapy is the gold standard forcervical cancer,nowadays,for SCC the response was effective significantly,but not significant in cervical ADC. As for CCRT in patients with cervical ADC,only few prospective studies were reported. Some retrospective studies revealed poorer OS than that of SCC. To improve the OS in CCRT some trials that concomitant agent changed with platinum and paclitaxel,or the subsequent CT with full dose paclitaxel and carboplatin.
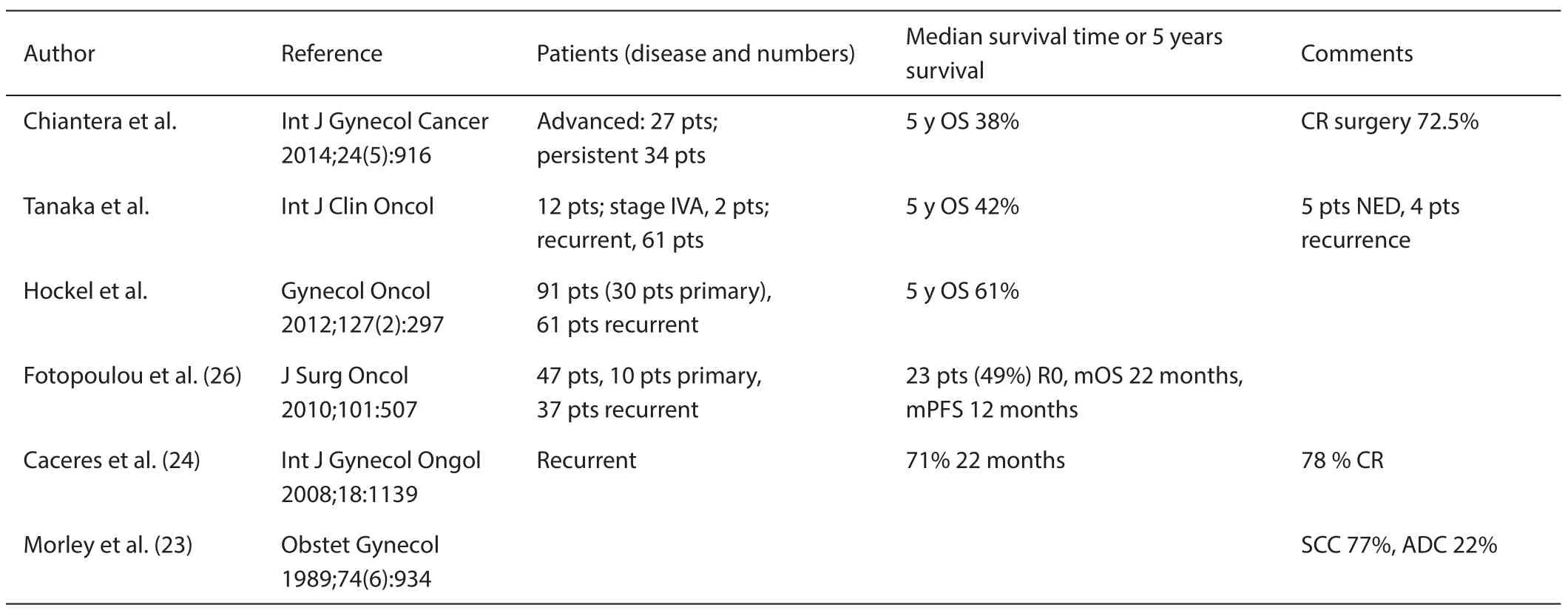
Table 3 Comparison of survival times pelvic exenteration and LEER
Systemic chemotherapy (CT)
From ESMO Clinical Practice Guideline (28),CT for advanced or metastatic/recurrent cervical cancer is palliative. Cisplatin is considered as the single most active cytotoxic agent. Only cisplatin combination doublet which had OS advantage than cisplatin single agent was cisplatin + topotecan regimen,but no significant difference among cisplatin + paclitaxel,cisplatin + gemcitabine,cisplatin + vinorelbine,and cisplatin + topotecan. Carboplatin had an advantage in lower adverse event such as nephrotoxicity Japanese Clinical Oncology Group (JCOG) trial of the RCT (JCOG0505) revealed no inferiority to cisplatin + paclitaxel regimen (29,30).
