Azo-bridged triazoles:Green energetic materials
Lemi TÜRKER*
Department of Chemistry,Middle East Technical University,Ankara,Turkey
Azo-bridged triazoles:Green energetic materials
Lemi TÜRKER*
Department of Chemistry,Middle East Technical University,Ankara,Turkey
In this short review,excerpts from the literature of azo-bridged triazoles (mainly 1,2,4-triazoles),some of their derivatives (chloromethyl,dinitro and trinitro pyrazole substituted ones,etc.)and some of their salts,have been presented focusing on the most recent investigations.These classes of compounds,known as high nitrogen compounds,are generally high energy density materials.Therefore,if available some of their ballistic properties were included.
Azo-bridged;Triazoles;Energetic materials;High nitrogen compounds
1.Nitrogen-rich compounds
A novel approach in the f i eld of energetic materials is to replace some conventional explosives with high-nitrogen compounds.These materials possess a higher proportion of nitrogen by mass,as compared to conventional explosives,so that they derive their energy output from this factor,in contrast to the redox reactions of fuel elements (carbon,hydrogen)as in the case of classical ones.When overall properties are considered,insensitivity toward destructive stimuli is also an important criterion forenergeticmaterials.However,mostazidofunctionalized compounds which generally possess high destructive power are sensitive to heat,impact,and friction. Hence,they are diff i cult to handle safely which is a great restriction for their further applications.Thus,the conf l ict between high energy and inherent instability of nitrogen-rich compounds requires a deeper understanding of the factors involved structurally and thermodynamically and consequently makes this research area challenging.After many decades of effort in the development of high-energy materials,the key concerns in weapon systems remain to be higher performance and lower sensitivity.Nowadays,the most desirable characteristics for new energetic materials include high positive heat of formation,high density,pressure and high detonation velocity,but high thermal stability and low sensitivity towardexternal forces such as mainly impact,shock,and friction. High-nitrogen compounds (e.g., azoles) in combination with energetic substituents such as nitro (—NO2),nitrato(—ONO2),nitramine (—NHNO2),and nitroimine (=NNO2)functionalitiesareofparticularinterestbecausethesecompounds additionally have satisfactory oxygen content.In high-nitrogen compounds,nitrogen gas (N2)is the major product of explosion;they burn more cleanly than other organic explosives,meanwhile producing less carbon monoxide,soot and other incompletely oxidized toxic explosive residues (e.g.,CO,NO,etc.).However,the requirements of insensitivity and high energy along with positive oxygen balance are most of the time contradictory to each other;hence,the development of new high energy density materials (HEDMs)is an interesting and challenging problem but diff i cult due to some synthetic handicaps.
Highly energetic compounds characterized by the presence of polynitro groups are one of the important classes of useful energetic materials.The involvement of nitro groups in structures generally tends to decrease the heat of formation but contributes markedlytotheoverallenergeticperformance.Also,thenitrogroup contributes to enhance the oxygen balance and density of the material,which are important in order to improve the detonation performance (pressure and velocity)[1-6].Traditional polynitro compounds produce energy primarily from the combustion of the carbon backbone while consuming the oxygen provided by the nitro groups (inter or intramolecular redox reactions)(Fig.1).Several well known explosives are triaminotrinitro benzene (TATB),1,3,5,-trinitrotriazacyclohexane (RDX),1,3,5,7-tetranitrotetraazacyclooctane (HMX),and 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclododecane (CL-20)(Fig.1).Highenergy materials possessing several numbers of nitrogen atoms,so-called “high-nitrogen” compounds,have been shown to derive energy from the presence of many energetic N—N and C—N bonds.
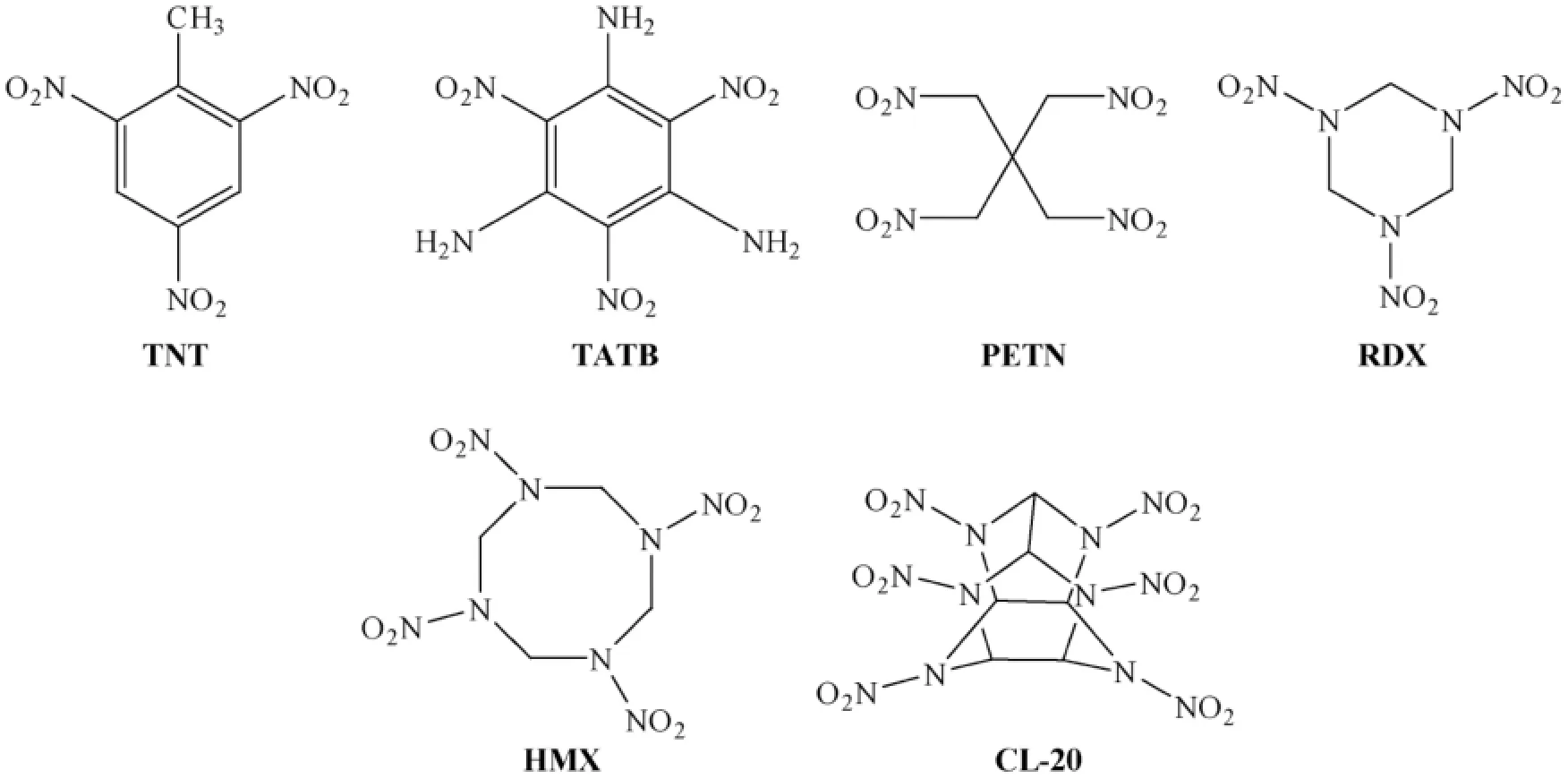
Fig.1.Traditional energetic polynitro compounds.
