Smallscale biodieselsynthesis from waste frying oiland crude methanol in Morocco
Fatiha Ouanji*,Marouane Nachid Mohamed KacimiLeonarda F.Liotta ,Fabtizio Puleo ,Mahfoud Ziyad 2
1 UniversitéMohammed V,Faculté des Sciences,Département de Chimie,Laboratoire de Physico-chimie des Matériaux et Catalyse(URAC26),Avenue Ibn Battouta,BP.1014,Rabat,Morocco
2 Hassan IIAcademy ofScience and Technology,Rabat,Morocco
3 Istituto per lo Studio deiMaterialiNanostrutturati(ISMN)-CNR,via Ugo La Malfa,153,90146 Palermo,Italy
1.Introduction
The biodieselis a mixture offatty acid methylesters(FAMEs).At the same time as ithas already proved its ef ficiency in urban transportation,its usage willcontinue to increase because of its renewability[1–4].It can be mixed with fossil fuels or used pure in diesel engines without any transformation[5].On the other hand,the biodieselrepresents a good approach to the problem ofgreenhouse gas emission.In Morocco,it willbe a way to introduce sustainable development in the ruralworld by using biodieselin water pumping for irrigation,heating,cooking and electricity production[6–8].Waste frying oils are daily generated in large quantities;therefore,their usage instead ofedible oils willlower the cost ofbiodieselproduction and in environment preservation[9–11].
Morocco is presently making huge efforts to acquire wind and solar farms in order to increase the part ofrenewable energies in its energetic mix.Politicaldecisions were taken so thatin 2020 the renewable energies representaround 42%of the globalenergy balance.On the otherhand,the improvementofenergy ef ficiency which obviously involves technological means and conscientious societal behaviour will allow Morocco to achieve in 2020 energy savings of15%.The success ofallthese renewable energy programmes relies on several major development projects in which Morocco is investing money and hopes(Agricultural Project:Green Morocco,Moroccan Solar Energy Project,NationalPactfor Industrial Emergence).These programmes are expected to generate important quantities of waste fats and biomass that can be recycled into first and secondary biodiesels,respectively.
Usually,waste frying oils contain impurities thatimpact the yield of biodieselproduction.The most undesired impurities are the free fatty acids(FFAs)and water because they lead to the formation ofsoap and subsequently to a decrease of biodiesel quality.The waste frying oil must be filtered and washed and the free fatty acid content ought to be reduced to the lowest level(<0.5 wt%)[12–14].However,these treatments increase signi ficantly the cost ofbiodieseland are actually under intense investigations for optimization[15].The cost ofbiodiesel also depends greatly on the price of the reactants.It is then worthy to search for a compromise between the costs of the puri fication process,the oil and the alcohol that could economically encourage biodiesel commercialization in Morocco.
The present work is essentially devoted to low cost small-scale biodieselproduction by transesteri fication ofwaste frying oils with methanol.The syntheses were carried out aiming to develop ways that ef ficiently reduce the cost of biodieselproduction.The methanolto oil molarratio,the concentration ofcatalyst(KOH),and reaction temperature were investigated.Specialattention was given to the biodieselproduction at smallscale(40 L)for socialapplications such as heating and electricity production.The purity and the properties of the products were studied measuring their acid value,viscosity,density and fatty acid methylester(FAME)concentration using FTIR,UV–visible and1H NMR spectroscopies and gas chromatography.TGA–TDA techniques were highlighted for monitoring transesteri fication reaction.
2.Materials and Methods
2.1.Materials
In this study,the feedstock used forbiodieselproduction was gathered from Kilimanjaro Environment Company at Casablanca.It is recovered from traditional Moroccan pastry shops and fish frying restaurants.The oil was pretreated by centrifugation then filtered and dehydrated at 115°C.This oilwillbe designated in the following as used frying oil(UFO).
Two methanolsamples differing by their purity were used to verify the impactof the alcoholquality and coston the production ofbiodiesel.The firstone is an analysis grade methanol(AGM)from Fluka Company.Its purity is equalto 99.8%,and its density is 0.791–0.792 kg·L-1.The second called puri fied crude methanol(PCM)is for industrialpurposes.Its costin Morocco is ten times lower than thatofanalysis grade methanol.Prior to use,itwas re fluxed in the presence ofmagnesiumand distilled under vacuum over a molecular sieve.The purity was found to be equalto 96.6%with a density around 0.802–0.805.Both alcohols samples willbe designated in the following as AGMand PCM,respectively.The other reagents used such as KOH(Merck),phenolphthalein indicator(Merck),ethanol,diethyl-ether,acetic acid,hydrochloric acid,sulphuric and phosphoric acids were analysis grade products.For comparison,complementary experiments were performed in the same conditions with re fined oil(also called here re fined frying oil(RFO)).
2.2.Methods
2.2.1.Characterization techniques
The physicaland chemicalproperties such as viscosity,density,peroxide value,iodine number and acid value of the reactants and the produced biodiesel were determined by the conventional methods and compared with the European standards of biodiesel[16,17].The free fatty acids(FFAs)in the oils and the products were analysed using a titration method[18].
The fatty acid concentration in the RFO and UFOwas determined measuring their corresponding methylester contentby a PeriChrom 2000 gas chromatograph(GC)equipped with a flame ionization detector(FID)and a capillary column DB-WAX(30 m×0.32 mm,0.23μm film thickness).Pure Helium was used as a carrier gas(1.5 ml·min-1).The column temperature was fixed at 210°C and that of the injector and detector at 250°C.An autosampler equipped with an automatic injection system provided quite an acceptable reproducibility.The quanti fication of the synthesised methylesters was carried out using the standard calibration curves using puri fied biodieselsample.The composition offatty acid methylesters(FAMEs)ofeach sample was obtained dissolving a known weight ofsample in 5 mlofn-hexane and injecting 0.1–0.5 μlof the solution in the GC.
The biodieselyield was calculated using Eq.(1)[18]:
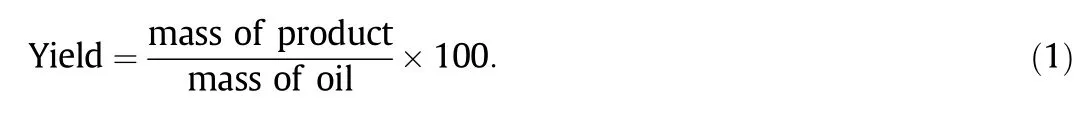
The1HNMR spectra were recorded at298 K with a pulse duration of 30°,a recycle delay of 1.0 s and 8 scans using a BRUKER AVANCE 300 MHz spectrometer.Deuterated chloroform(CDCl3)was used as a solvent.The13C NMR(75 MHz)spectra were obtained with a pulse duration of30°,a recycle delay of1.89 s and 160 scans.
Infrared spectraldata were collected in the 4000–650 cm-1range by co-adding 16 scans ata resolution of4 cm-1on a VERTEX 70 spectrometerequipped with an ATRMIRACLEDIAMANT technique.Spectra were recorded as absorbance values at each data point in triplicate.
Thermogravimetry analyses(TGA)were performed using a SHIMADZUTGA50H thermobalance.The oils and prepared biodiesel were completed with 5–15 mg ofsamples which was heated at a constant rate of 10 °C·min-1in an atmosphere of helium at a constant rate of 60 ml·min-1.The temperature range employed was 25–600 °C.TGA–TDA analyses are also used as an improved technique for monitoring the transesteri fication reaction.
2.2.2.Biodieselproduction
The biodiesellaboratory scale synthesis was performed according to the conventionalprocedure described in the literature[1–4].The oil,preheated at60°C wasadded with stirring to a solution ofKOHin methanolatdifferenttemperatures,and times ofreaction.The resulting mixture was stored for 8 h in a separating funnelto isolate glycerolfrom biodiesel.The unreacted alcohol and water were removed from the mixture by vacuum distillation using a BÜCHI Rotavapor R-114 equipped with a BÜCHIWater-bath B-48.The recovered methylester phase was puri fied using aqueous phosphoric acid solution(4%v/v)and water steam bubbling.The obtained liquid was dried at 80°C and stored before analysis.The reactions as wellas the washings were carried out in a systematic manner.