Issues of adjuvant chemotherapy (CT) and CCRT
Shimada et al. reported a retrospective study of cervical ADC in comparing adjuvant RT (including CCRT) and adjuvant CT. It revealed favorable 5-year survival in CT rather than RT (+ CCRT) such as 79.2%,70.9%,respectively. RT followed by CT group had worst 5-year survival such as 66.2% (31). Chinese study of 424 pts revealed adjuvant CT had longer recurrence free survival time (15).
Issues of neo-adjuvant chemotherapy (NACT)
He et al. reported a meta-analysis of 2 RCTs and 9 observational studies about the efficacy of NACT in different histological types of cervical cancer. The results were as follows: the short-term efficacy estimated by response [complete response (CR) + partial response (PR) or CR alone] was no difference between SCC and non-SCC,and in RCTs higher response in SCC than that of non-SCC. Long-term outcome of NACT,SCC had a significant longer 5-year OS (HR=1.47,95% CI,1.06—2.06) and PFS (HR=1.96,95% CI,1.61—2.38) than those of non-SCC. It became more obvious if the stage in IIB in subgroup analysis (HR=2.06,95% CI,1.79—2.36),but stage IB—IIB it became no significant (HR=1.33,95% CI,0.99—2.38). In conclusion,in early stages such as IB and IIB,there was no signifi cant diff erence in OS between SCC and non-SCC (32). Italian group,Landoni et al.reported a retrospective study of eff i cacy of neo-adjuvant CT followed by radical hysterectomy. The optimal responders by NACT and surgery in FIGO stage IB—IIB cervical cancer do not need any further treatment,and for patient with suboptimal response and intra-cervical residual disease,additional cycles of CT would be of benefi t (33).
ARCT of CCRT with CDDP vs. CDDP and tirapazamine (TPZ) in patients with advanced ADC revealed no superiority of additional TPZ. Three-year PFS for the TPZ/CIS/RT and CIS/RT arms were 63.0% and 64.4% (log-rank P=0.7869). Three-year OS for the TPZ/CIS/RT and CIS/RT arms were 70.5% and 70.6%,respectively (log-rank P=0.8333) (34).
Tang et al. reported in patients with stage IIB—IVA cervical ADC a randomized phase III study comparing CCRT alone vs. one cycle of paclitaxel + cisplatin (TP)therapy before CCRT followed by two cycles of TP therapy. The disease free survival of CCRT alone and CCRT with adjuvant CT were 60.4% vs. 71.4% (P<0.05). Recurrence were 39.6% vs. 28.6% (P<0.05),respectively. OS were about 60% vs. 73% (P<0.04,log-rank test: data were not elucidated in true value,only Kaplan-Meier curves were demonstrated) (35).
Poujade et al. studied about neo-adjuvant (NAC) CCRT for stage IB2 to IIIB ADC of the cervix,and revealed the pathological CR was obtained 33% of the patients. Prognostic factors of residual viable tumor were patients with post menopause,parametrial invasion (PMI),LVSI,and mucinous subtype (36). A total of 66.7% of patients treated CCRT had residual disease at least. Shibata et al. reported 26% of pathological CR after NAC CCRT with 70 mg/m2cisplatin and 700 mg/m25-FU 5 days continuous infusion. A total of 74% had residual viable tumors,as well (37). Here we can find the limitation of CCRT with cisplatin.
For advanced stage,Katanyoo et al. reported retrospective study for the treatment outcomes in patients with advanced (stage IIB—IVA) ADC of cervix compared with the same stage-matched SCC of cervix. It revealed that the significant worse CR rate of ADC than that of SCC (P=0.004). Complete pathological response rates were 42.1% and 73.7% in ADC and SCC,respectively,it seemed less effective in ADC,as well. As for CCRT,used agents were cisplatin alone,carboplatin alone,and carboplatin with 5-FU,so more precise information such as dosage and course number was not shown,37% of the patients were treated such CCRT (38).