Material scientists,interesting in energetic materials,are after high-energy density compounds with high detonation properties and low sensitivities [1-6].Polynitropyrazole families,such as 3,4-dinitropyrazole,3,5-dinitroprazole,4-amino-3,5-dinitropyrazole, and 3,4,5-trinitropyazole, have been widely used in the development of energetic materials because they have good thermal stabilities,moderate detonation properties,and low friction and impact sensitivities [7-11].Unfortunately,because of their acidity and water sensitivity,they have no use in the daily energetic world,unless the corresponding conjugate bases are used [12].
2.High-energy nitrogen-rich compounds
Certain classes of nitrogen-rich compounds are the most promising candidates for high energy density materials(HEDM),as they are environmentally benign and possess high energy density [9,13-24].In recent years,nitrogen-rich compounds containing long catenated nitrogen atom chains have attracted considerable interest in research areas such as propellants,explosives,and pyrotechnics.This attraction is mainly due to the high positive heat of formation which is the unique feature of energetic compounds containing catenated N—N bonds.Recently,some energetic azo compounds containing eight-nitrogen and ten-nitrogen chains have been prepared and characterized (Fig.2)[25-28].
In the f i eld of high-energy materials research,nitrogen-rich compounds based on C/N heteroaromatic rings with highnitrogen content are at the forefront [21-28].Recently,the combination of an azo group with high-nitrogen heteroaromatic rings has been extensively studied.The azo linkage not only desensitizes but also dramatically increases the heat of formation of high-nitrogen compounds such as DAAT (5)and TAAT(6)(Fig.3),in which the azo group is bonded to carbon [29,30]. Such azo compounds (e.g.,azobenzene-based compound 7)[31]are well known as diazoic dyes and thermally reversiblephotochromic materials [32]and are now being used in the f i elds of optical recording memory,photorefractive materials,photo optical switches,and molecular machines [33,34]. However,if the azo groups are attached to the nitrogens of heteroaromatic rings to create a rather long chain of catenated nitrogens,such a structure could result in having unique properties [35-37].Moreover,in contrast to the toxicity of many azobenzene-based compounds,these high-nitrogen azo compounds are nontoxic and harmless.
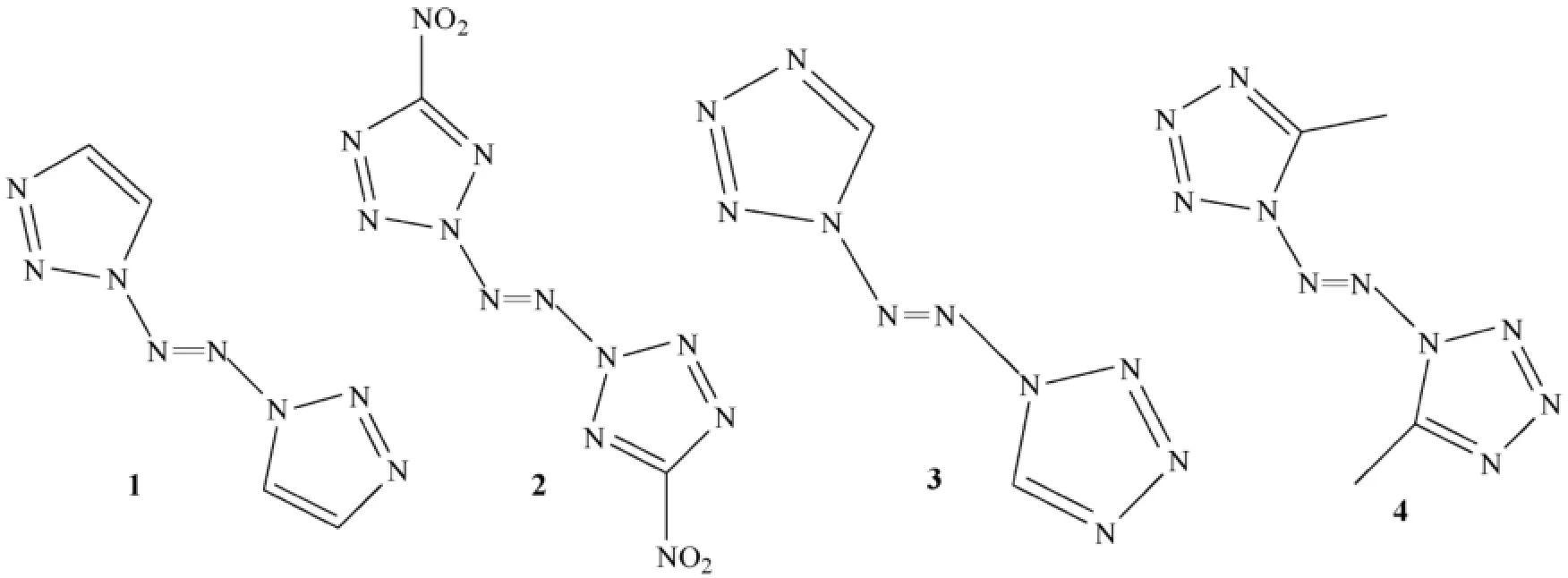
Fig.2.Some energetic azo compounds.