The low-cost smallscale biodieselproduction of40 L was undertaken in a home-made tank reactor.The production conditions are identical to those of the optimal lab-scale synthesis.The analyses of the reaction products were carried out with1H NMR and FT-IR spectroscopies and TGA–TDA analyses.Physico-chemicalcharacteristics of the synthesised biofuelwere evaluated using the standard methods.
2.3.Experimentaldesign
The impact of the raw materialpurity on the finalbiodieselwas investigated using severalbatches and series ofexperiments differing by the quality of the oiland the purity of the methanolsample.Table 1 reports the composition of the different batches.The transesteri fication reaction was performed in the same optimum conditions in each of the batch.The smallscale syntheses were performed on the cheapest mixture using UFO and PCM.

Table 1 Experimentaldesign:description of the batches
3.Results and Discussion
3.1.Analyses of the used frying oil(UFO)
3.1.1.Chromatographic analyses

Fig.1.Fatty acid methylester composition of UFO(a)and RFO(b).
The chromatograms of the RFOand UFOare presented on Fig.1.They show that the majority of fatty acids contained in RFO and UFO range between C14 and C24.The mostabundantone isthe linoleic acid methyl ester(C18:2)with percentages of 57.4%and 53.9%in RFO and UFO respectively(Table 2).Table 3 reports the viscosities,the densities,the acid values,the peroxide values and the iodine numbers of the RFO and UFO.The comparison of these results with the existing data in the literature con firms thatoilheating in the presence offood and water increases its contentoffree fatty acids engendered through the hydrolysis of the triglycerides[14,15].Acid value of the UFO remains above the critical FFA concentrations that would prevent the alkali-catalysed transesteri fication,but this acidity with a limit of applicability of3.5%FFA in the starting material,can lead to a satisfactory yield and a biodieselofgood quality[15].
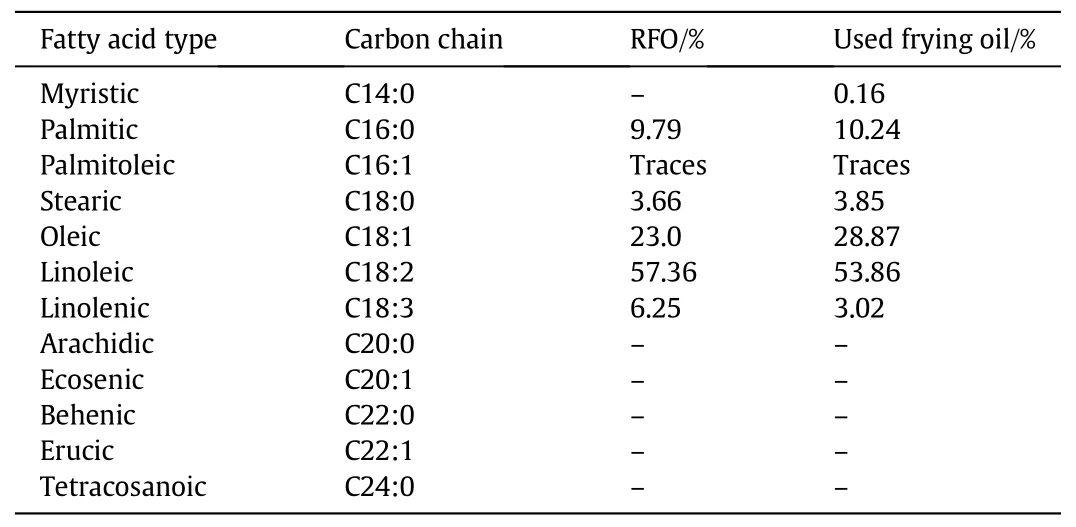
Table 2 Fatty acid methylester composition ofre fined fraying oiland used frying oils

Table 3 Physicalproperties ofre fined fraying oiland frying oils
3.1.2.NMR analyses of the oilsamples
The1H NMR spectra of the RFO and UFO are,as shown on Fig.2A,similar.The assignment of the chemicalshifts to different kinds ofhydrogens of fatty acyl chains is quite easy since the literature contains most of the needed data[19–22].The massifs appearing between 4 and 4.3 are attributed to –CH2–O– and the peak at 5.2 assigned to –CH–O groups of the triacylglycerols.These groups play an important role in1H NMR monitoring oftransesteri fication reaction[21].The absence ofsignals between 3.5 and 3.8 indicates that the oils do not contain methylesters[22].
Fig.2B displays13C NMR similarspectra for RFOand UFO.The signals appearing around 131.88 and 127.08 belong to the unsaturated carbons ofmethylesters.The peaks at 22–34 are attributed to methylene carbons of the long chains offatty acid methylesters.No typicalpeaks belonging to ester carbonyl(–COO–)and C–O appearing at 174.26 and 51.38 ppm respectively were observed[19].
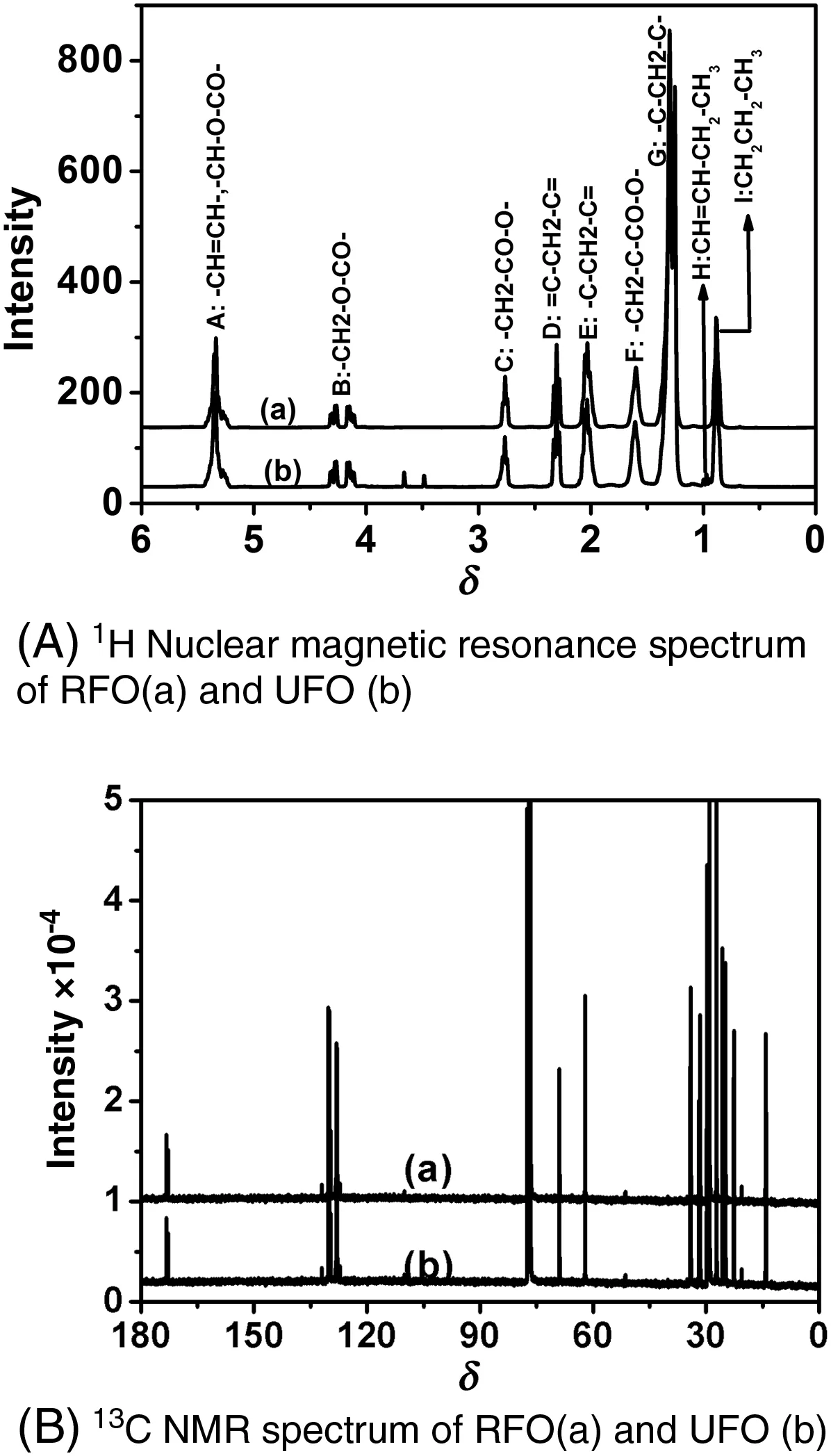
Fig.2.1H NMR and 13C NMR spectrum of RFO and UFO.