Radiotherapy (RT) and CCRT
Adeno- and adenosquamous carcinomas of the cervix are associated with worse OS when treated with radiation alone but with similar progression-free and OS compared to SCCs of the cervix when treated with cisplatin based chemoradiation (1).
In Japan,Niibe et al. reported that the stage IIIB cervical ADC treated with RT alone revealed 20.2% in 5-year survival (39).
In Asia,from Taiwan,retrospective study of CCRT for stage IB—IIB cervical cancer was performed and ADC/ADSQ (n=21) showed worse 5-year progression free survival (PFS)(30.0% vs. 47.6%,P=0.044),worse 5-year distant metastasisfree survival (41.5% vs. 69.9%,P=0.005),and trends toward worse 5-year local recurrence-free survival (64.4% vs. 76.2%,P=0.165),and worse 5-year OS (41.3% vs. 58.1%,P=0.090)than patients with SCC (n=109) (40).
In summary,it is very difficult to diagnose in precancerous neoplasia such as AIS or LEGH by cytology or curettage. AGC in Bethesda system is one of trial of screenings for early diagnosis with the measurement of CAIX,or HPV18,p16INK4,and Ki-67. It has been a GOG0237 study which has been on going,would be useful to improve the diagnostic accuracy.
Oncogene mutation would be detected by cytology in future and it would be useful to make diff erential diagnosis between usual ADC between GAS.
New entity of GAS was chemo-resistant rather than usual ADC. If the ADC were able to diagnose in early stage,surgical treatment with or without CCRT is selected,which are eff ective for early stages in treatment of ADC.
Which is better CCRT or RT alone as adjuvant RT?The answer has not been determined. Now,we are accruing GOG 263 trial,the RCT of RT alone or CCRT with cisplatin for intermediate risk in patients with stage IB-IIA cervical cancer. The result of GOG263 would be informative first randomized study as for adjuvant CCRT in patients with intermediate-risk of cervical cancer.
For advanced,metastatic,and recurrent disease,there was no effective treatment except for exenteration and LEER.
CCRT was the standard treatment without distant metastasis but it was not necessary appropriate treatment. Sometimes further therapy such as CT or extended RT was necessary. To investigate more effective treatments,neoadjuvant systemic chemotherapy (NACT),or following systemic CT (paclitaxel + carboplatin) was tried. RCT hasnot been performed yet.
Possibility of molecular targeted therapy (MTT) for ADC
ADC specific gene mutations were detected by Genome Wide Association Study. From the two datasets,ADC specific gene mutations were ELF3,CBFB,STKII,and Kras mutations. Both SCC and ADC had ERBB2,P53and PIK3CA mutations. From these knowledges,new targeted therapy would be planed.
Conclusions
Few RCTs were conducted and some improvements were demonstrated in this disease,but the prognoses in advanced patients were still poorer. Further randomized study will be mandatory including new molecular targeted agents.
Acknowledgements
None.
Footnote
Confl icts of Interest: The author has no confl icts of interest to declare.
References
1. Rose PG,Java JJ,Whitney CW,et al. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol 2014;135:208-12.
2. Shimada M,Nishimura R,Nogawa T,et al. Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU Intergroup Surveillance. Mol Clin Oncol 2013;1:780-4.
3. Nguyen TL,Nguyen DC,Nguyen TH,et al. Surveybased cancer mortality in the Lao PDR,2007-08. Asian Pac J Cancer Prev 2011;12:2495-8.
4. Annual Cancer Registry Japan. Mortality of female malignancy adjusted age. 2013,National Cancer Institute Central Hospital; bio statistics and information: Tokyo. Available online: http://ganjoho.jp/reg_stat/statistics/dl/ index.html
5. Jemal A,Bray F,Center MM,et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
6. Tumor committee of Japan Society of Obstetrics and Gynecology (JSOG),The registry of gynecologic malignancy. Acta obst et gynaecol Japonica 2013;64:1078-97. Available online: http://fa.kyorin.co.jp/ jsog/readPDF.php?file=68/3/068031117.pdf
7. Glandular carcinomas of thecervix,related tumors and their precursors. In: Clement PB,Young RH,editors. Atlas of Gynecologic Surgical Pathology. Philadelphia,PA: WB Sauders,2000:109-31.