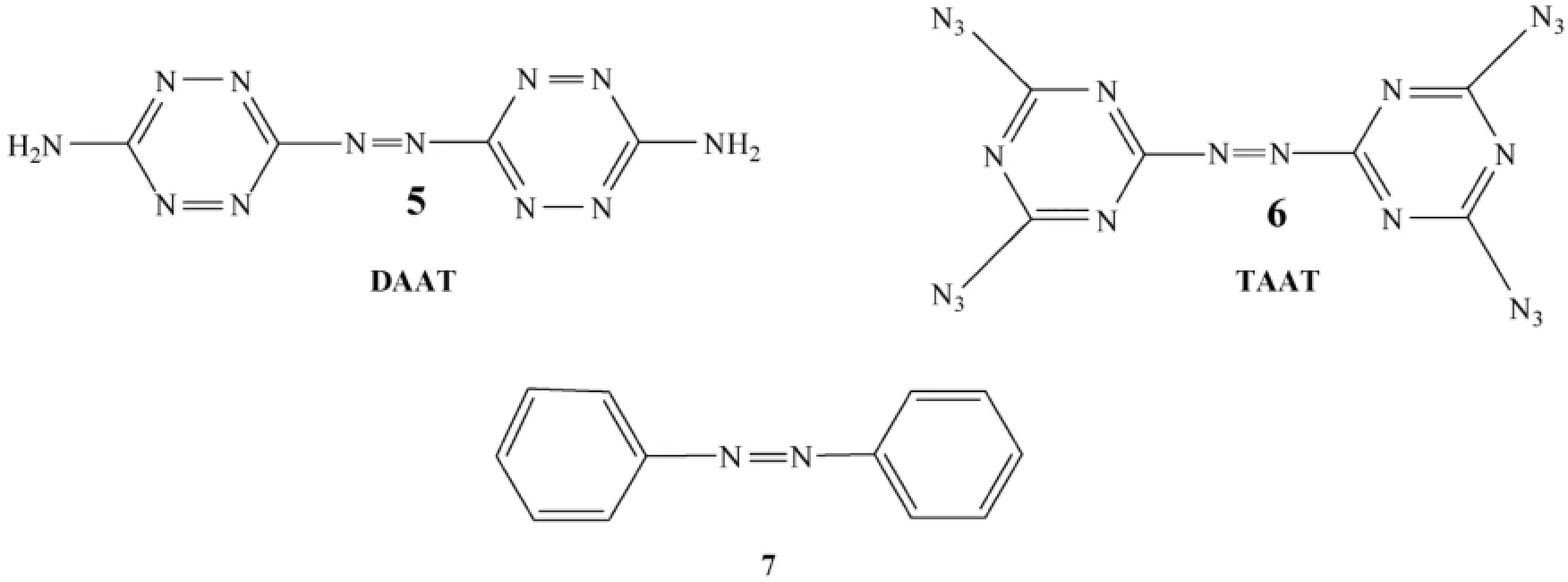
Fig.3.Some azo-bridged high energy nitrogen rich compounds.
The presence of certain bridges in energetic compounds is known as groups which give rise to some additional properties or property changes.For instance,links like —N=N—[27,31,38],—N=N(O)— [39],—NH [40,41],—NH—NH—[42]and —CH2—N(NO2)—CH2[43]generally are known to enhance the heat of formation,whereas the bridges such as—CH2—CH2[44]and —O—CH2—CH2—O— [45]cause a decrease in sensitivity to impact and friction.Thus,new high nitrogen containing energetic compounds with bridging moieties attract the attention of scientists for various purposes.
On the other hand,nitrogen-rich energetic salts also have attracted attention because they are environmentally friendly high-energy-density materials (HEDMs) and they have attracted considerable interest due to their lower vapor pressures,higher heats of formation,and enhanced thermal stabilities as compared to their atomically similar nonionic analogs.
Shreeve et al.considered energetic compounds having N-azo bridged pyrazoles and oxadiazole such as compounds 8 and 9 and 1,2,4-triazole links such as 10-12 along this line (see Fig.4)[7,46,47].
3.Azo-bridged triazoles and their derivatives
They also obtained azo-bridged 1,2,4-triazole containing systems (13)-(15).Fig.5 shows the synthesis of some azobridged triazole compounds [48].The structures contain substituted (NO2,NH2)pyrazole units as substituents linked to the triazole moieties which f l ank the azo-bridge.
Triazole links and polynitropyrazole rings give rise to compounds with energetic properties.In the study,these materials were fully characterized by NMR and infrared spectroscopy,elemental analysis,and differential scanning calorimetry(DSC).In addition,the structure of compound 13 was conf i rmed by single-crystal X-ray diffraction analysis.The detonation properties of these triazole-linked energetic compounds were calculated by using the EXPLO5 (6.01)program [49].
Detonation properties,calculated from heats of formation and experimental densities,thermal stabilities,and impact and friction sensitivities,support the potential use of these materials for explosive applications (see Table 1).Compound 13,with amino substituted pyrazoles,is insensitive with impact sensitivities of 36 J.Compound 15 has D:8637 m s-1;P:32.3 GPa with impact sensitivities of 10 J and friction sensitivities of 360 N.
15N NMR spectrum of compounds 13 and 14 showed that N-azo bridge occurs at the lowest f i eld (δ:17-20 ppm).The amino nitrogen in 13 happens at-314 ppm.The thermal stabilities were also determined.
The calculated detonation parameters of all these new energetic compounds (EXPLO5 6.01)provide detonation pressures and velocities in the ranges of 25.8-34.1 GPa and 7990-8797 m/s,respectively.They reported some other types of compounds as well and also performed DFT calculations (geometry optimizations and frequency analysis were achieved at the level of (B3LYP/6-31+G(d,p)and single point energies were calculated at the MP2/311++G(d,p)level,atomization energies by the G2 method of calculations)[47].The incorporation of triazole links and azo-bridges results in higher heats of formation and moderate sensitivities.
Some new polynitro-1,2,4-triazoles containing a trinitromethyl group were synthesized by Shreeve et al.via straightforward routes [46,47].The authors fully characterized these high-nitrogen and oxygen-rich compounds using IR and multinuclear NMR spectroscopy,elemental analysis,natural bonding orbital(NBO)analysis,and differential scanning calorimetry (DSC).In the study,the heats of formation for all compounds were calculated with Gaussian 03 (revision D.01)and then combined with experimentally determined densities to estimate the detonation pressures (P)and velocities (D)of the energetic materials considered (Cheetah 5.0).It was found that the compounds studied exhibit high density,good thermal stability,acceptable oxygen balance,positive heat of formation,and excellent detonation properties,so that,in some cases,they are superior to those of TNT,RDX,and HMX.