3.1.3.FTIR analyses
Fig.3 shows the FT-IR spectra of RFO and URO.The band located at 3007 cm-1is due to the stretching vibration of the cis ole finic CHdouble bond(cis C=CH).The bands at 2919.9 cm-1and 2853.7 cm-1are assigned to the symmetricaland asymmetricalstretching vibrations of the saturated carbon–carbon bond(–CH2-asymmetric and –CH2-symmetric).The band at 1737.8 cm-1is attributed to the stretching vibration of the C=O group of the triglycerides.The cis C=C bond gives rise to a smallband centred on 1652.2 cm-1.The bands at 1464.5 and 1377.5 cm-1resultfromthe bending vibrations of CH2and CH3aliphatic groups[23].The spectra also show peaks located at1236.91,1157.7 and 1090.86 cm-1.Some of them could be assigned to the stretching vibrations of the(C–O)ester group.The band near 724.7 cm-1is due to the overlapping of the CH2and the out-of-plane vibration CH wag of cis-di-substituted ole fins[23].
3.1.4.TGA-TDA analyses
Recently,it is worth mentioning that the TGA–TDA analyses can be used as a good method for the characterization of oils and biodiesel[24–27].It was even used in the calculation of conversion of the transesteri fication reaction for production ofbiodiesel[27],
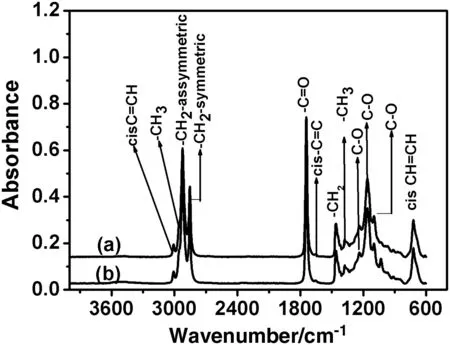
Fig.3.ATR IR-FT spectrum of RFO(a)and UFO(b).
Fig.4 reports the TGA results for RFO and UFO.No difference between the weight loss temperatures of these two compounds was observed.The mass of the oil samples starts to decrease approximately at 225°C,and it continues to decrease untilallthe oils present in the samples are vaporized at about 440°C.Also,the figure shows the DTG curves that con firm the similar degradation temperature of the two samples(RFOand UFO).However,very lower weightloss was observed for the UFO.Itcan be assigned to FFApresentin UFO.In orderto con firm this assignation,a TGA of the UFO acidi fied with oleic acid was performed(5%).The obtained curve shows a smallweightloss corresponding to the DTG peak at 295°C(Fig.4).Therefore,the first observed weight loss is due to the presence of the FFA.It can be concluded that TGA–DTG techniques can discriminate between the RFO and the UFO.But,supplementary investigations willbe necessary to further con firm this hypothesis.
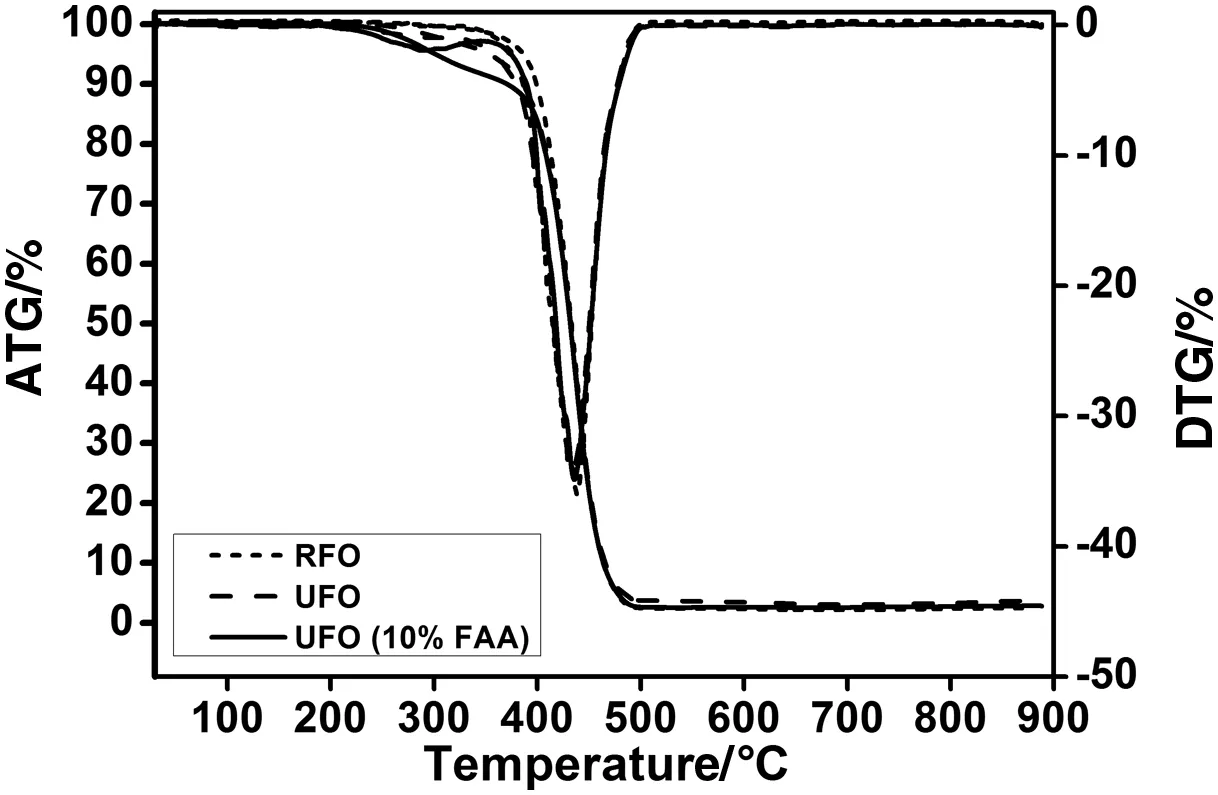
Fig.4.TGA–TDA analyses:comparison between RFO,UFO,and UFO(10%FAA).
3.2.Factors affecting the methylester yield
The in fluence of the catalystconcentration and oil/methanolratio on the yield of biodieselwas studied with the aim ofbalancing the acid value of the oils.For instance,methanol/oilratios exceeding 6 increase the amount of glycerol dissolved in the biodiesel and decrease the methylester yield without impacting negatively the quality of the biodiesel(as it willbe shown below)[28].Also,large amounts of the catalystfavourthe conversion offree fatty acids into soap.The quality of the oilemployed remains the key factor in the biodieselproduction.However,adjustments of reaction conditions to the feedstock properties are necessary to avoid the inconsistentyields often reported in the literature.The experimentalconditions for the transesteri fication must be adapted to the composition of the UFO to produce biodieselwith reasonable finalcost.
3.2.1.Effectof the reaction time
The effectoftransesteri fication duration on the biodieselproduction was investigated in the following conditions:1.2%ofKOH(based on the oilmass),a methanol/oilmolar ratio of6:1 and a reaction temperature of 65°C.Fig.5 shows a rapid increase of the biodieselyield in the first 15 min,whatever the oilor methanolquality is.After this increase,a decrease of the oiland methanolpurities results in a yield decrease that can be as high as 20%(Fig.5d)essentially due to soap production.If the transesteri fication is carried out utilising USO and methanol free of water then the degree of purity of the alcohol does not seem to play a crucialrole.Therefore,employing crude methanolreduces the cost and the yield of biodiesel but the price of the reactants is also lowered.Analysis grade methanol,for instance,is ten times more expensive than the crude methanolin Morocco.