8. Ojesina AI,Lichtenstein L,Freeman SS,et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014;506:371-5.
9. Kojima A,Mikami Y,Sudo T,et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 2007;31:664-72.
10. Wright AA,Howitt BE,Myers AP,et al. Oncogenic mutations in cervical cancer: genomic diff erences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer 2013;119:3776-83.
11. Kobayashi Y,Masuda K,Kimura T,et al. A tumor of the uterine cervix with a complex histology in a Peutz-Jeghers syndrome patient with genomic deletion of the STK11 exon 1 region. Future Oncol 2014;10:171-7.
12. Kurman RJ,Carcangiu ML,Herrington S,et al,editors. WHO Classifi cation of Tumours of Female Reproductive Organs. Lyon: IARC Press,2014.
13. Matsuo K,Mabuchi S,Okazawa M,et al. Utility of riskweighted surgical-pathological factors in early-stage cervical cancer. Br J Cancer 2013;108:1348-57.
14. Noh JM,Park W,Kim YS,et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13-10). Gynecol Oncol 2014;132:618-23.
15. Wang H,Zhu L,Lu W,et al. Clinicopathological risk factors for recurrence after neoadjuvant chemotherapy and radical hysterectomy in cervical cancer. World J Surg Oncol 2013;11:301.
16. Mikami Y,Kiyokawa T,Sasajima Y,et al. Reappraisal of synchronous and multifocal mucinous lesions of the female genital tract: a close association with gastric metaplasia. Histopathology 2009;54:184-91.
17. Kawauchi S,Kusuda T,Liu XP,et al. Is lobular endocervical glandular hyperplasia a cancerous precursorof minimal deviation adenocarcinoma?: a comparative molecular-genetic and immunohistochemical study. Am J Surg Pathol 2008;32:1807-15.
18. Mikami Y,Minamiguchi S,Teramoto N,et al. Carbonic anhydrase type IX expression in lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Pathol Res Pract 2013;209:173-8.
19. Pirog EC. Diagnosis of HPV-negative,gastric-type adenocarcinoma of the endocervix. Methods Mol Biol 2015;1249:213-9.
20. Takatsu A,Shiozawa T,Miyamoto T,et al. Preoperative diff erential diagnosis of minimal deviation adenocarcinoma and lobular endocervical glandular hyperplasia of the uterine cervix: a multicenter study of clinicopathology and magnetic resonance imaging fi ndings. Int J Gynecol Cancer 2011;21:1287-96.
21. Baek MH,Park JY,Kim D,et al. Comparison of adenocarcinoma and adenosquamous carcinoma in patients with early-stage cervical cancer after radical surgery. Gynecol Oncol 2014;135:462-7.
22. Yamauchi M,Fukuda T,Wada T,et al. Comparison of outcomes between squamous cell carcinoma and adenocarcinoma in patients with surgically treated stage I-II cervical cancer. Mol Clin Oncol 2014;2:518-24.
23. Morley GW,Hopkins MP,Lindenauer SM,et al. Pelvic exenteration,University of Michigan: 100 patients at 5 years. Obstet Gynecol 1989;74:934-43.
24. Caceres A,Mourton SM,Bochner BH,et al. Extended pelvic resections for recurrent uterine and cervical cancer: out-ofthe-box surgery. Int J Gynecol Cancer 2008;18:1139-44.
25. Mourton SM,Sonoda Y,Abu-Rustum NR,et al. Resection of recurrent cervical cancer after total pelvic exenteration. Int J Gynecol Cancer 2007;17:137-40.
26. Fotopoulou C,Neumann U,Kraetschell R,et al. Longterm clinical outcome of pelvic exenteration in patients with advanced gynecological malignancies. J Surg Oncol 2010;101:507-12.