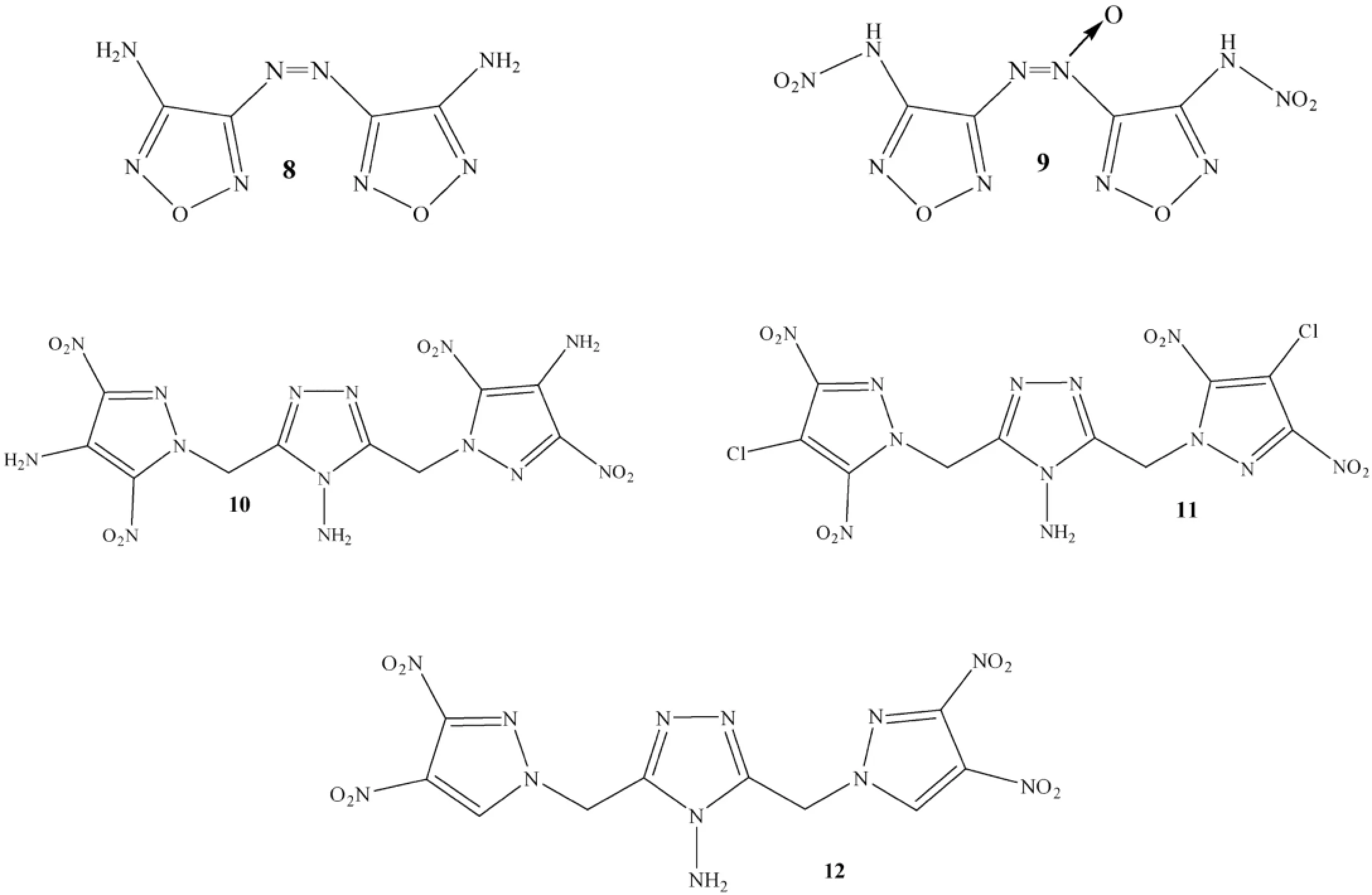
Fig.4.Some high-energy nitrogen rich compounds.
Azoles with more than two nitro groups are highly powerful,and as a result,a large variety of nitroazoles have been prepared[46,51-53].Heterocyclic compounds with high nitrogen content are also environmentally friendly and have high heats of formation and are endothermic.The high nitrogen content of these compounds often leads to high crystal density,which is associated with increased performance.Note that incorporation of a triazole ring into a compound is a known strategy for increasing thermal stability.Many triazole compounds show high thermal sensitivity coupled with low sensitivity to shock and impact[54]. Figs.6 and 7 show the synthesis of some compounds considered in the article [46].The amino triazolylacetic acid 16 was convertedintotheazocompound,17,bytreatingwithalkalinepotassium permanganate acting as an oxidizer.Then,compound 17 was reacted with mixed acids at room temperature in order to form 5,5′-bis(trinitromethyl)-3,3′-azo-1,2,4-triazole (18).When an attempt was made to convert the trinitromethyl groups of hexanitro compound (18)to the corresponding dinitromethylene groups,by treating with alkaline hydroxylamine followed by acidif i cation,alowyieldmixtureofcompoundswasformed.The reaction also failed with potassium iodide in methanol.Methylation of hexanitro 18 was carried out using various reagents;however, reaction was successful with trimethylsilyl diazomethane to produce 1,1′-dimethyl-5,5′-bis-(trinitromethyl)-3,3′-azo-1,2,4-triazole (19)(Fig.7).
Impact sensitivity measurements were made using Standard BAM Fallhammer techniques [55].For all of the compounds studied,the impact sensitivities range from those of the relatively less sensitive compound (19)(between 5.5 and 13 J)to the very sensitive compound 18 (1.5 J).N-Methyl derivative(19)is less sensitive than the corresponding N—H compound 18.Thermal stabilities of the energetic compounds were determined with differential scanning calorimetry (DSC)at a scan rate of 5 °C min-1.Azo compounds 18 and 19 decomposed at 150 °C and 165 °C,respectively,without melting.