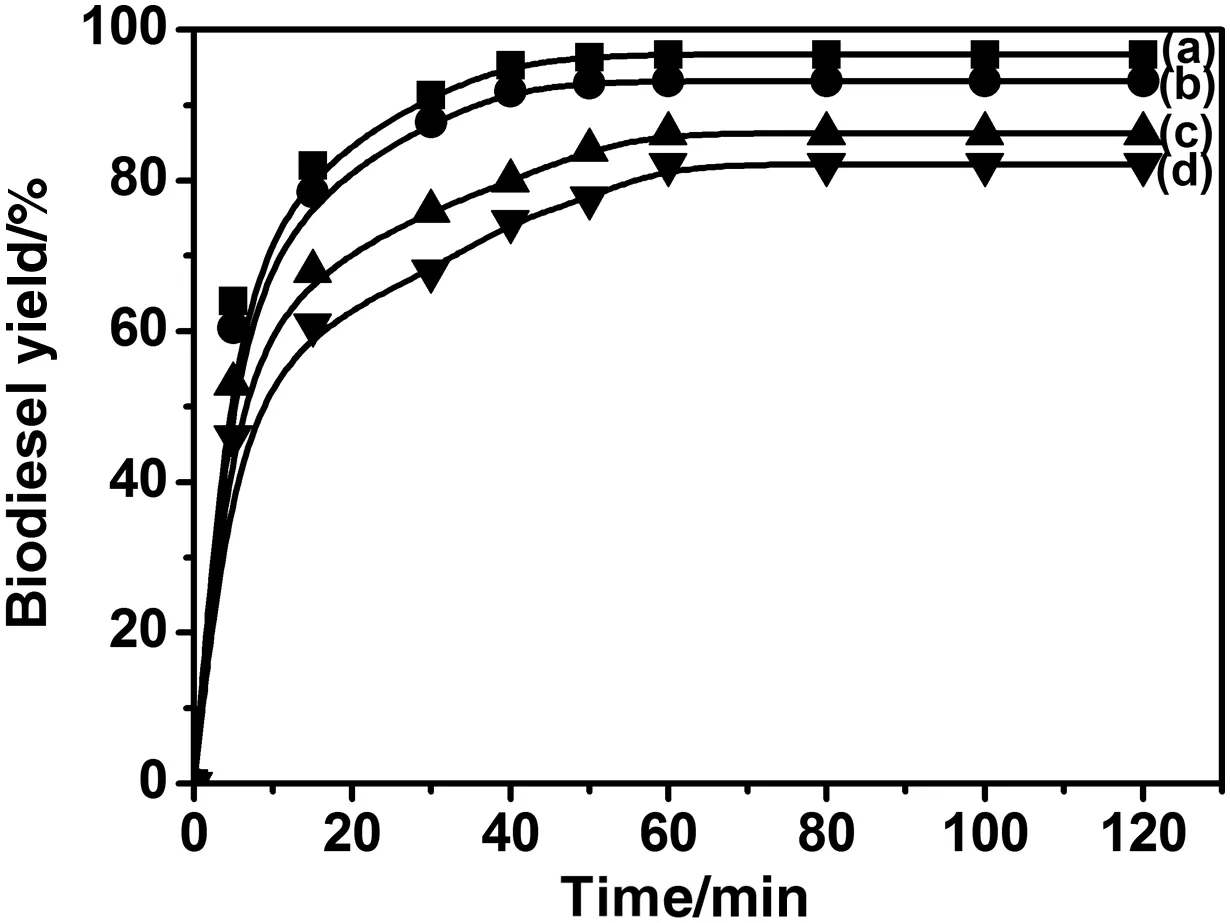
Fig.5.Effect of reaction time on biodieselyield:UFR/AGM(a),RFO/PCM(b);UFO/AGM(c);and UFO/PCM(d).(Reaction conditions:KOH 1.2 wt%,reaction temperature 60°C,reaction time 2 h and molar ratio 6:1.)
3.2.2.In fluence of the catalystconcentration
In fluence of the catalyst concentration was studied using KOH instead of NaOH because it can be recycled as a fertiliser or recovered as K3PO4[29].The results are exhibited in Fig.6.It clearly shows that the in fluence of the catalyst amounton the yield depends on the purity of the oil.In the case of UFO,the required amount for reaching the maximum yield is 1.2%.An overtaking of the catalyst results in a signi ficant decrease of the reaction yield because KOH favours then the saponi fication of the triglycerides[28].The results also indicate thatthe quality of water free methanolhas a less importantimpacton the biodieselyield than the oil.With RFO,the maximum yield is achieved for less than 1%of KOH.Therefore,the transesteri fication optimalconditions seem to depend essentially on the quality of the oiland the heating processes it went through.
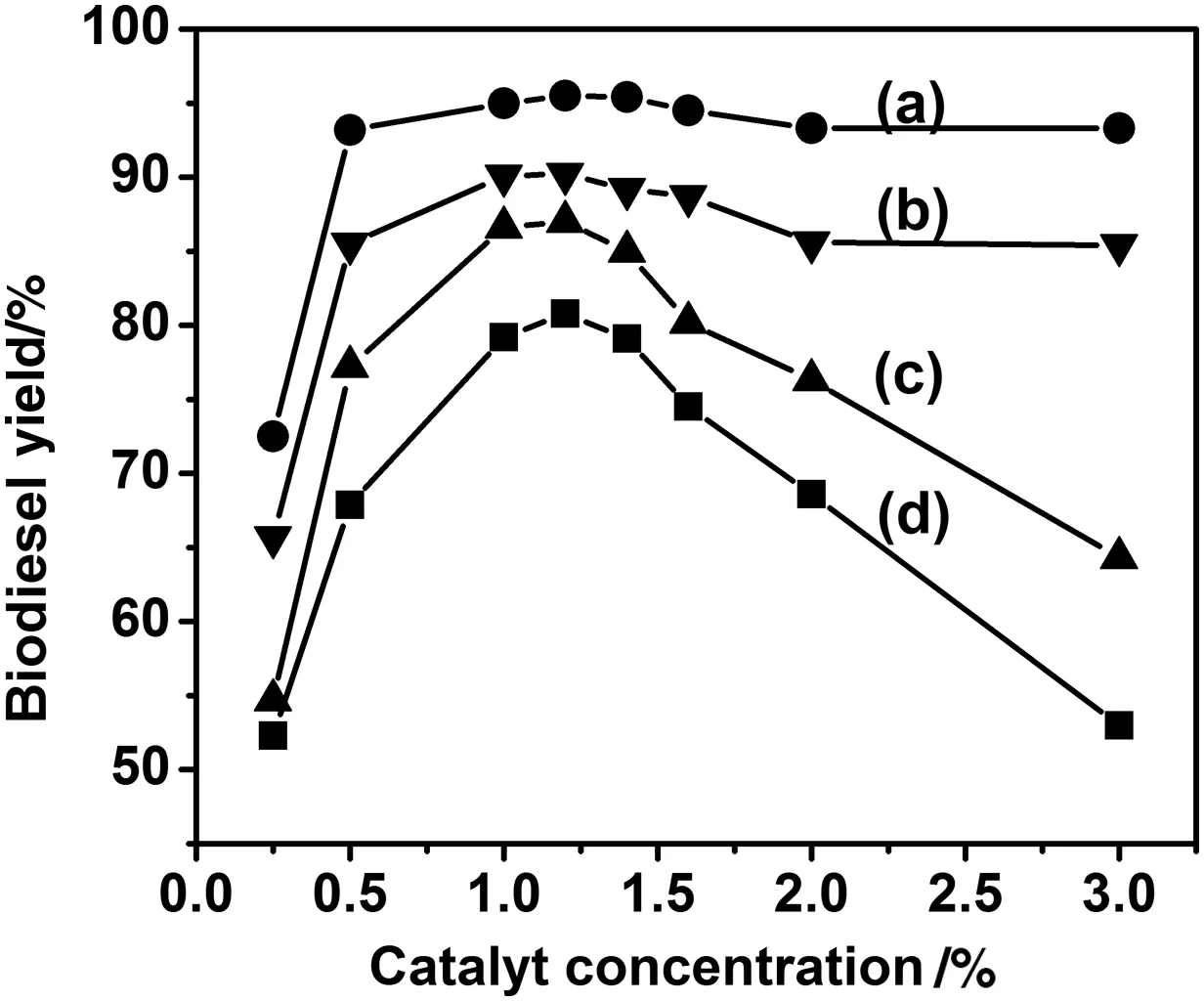
Fig.6.Effectofcatalystconcentration on biodieselyield RFO/AGM(a);RFO/PCM(b);UFO/AGM,(c);and UFO/PCM(d).(Reaction conditions:reaction temperature 60°C,reaction time 2 h and molar ratio 6:1.)