27. Höckel M. Laterally extended endopelvic resection. Novel surgical treatment of locally recurrent cervical carcinoma involving the pelvic side wall. Gynecol Oncol 2003;91:369-77.
28. Colombo N,Carinelli S,Colombo A,et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii27-32.
29. Saito I,Kitagawa R,Fukuda H,et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB,persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Jpn J Clin Oncol 2010;40:90-3.
30. Kitagawa R,Katsumata N,Shibata T,et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol 2015;33:2129-35.
31. Shimada M,Nishimura R,Hatae M,et al. Comparison of adjuvant chemotherapy and radiotherapy in patients with cervical adenocarcinoma of the uterus after radical hysterectomy: SGSG/TGCU Intergroup surveillance. Eur J Gynaecol Oncol 2013;34:425-8.
32. He L,Wu L,Su G,et al. The eff i cacy of neoadjuvant chemotherapy in diff erent histological types of cervical cancer. Gynecol Oncol 2014;134:419-25.
33. Landoni F,Sartori E,Maggino T,et al. Is there a role for postoperative treatment in patients with stage Ib2-IIb cervical cancer treated with neo-adjuvant chemotherapy and radical surgery? An Italian multicenter retrospective study. Gynecol Oncol 2014;132:611-7.
34. DiSilvestro PA,Ali S,Craighead PS,et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2,IIA,IIB,IIIB,and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J Clin Oncol 2014;32:458-64.
35. Tang J,Tang Y,Yang J,et al. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol 2012;125:297-302.
36. Poujade O,Morice P,Rouzier R,et al. Pathologic response rate after concomitant neo-adjuvant radiotherapy and chemotherapy for adenocarcinoma of the uterine cervix:a retrospective multicentric study. Int J Gynecol Cancer 2010;20:815-20.
37. Shibata K,Kajiyama H,Yamamoto E,et al. Eff ectiveness of preoperative concurrent chemoradiation therapy (CCRT) for locally advanced adenocarcinoma of cervix. Eur J Surg Oncol 2009;35:768-72.
38. Katanyoo K,Sanguanrungsirikul S,Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol 2012;125:292-6.
39. Niibe Y,Kenjo M,Onishi H,et al. High-dose-rate intracavitary brachytherapy combined with external beam radiotherapy for stage IIIb adenocarcinoma of the uterine cervix in Japan: a multi-institutional study of Japanese Society of Therapeutic Radiology and Oncology 2006-2007 (study of JASTRO 2006-2007). Jpn J Clin Oncol 2010;40:795-9.
40. Chen JL,Huang CY,Huang YS,et al. Diff erential clinical characteristics,treatment response and prognosis of locally advanced adenocarcinoma/adenosquamous carcinoma and squamous cell carcinoma of cervix treated with defi nitive radiotherapy. Acta Obstet Gynecol Scand 2014;93:661-8.
Cite this article as: Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res 2016;28(2):254-262. doi: 10.21147/j.issn.1000-9604.2016.02.11
Correspondence to: Satoshi Takeuchi,MD,PhD. Department of Obstetrics and Gynecology,School of Medicine,Iwate Medical University,19-1 Uchimaru Morioka,Iwate Prefecture 0208505,Japan. Email: stakeuch@iwate-med.ac.jp.
doi:10.21147/j.issn.1000-9604.2016.02.11
 Chinese Journal of Cancer Research2016年2期
Chinese Journal of Cancer Research2016年2期
- Chinese Journal of Cancer Research的其它文章
- Risk prediction models for hepatocellular carcinoma in diff erent populations
- Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma
- Comparison of the short-term and long-term outcomes of laparoscopic hysterectomies and of abdominal hysterectomies: a case study of 4,895 patients in the Guangxi Zhuang Autonomous Region,China
- Phase I study of chimeric anti-CD20 monoclonal antibody in Chinese patients with CD20-positive non-Hodgkin’s lymphoma
- Clinical and functional comparison of endoprosthetic replacement with intramedullary nailing for treating proximal femur metastasis
- Evolution of radical hysterectomy for cervical cancer along the last two decades: single institution experience