Oxygen balance (OB)stands for the index of the def i ciency or excess of oxygen in a compound required to convert all carbon atoms into carbon dioxide and all hydrogens into water. For a compound having the molecular formula of CaHbNcOd,OB is expressed as OB (%)= (d-2a-b/2)/MW.Positive oxygen balance has signif i cance in explosive materials,of which can be used as oxidizers.The oxygen balance of 18 is_8.6%,which is superior to RDX (-22%)and HMX (-22%).
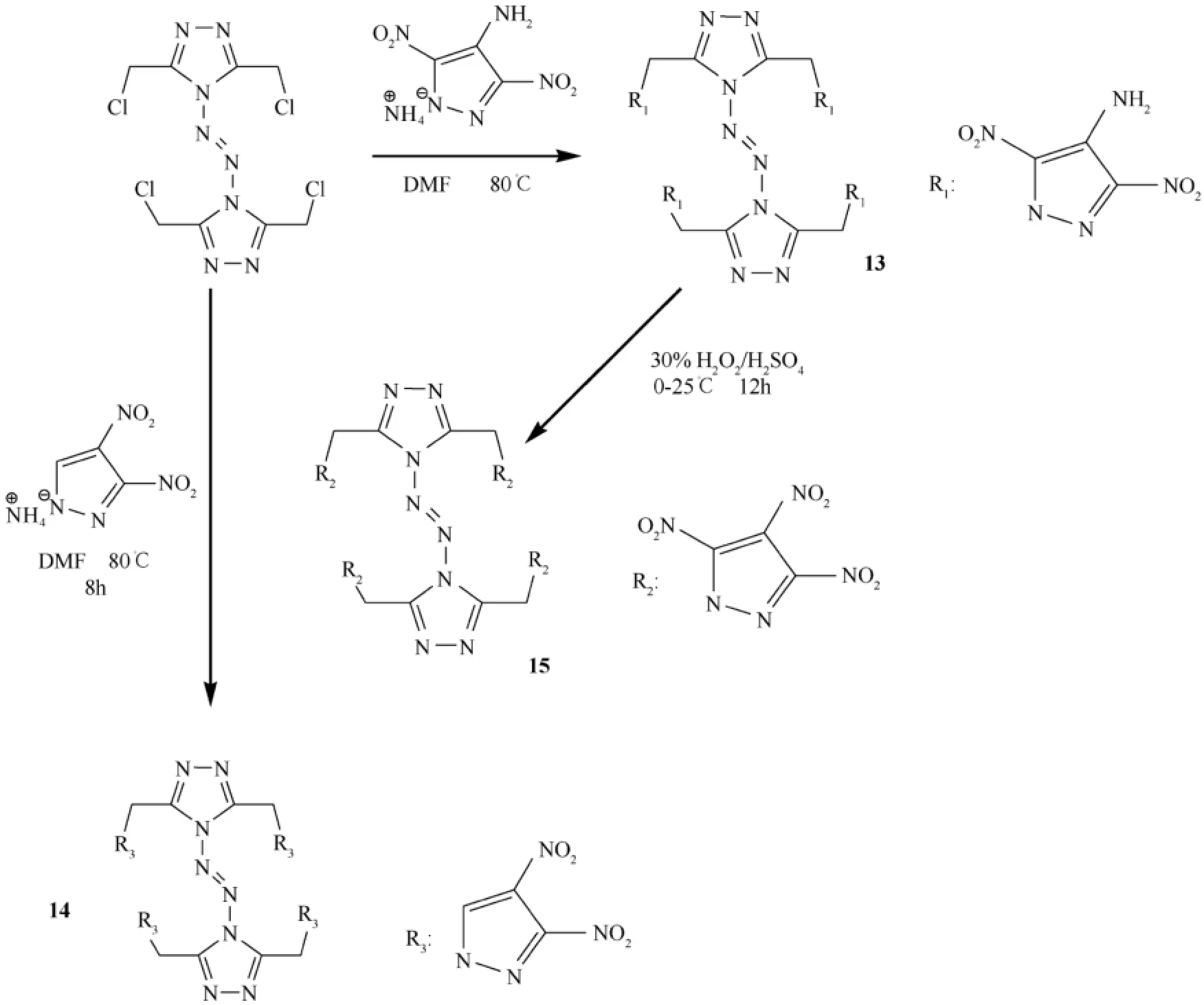
Fig.5.Synthesis of some azo-bridged triazole compounds [48].
By using the calculated values of the heats of formation and the experimental values for the densities (gas pycnometer,25 °C)of the new energetic polynitro triazoles obtained,the authors got the detonation pressures (P) and detonation velocities (D)on the basis of traditional Chapman-Jouguet thermodynamicdetonationtheoryusingCHEETAH 5.0 program.The detonation pressures of polynitro triazoles were in the range between P=33.83 and P=38.41 GPa (as compared toTNT 19.53 GPa,RDX 35.2 GPa,and HMX 39.6 GPa). Detonation velocities lie between D=8742 and D=9229 m s-1(note that TNT 6881 m s-1,RDX 8997 m s-1,and HMX 9320 m s-1).The specif i c impulse values (CHEETAH 5.0)of these polynitrotriazoles were found to be in the range of 233 and 264 s,which suggests that these compounds may have propellant possibilities [46].Based on the calculated properties and the rather high thermal and hydrolytic stabilities of those compounds,the article claims that these high nitrogen,oxygenrich materials may be attractive candidates for energetic applications.
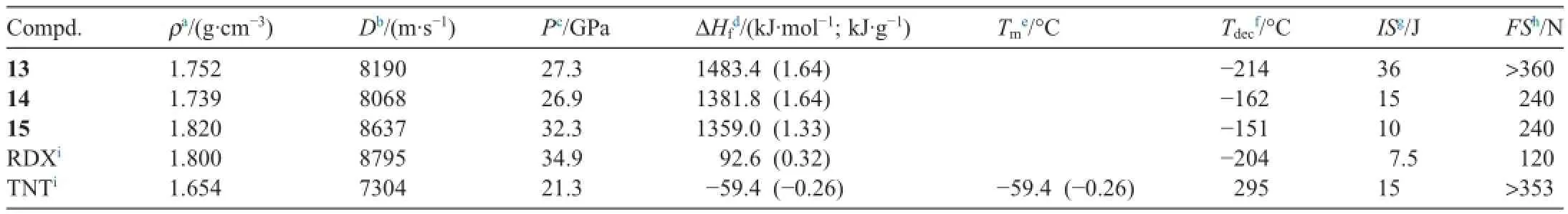
Table 1Some properties of compounds 13-15·H2O [47].