The literature is abundanton this subject,however,despite the numerous studies,the results stillsuffer from incoherence,probably because the composition of UFO is intimately related to the conditions of its use[30].In the following experiments,the amount of KOH was set at 1.2 wt%of oil.
3.2.3.In fluence ofmethanol/oilratio
Fig.7 shows the variations of biodiesel yield versus methanol/oil ratio for different batches.The increase of methanol/oilratio leads to an increase of the reaction yield due to the equilibrium shift towards the methylester production[26].Another possible interpretation of this result might be the lowering of the UFO viscosity by the excess of methanolthat increases the oilsolubility in the reaction mixture.As a consequence,the interactions between the molecules of the reactants are made easier,and the conversion increases.On the other hand,methanol/oilratio is the key factor in this study because of their in fluence on lowering the cost ofbiodiesel.
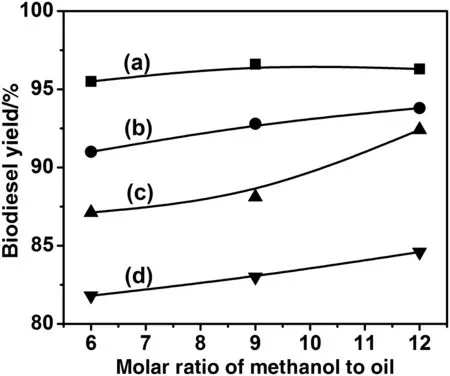
Fig.7.Effectmethanol/oilratio on biodieselyield:RFO/AGM(a);RFO/PCM(b);UFO/AGM(c);and UFO/PCM(d).(Reaction conditions:KOH 1.2 wt%,reaction temperature 60°C,reaction time 2 h.)
3.3.Physicaland chemicalproperties of the produced biodiesel
Table 4 displays a comparison of the produced biodieselwith the biodieselsynthesised according to the speci fic European standards.As previously shown,the biodieselyield is impacted by the presence in the oiloffree fatty acids and other impurities which mightlead to the formation ofsoap.Itis worthy to notice thatthe properties of the differentbatches ofbiodiesel(Table 4)prepared using low-costrawmaterials(UFOmixture and crude puri fied methanol)are nottoo farfrom those of biodieselobtained with RFO.The reaction yield is slightly lower(80.8%,batch d),butallthe otherparameters are as good as those of the biodieselobeying the European standards(Table 4).The standards presently in use were established for the existing dieselengines.Perhaps modi fications can be broughtto those engines and standards to lower the cost of biodieselproduction.
3.4.Biodieselanalyses
NMRis the appropriate technique for the characterization ofvegetable oils before and after their conversion into methyl esters.The transesteri fication progression and the quality of the finalproduct can be traced easily by this technique.The first1H NMR characterization was reported by Gelbard et al.[31].The authors used the protons of the methylene group adjacentto the ester moiety in the triacylglycerols and the protons in the alcoholmoiety of the methylesters to monitor the biodieselyield.Thus,NMR spectra can be used to evidence the biodieselquality.
Fig.8A displays the typical1H NMR spectrum of the low cost biodieselsample prepared in the following optimalconditions:1.2%ofKOH,reaction time=2 h at60°C and differentcrude methanol/oilratios(6,9and 12).The proton of the CH–O group of the glycerolusually appears at 5.3 but disappears at the end of the reaction.As a matter of fact,the1H NMR spectrum of the transesteri fication productdoes notshow any signals at4.0–4.5 assigned to the protons of the triacylglycerols.Biodiesel production can,therefore,be monitored through the decrease of these peaks.The strong singletat3.658 indicates the formation of the methoxy group ofmethylester(–CO2CH3).The signals at2.26 resultfrom the protons of CH2– groups adjacent to(–CH2–COO–CH3).The comparison of the areas ofthose peaks con firms that the transesteri fication reaction is complete in the chosen experimentalconditions.
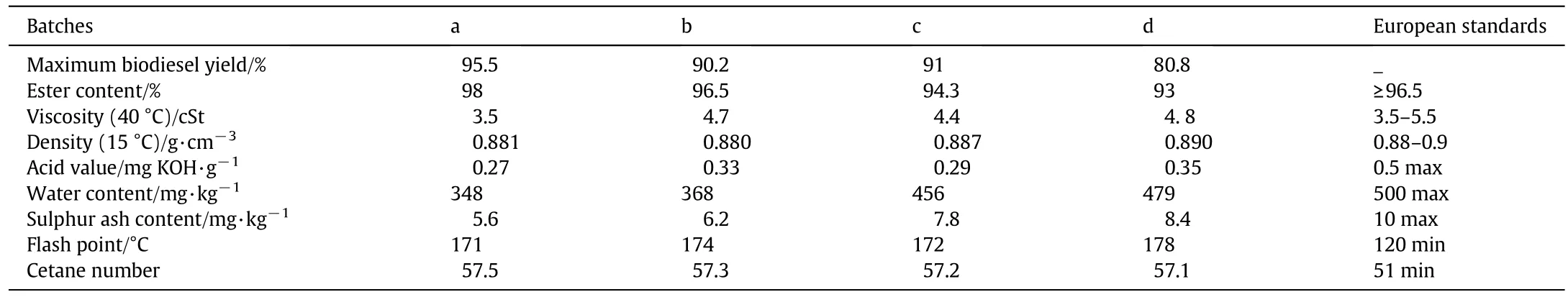
Table 4 Physicochemicalproperties ofbiodieselobtained from the different batches
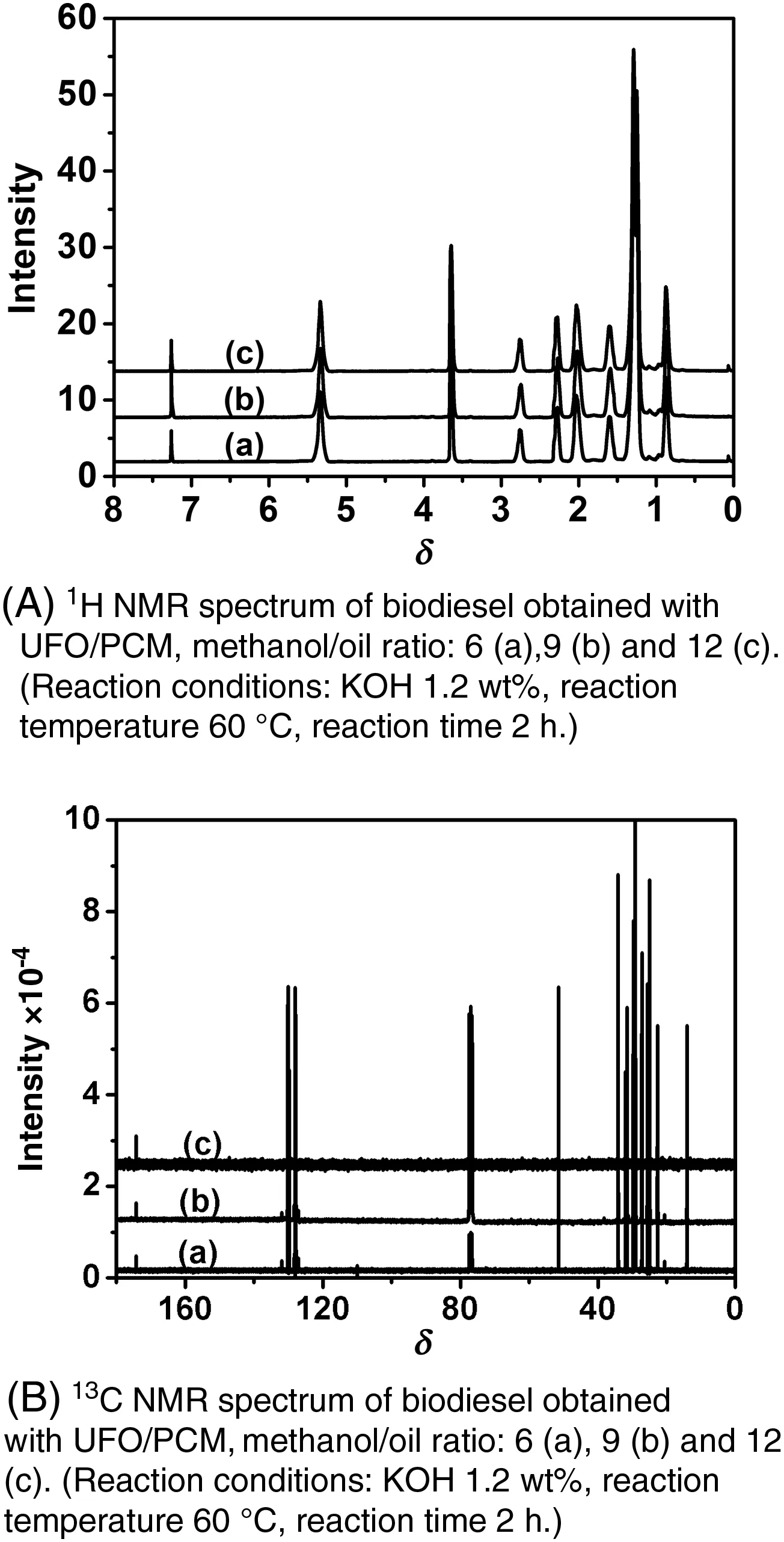
Fig.8.1H NMR and13C NMR spectrum ofbiodieselobtained with UFO/PCM.
The results also show that the catalyst concentration,the reaction temperature and the methanol/oil ratio play a more important role than that played by the quality of the used frying oiland the impurities introduced by the culinary habits.Therefore,it can be concluded that a biodieselofacceptable quality and reasonable price can be produced from the Moroccan UFO and a puri fied crude methanol.This result is also supported by the spectrum of the biodiesel synthesised using low-cost raw materials(UFO and PCM)as it has been reported in Table 1.The spectrum(Fig.8A),as shown by other authors,does not contain any dior monoglyceride suggesting thatthe conversion is completed[32–36].This was also con firmed by the13C NMR spectrum of biodieselobtained from used frying oil(Fig.8B),which displays typical peaks of ester carbonyl(–COO–)and C–O located at 174.26 and 51.38,respectively[32–34].The other signals appearing around 131.88 and 127.08 belong to the unsaturated carbons ofmethylesters.The peaks at 22–34 are attributed to methylene carbons of the long chains of fatty acid methyl esters.The results con firm that the conversion reaches 96.5%forallthe batches reported in Table 1.Practically,the produced biodieselmeets the internationalstandards(Table 4).The percentage of FAMEs is around 94%of the final product.The low yield obtained in batch d(Table 4)is essentially due to soap formation.This was con firmed by the FTIR analyses performed before and after the elimination ofsoap by washing(see Table 4 and Fig.9).Moreover,the FTIR spectra recorded after the biodiesel washing(Fig.10)show a band centred on 1435 cm-1(highlighted in Fig.11),which is often selected to quantify the methylesters contained in biodiesel,petrodiesel and blended oils[37].
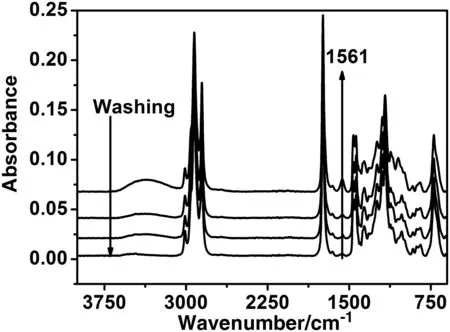
Fig.9.Spectra of biodiesel:UFO/PCM,washing effect on soap elimination.(Reaction conditions:KOH 1.2 wt%,reaction temperature 60°C,reaction time 2 h,molar ratio 6:1.)

Fig.10.FT-IR spectra ofsamples after washing.UFO(a).Biodieselbatches:RFO/AGM(b);RFO/PCM(c);UFO/AGM(d);and UFO/PCM(e).(Reaction conditions:KOH 1.2 wt%,reaction temperature 60°C,reaction time 2 h and molar ratio 6:1.)
In order to evaluate the technicalaspects of the low-cost biodiesel(Table 1,batch d),we have synthesised 40 L in a home-made batch reactor.The reaction was monitored by1HNMR spectroscopy.The spectra were recorded during the reaction.The obtained results are shown in Fig.12.The conversion reaches 86%after 20 min of reaction and 96%after 80 min.TGA–DTA results obtained for the biodieselare reported in Fig.13.Thermogravimetric analysis showed the good quality of the small-scale prepared biodiesel.In addition,the results con firm the ability of the TGA technique in the characterization of the biodieselquality.
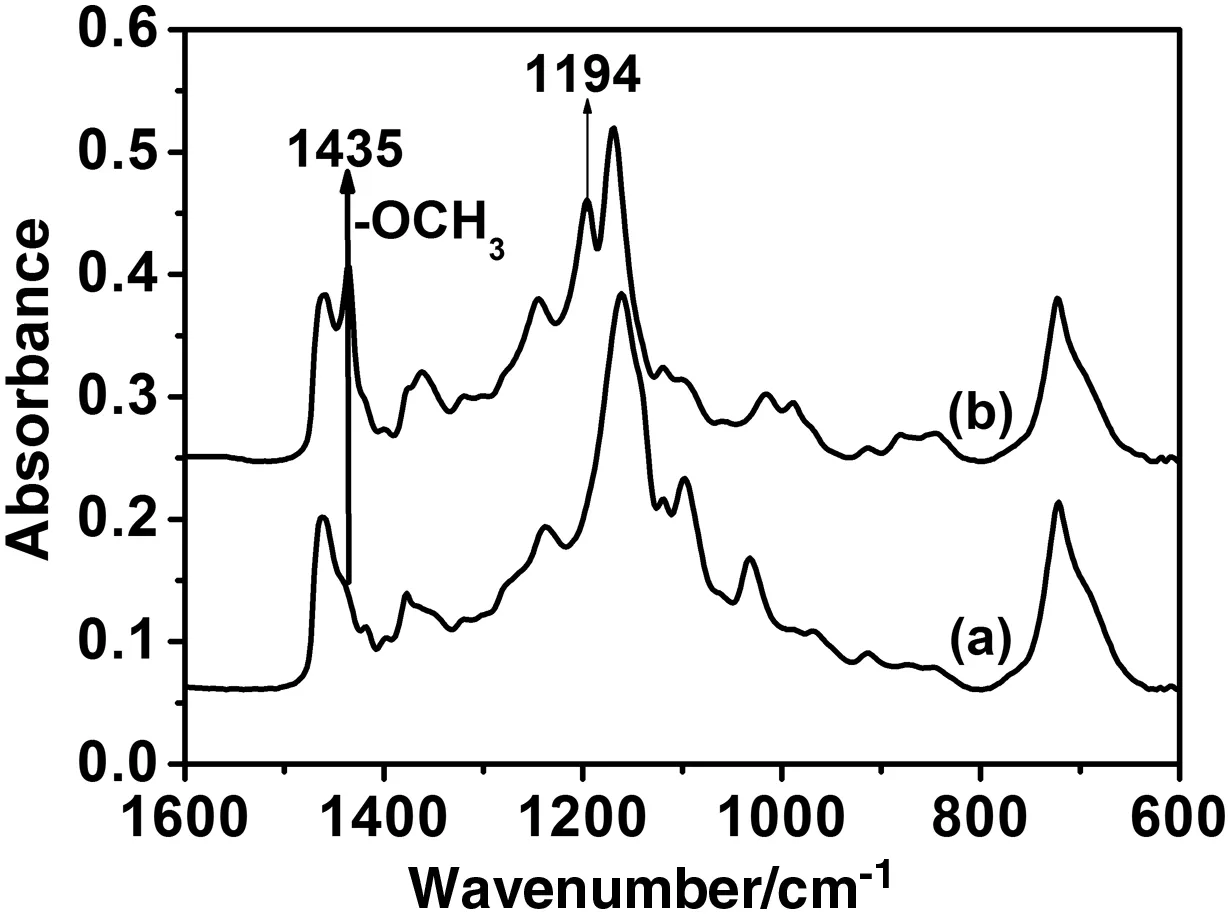
Fig.11.The fingerprint of the biodieselsamples after washing.UFO(a).Biodieselbatch d(b).(Reaction conditions:KOH 1.2 wt%,reaction temperature 60°C,reaction time 2 h and molar ratio 6:1.)