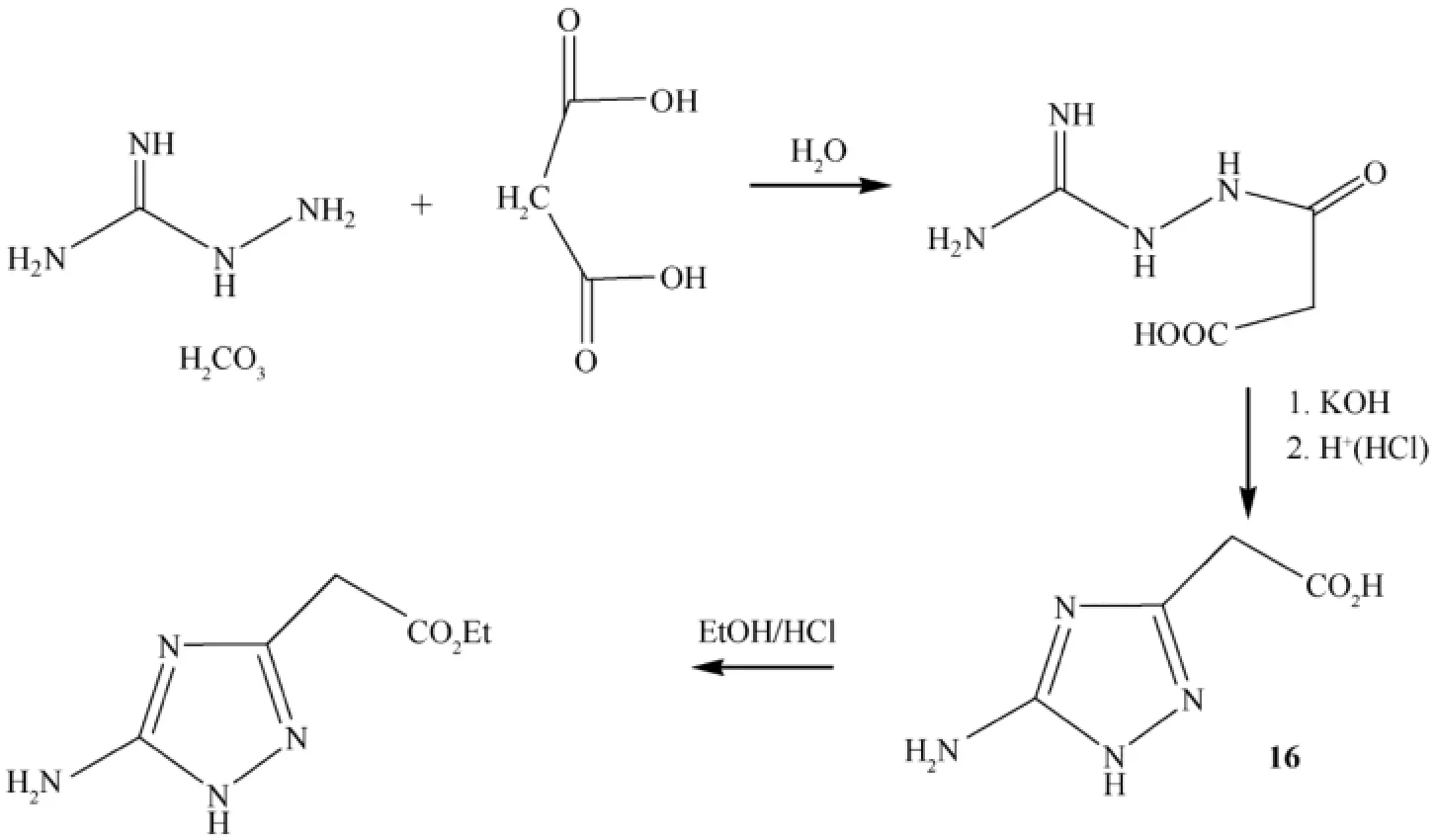
Fig.6.Synthesis of the starting materials used in the synthesis of azo bridged triazole derivatives.
On the other hand,natural bond orbital analysis provides an eff i cient method for investigating charge distribution in molecular systems.The authors obtained the charge distribution data calculated by the NBO method for optimized geometries of the compounds studied [46].The NBO analysis shows that the C—NO2negative nitro charges (QNitro)correspond to the more stable nitro compounds [56]because there are more than one nitro group in their synthesized molecules.It is found that average QNitrodecreases when the methyl group was introduced into the molecule for compound 18 (-0.164 as compared to -0.166 of 19).The more negative average QNitrovalue indicates that compound 19 is more stable than compound 18,which is similar to the results for impact sensitivity tests or thermal decomposition results.The following equations were used for the above mentioned charge calculations.
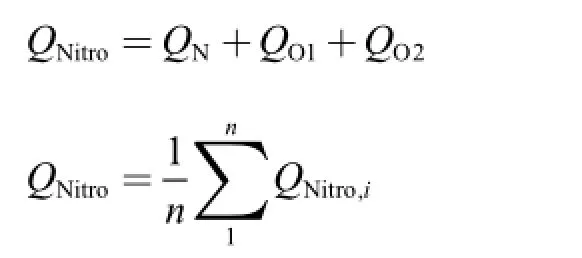
The syntheses of high energy density polynitro triazoles with an azo bridge,5,5′-bis(trinitromethyl)-3,3′-azo-1,2,4-triazole(18),and 1,1′-dimethyl-5,5′-bis(trinitromethyl)-3,3′-azo-1,2,4-triazole (19)were carried out,as shown in Fig.7,and their physical and detonation properties were determined.Thesecompounds exhibit good physical and detonation properties,such as moderate thermal stabilities,high densities,high heats of formation,and high detonation pressures and velocities. Calculated detonation values for these compounds are comparable to those of explosives such as TNT,RDX,and HMX.