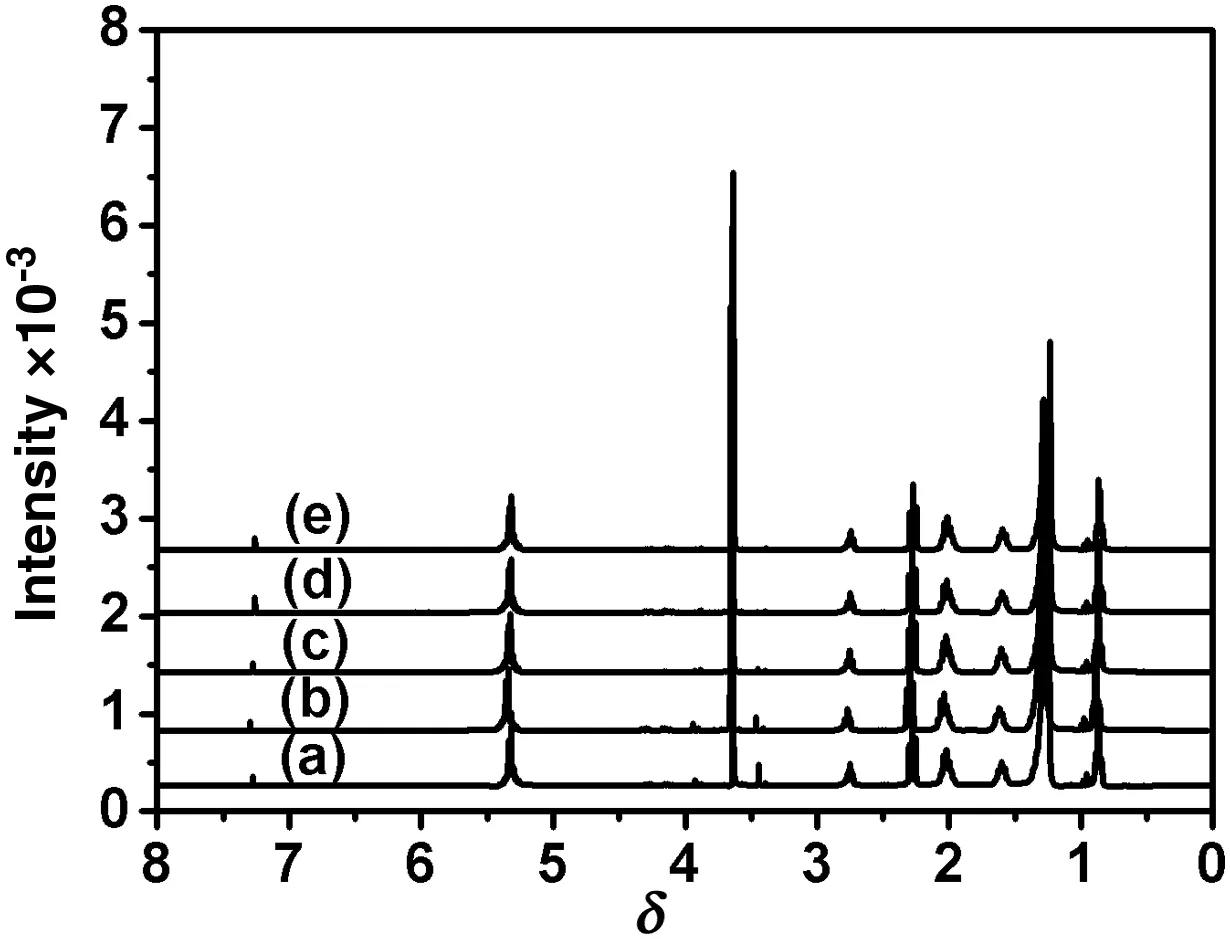
Fig.12.Evolution with time of1H NMR spectrum oflow scale biodieselbatch:used frying oil/PCM:20(a),30(b),45(c),60(d)and 80 min(e).(Reaction conditions:KOH 1.2 wt%,reaction temperature 65°C and molar ratio=6.)

Fig.13.TGA–DTA analyses of UFA and biodiesel(FAME):batch d(reaction conditions:KOH 1.2 wt%,reaction temperature 60°C and molar ratio 6:1).
The characteristics of the prepared biodieselwere summarized in Table 5.They clearly indicated that the synthesised biodieselobeys almost the internationalstandards.Therefore,in the regions of Morocco where populations are isolated,biodieselcould play an important role in the improvement of the quality oflife of the populations.The small production units reduce the dependence of the population ofconventionalpower distribution circuits.
4.Conclusions
From the collected results the following conclusions might be drawn:
·The optimalconditions for the transesteri fication reaction depend on the origin and the quality of the frying oil.

Table 5 Characteristics ofsmallscale prepared biodiesel
·The biodieselyield ranges between 80%and 95 wt%depending on the quality and the purity of the oil.An ef ficient but costly puri fication of the waste oilincreases the biodieselyield.
·The1H NMR,13C NMR and FTIR,gas chromatography and TGA analyses of the final product con firmed that:(1)the reaction was total,(2)the biodiesel did not contain any trace of glycerol and(3)it meets the required internationalstandards.
·Analyses of the biodiesel produced at smallscale(40 L)con firmed that it can be synthesised at an acceptable quality and cost.
Acknowledgements
The authors acknowledge Hassan IIAcademy of Science and Technology for the financialsupport kindly provided to this research.Our thanks go also to the CNRST for the offered open access to the facilities to perform NMR analysis.The bilateral project CNRST–CNR(2014–2015)is also acknowledged for the financial support and people exchange.
[1]H.W.Huber,S.Iborra,A.Corma,Synthesis of transportation fuels from biomass:Chemistry,Catal.Eng.Chem.Rev.106(2006)4044–4098.
[2]M.M.Azam,A.Waris,N.M.Nahar,Prospects and potentialoffatty acid methylesters ofsome non-traditionalseed oils for use as biodieselin India,Biomass Bioenergy 29(2005)(2005)293–302.
[3]Y.C.D.Leung,Xuan Wu,M.K.H.Leung,A review on biodieselproduction using catalyzed transesteri fication,Appl.Energy 87(2010)1083–1095.
[4]S.Leduc,K.Natarajan,E.Dotzauer,I.McCalluma,M.Obersteiner,Optimizing biodieselproduction in India,Appl.Energy 86(2009)125–131.
[5]J.P.Szybist,J.Song,M.Alam,A.L.Boehman,Biodiesel combustion,emissions and emission control,FuelProcess.Technol.88(2007)679–691.
[6]W.M.J.Achten,J.Almeida,V.Fobelets,E.M.Bolle,E.Virendra,P.Singh,D.N.Tewarie,L.V.Verchot,B.Muys,Life cycle assessment of Jatropha biodieselas transportation fuelin rural India,Appl.Energy 87(2010)3652–3660.