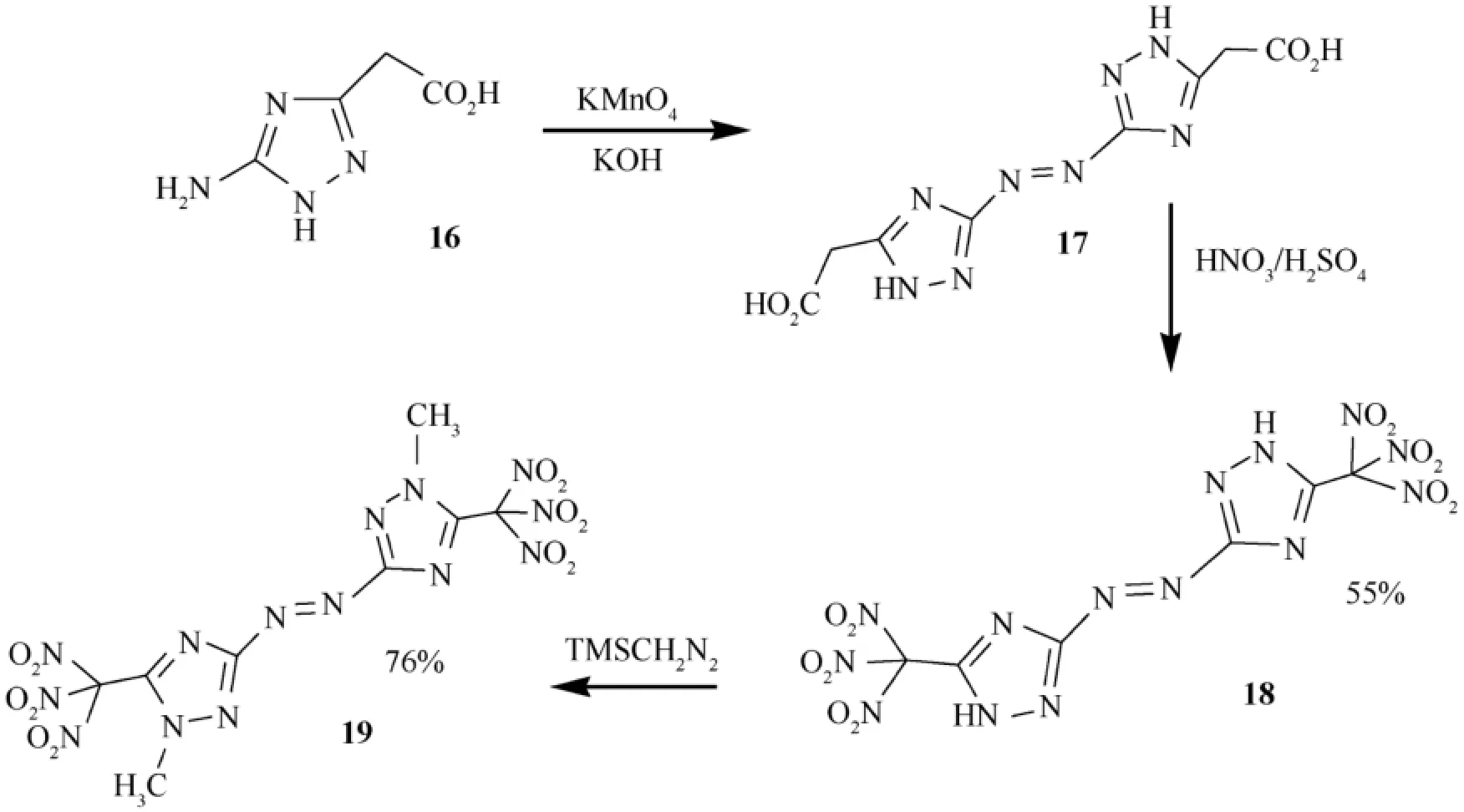
Fig.7.Synthesis of some azo bridged triazoles.R:Triazolone,triazole.
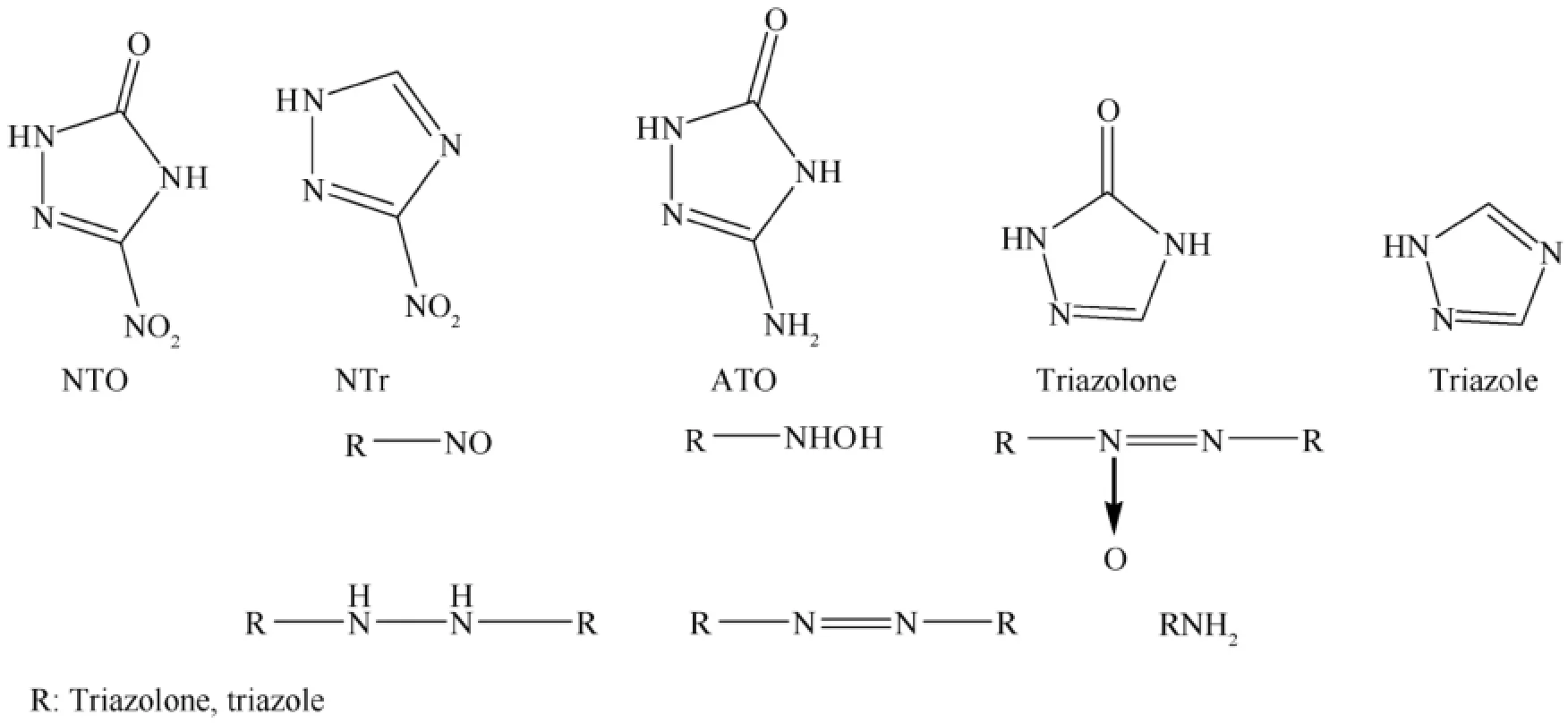
Fig.8.Structures of NTO and Ntr and their possible reduction products.