[7]C.Ilkılıç,S.Aydın,R.Behcet,H.Aydin,Biodieselfrom saf flower oiland its application in a dieselengine,Fuel Process.Technol.92(2011)356–362.
[8]A.Murugesan,C.Umarani,R.Subramanian,N.Nedunchezhian,Biodieselas an alternative fuelfor dieselengines–A review,Renew.Sust.Energ.Rev.213(2009)653–662.
[9]T.W.Charpe,V.K.Rathod,Biodieselproduction using waste frying oil,Waste Manag.31(2011)85–90.
[10]A.Dhar,R.Kevin,A.K.Agarwal,Production ofbiodieselfrom high-FFA neem oiland its performance,emission and combustion characterization in a single cylinder DICI engine,FuelProcess.Technol.97(2012)118–129.
[11]M.G.Kulkarni,A.K.Dala,Waste cooking oils An economical source for biodiesel:A review,Ind.Eng.Chem.Res.45(2006)2901–2913.
[12]L.Lin,H.Cui,S.Vittayapadung,Z.Xiao,W.Wu,A.Zhang,W.Wael Mamdouh,C.Changzhu Li,Synthesis of recyclable hollow Fe/C—SO3H fiber as a catalyst for the production of biodiesel,Environ.Prog.Sustainable Energy 33(4)(2014)1432–1437.
[13]J.M.Encinar,J.F.Gonzalez,A.Rodrıguez-Reinares,Biodieselfrom used frying oil.Variables Affecting the Yields and Characteristics of the Biodiesel,Ind.Eng.Chem.Res.44(2005)5491–5499.
[14]Z.Jurac,V.Zlatar,Optimization ofraw materialmixtures in the production ofbiodieselfrom vegetable and used frying oils regarding quality requirements in terms of cold flow properties,FuelProcess.Technol.106(2013)108–113.
[15]A.Cukalovic,J.M.Monbaliu,Y.Eeckhout,C.Echima,Roland Verhe,R.,Geraldine Heynderickx,G.,C.V.Stevens,Development,optimization and scale-up ofbiodiesel production from crude palm oiland effective use in developing countries,Biomass Bioenergy 56(2013)62–69.
[16]Biodieselfuelquality standard,http://www.svlele.com/biodiesel_std.htm.
[17]M.R.Monteiro,A.R.P.Ambrozin,L.M.Lião,A.G.Ferreira,Criticalreview on analytical methods for biodieselcharacterization,Talanta 77(2008)593–605.
[18]M.Charoenchaitrakool,J.Thienmethangkoon,Statisticaloptimization for biodiesel production from waste frying oilthrough two-step catalyzed process,Fuel Process.Technol.92(2011)112–118.
[19]J.K.Satyarthi,D.Srinivas,P.Ratnasamy,Estimation of free fatty acid content in oils,fats,and biodieselby1H NMR Spectroscopy,Energy Fuel23(2009)2273–2277.
[20]Y.Miyake,K.Yokomizo,N.Matsuzaki,Determination ofunsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy,J.Am.Oil Chem.Soc.75(1998)1091–1094.
[21]M.Morgenste,J.Cline,S.Meyer,S.Cataldo,Determination of the kinetics ofbiodiesel production using proton nuclear magnetic resonance spectroscopy(H-NMR),Energy Fuel20(2006)1350–1353.
[22]M.Tariq,S.Saqib Ali,F.Ahmad,M.Ahmad,M.Zafar,N.Khalid,M.A.Mir Ajab Khan,Identi fication,FT-IR,NMR(1H and13C)and GC/MS studies of fatty acid methylesters in biodieselfrom rocket seed oil,FuelProcess.Technol.92(2011)336–341.
[23]Z.Ullah,M.A.Bustam,Z.Man,Characterization ofwaste palm cooking oilfor biodieselproduction,Int.J.Chem.Eng.Appl.5(2014)134–137.
[24]F.P.Sousa,M.A.Luciano,V.M.D.Pasa,Thermogravimetry and viscometry for assessing the Ester Content(FAME and FAEE),Fuel Process.Technol.109(2013)133–140.
[25]P.Chand,C.V.Reddy,J.G.Verkade,T.Wang,D.Grewell,Thermogravime-tric quantification ofbiodieselproduced via alkalicatalyzed transesteri-fication ofsoybean oil,Energy Fuel23(2009)989–992.
[26]J.F.Puna,M.J.Neiva Correia,Soares Dias A.P.,J.Gomes,J.Bordado,Bio-dieselproduction from waste frying oils over limecatalysts,React.Kinet.Mech.Catal.109(2013)405–441.
[27]H.A.Farag,A.El-Maghraby,N.A.Taha,Transesteri fication of esteri fied mixed oilfor biodieselproduction,IJCBS 2(2012)105–114.
[28]S.T.Keera,S.M.ElSabagh,A.R.Taman,Transesteri fication of vegetable oilto biodieselfuelusing alkaline catalyst,Fuel90(2011)42–47.
[29]G.Guan,K.Kusakabe,S.Yamasaki,Tri-potassium phosphate as a solid catalyst for biodieselproduction from waste cooking oil,FuelProcess.Technol.90(2009)520–524.
[30]M.J.Ramo,C.M.Fernández,A.Casas,L.Rodríguez,Á.Pérez,In fluence of fatty acid composition of raw materials on biodiesel properties,Bioresour.Technol.100(2009)(2009)261–268.
[31]G.Gelbard,O.Brès,R.M.Vargas,F.Vielfaure,U.F.Schuchardt,1H nuclear magnetic resonance determination of the yield of the transesesteri fication of rapeseed oil with methanol,J.Am.Oil Chem.Soc.72(1995)1239–1241.
[32]G.Knothe,Monitoring the turnover of a progressing transesteri fication reaction by fiber-optic NIR Spectroscopy with correlation to1H-NMR spectroscopy,J.Am.Oil Chem.Soc.77(2000)489–493.
[33]M.R.Monteiro,A.R.P.Ambrozin,L.M.Lião,A.G.Ferreira,Determination of biodiesel blend levels in different dieselsamples by1H NMR,Fuel88(2009)691–696.
[34]G.Knothe,Determining the blend levelof mixtures of biodiesel with conventional dieselfuelby fiber-optic near-infrared spectroscopy and1H nuclear magnetic resonance spectroscopy,J.Am.Oil Chem.Soc.78(2001)1025–1028.
[35]G.F.Ghesti,J.L.de Macedo,I.S.Resck,J.A.Dias,S.C.L.Dias,FT-Raman spectroscopy quanti fication of biodieselin a progressive soybean oiltransesteri fication reaction and its correlation with1H NMR spectroscopy methods,Energy Fuel21(2006)2475–2480.
[36]F.Jin,K.Kawasaki,H.Kishida,T.Tohji,K.Moriya,H.Enomoto,1H-NMRspectroscopy study on methanolysis reaction of vegetable oil,Fuel86(2007)1201–1207.
[37]C.Baroi,E.K.Yanful,M.A.Bergougnou,Biodieselproduction from jatro-pha curcas oilusing potassium carbonate as an unsupported catalyst,Int.J.Chem.React.Eng.7(2009)1–18.
 Chinese Journal of Chemical Engineering2016年9期
Chinese Journal of Chemical Engineering2016年9期
- Chinese Journal of Chemical Engineering的其它文章
- In situ synthesis ofhydrophobic magnesium hydroxide nanoparticles in a novelimpinging stream-rotating packed bed reactor☆
- Enhancing the hydration reactivity ofhemi-hydrate phosphogypsum through a morphology-controlled preparation technology☆
- Synthesis and characterization ofcopolymers ofpoly(m-xylylene adipamide)and poly(ethylene terephthalate)oligomers by melt copolycondensation
- Improvement of CO2 capture performance ofcalcium-based absorbent modi fied with palygorskite☆
- Adsorption behavior ofcarbon dioxide and methane in bituminous coal:A molecular simulation study☆
- Characterization of the adsorption behavior ofaqueous cadmium on nanozero-valent iron based on orthogonalexperiment and surface complexation modeling☆