4.Electrochemical syntheses of azo-bridged triazoles and derivatives
Wallace et al.investigated the electrochemical reduction of nitrotriazoles in aqueous media as an approach for the synthesis of new green energetic materials [42].In one of their publications they reported the synthesis of new azo and azoxy compounds via electrochemical reduction of nitrotriazoles in aqueous media using nitrotriazolone (NTO)and nitrotriazole(NTr)as representative substrates.Reduction of NTO produces mainly solid azoxytriazolone (AZTO),with azotriazolone(azoTO)and aminotriazolone (ATO)as minor products,while 3-hydroxylaminotriazole is the major product formed from NTr(see Figs.8 and 9).These compounds are of interest as new green high-nitrogen compounds for use as insensitive high explosives (IHE).The effect of varying reaction conditions such as pH and substrate concentration was evaluated,and a mechanism was proposed accounting for the experimental observations.The study in particular revealed that the ratio of azoxy to azo in the solid product was inf l uenced by pH and temperature,and the minor product ATO was formed not via direct reduction of NTO but via a novel thermal disproportionation reaction of the hydrazotriazolone intermediate.Conditions of high substrate concentration and low cell temperature maximize the azoxy yield and minimize the formation of minor products.The results have indicated that this green electrosynthetic approach may be generally useful for the synthesis of new azoxy and azo triazoles from suitable substrates.
Electrochemical methods of synthesis and waste remediation have been increasingly favored recently as they are often considered to be economical and environmentally friendly[57-59].The employment of electrons as reagents enables synthetic chemists to avoid usage of potentially toxic and/or expensive oxidizing or reducing agents.Moreover,the reaction conditions are generally mild,and the electrodes can be considered as heterogeneous catalysts and can be readily re-used. Previously,the same authors had reported on the formation of the new high nitrogen compound azoxytriazolone (AZTO),which was produced by electrochemicalreduction of nitrotriazolone (NTO)in acidic aqueous solution [60].NTO is quite a new insensitive high explosive (IHE)promising as a safer replacement for standard explosive materials in several applications [61].One of the problems with its manufacture is treatment of the wastewater,since the very high aqueous solubility of NTO simply means that the wastewater in the process cannot be treated by conventional methods.In one of their previous works,they investigated the direct electrolysis of aqueous NTO solutions as a means of removing the soluble organic material from waste streams in the manufacture[57-59].That research led to the discovery of AZTO,which precipitates from solution in good yield [60].The structure and formula ofAZTO have indicated that it also has the potential to be used as an IHE.Indeed some preliminary results have conf i rmed that it acts as a typical IHE in small-scale tests [62].For a typical AZTO sample,it has been determined that the sensitivity to impact and electrostatic discharge (ESD)was identical to that measured for NTO [63](impact f i gure of insensitivity=100,ESD ignition at 4.5 J not 0.45 J)and the friction sensitivity was lower (>360 N compared to 252 N for NTO).In particular,AZTO exhibited greater thermal stability than the parent NTO,as is often the case for azoxy and azo materials[64].Thus,the authors claimed that the formation of AZTO from waste NTO solutions was proved to be a very economical method of remediation.
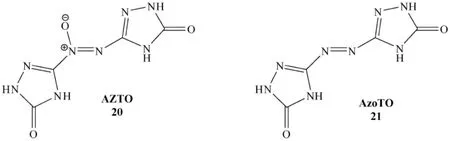
Fig.9.Structures of AZTO and AzoTO.
Upon reduction at-1.2 V vs.SCE in 0.1 mol L-1H2SO4,NTO solutions f i rst turned into green,then yellow,then a thick yellow precipitate was formed.NTr (nitrotriazole)solutions turned light blue initially,then pale yellow,and a small amount of yellowish white precipitate was formed.The green (NTO) and blue (NTr)colors observed in the initial stages of reduction could be attributed to intermediate nitroso species,or to anion radicals,which would be expected to hydrolyze rapidly in the aqueous medium.The HPLC analysis showed the presence of both NTO and NTr,all substrates were consumed within 5 h.
They investigated the effect of various factors on the formation of products using HPLC and various spectral techniques.In particular,they wished to assess whether it was possible to obtain pure samples of AZTO and/or azoTO by controlling the cell parameters.UV-vis spectroscopy indicated that AZTO/ azoTO was present in product solutions at concentrations representing <2%of NTO consumed under standard conditions. Since these products were not observed by HPLC,it seems that they had a strong aff i nity for the column and were not readily eluted.Oxidative mineralization is therefore the principal fate of substrate,although under certain conditions much more signif i cant concentrations of the dissolved azo,azoxy or hydrazo species could also be present.
In a “typical”electrolysis,NTO (10 g L-1)was reduced at -1.2 V in 0.1 mol L-1H2SO4at room temperature to give the products.Under these conditions,ATO accounts for 14%of the NTO consumed,and the precipitate accounts for a further 69%(solid contains AZTO and azoTO in 5.7:1 ratio).They also investigated the effects of cell potential,lower cell temperature,substrate concentration as well as the effect of increased cell pH.
In the experiments,three different species (AZTO,azoTO and ATO)were produced from the reaction,all of which have useful energetic properties.The authors have observed that the reaction conditions can be chosen to maximize the yield of AZTO (in the case of high NTO concentration and low temperature).In a slightly cooled cell,the conversion of NTO to AZTO occurs with very high eff i ciency.On the other hand,AzoTO and hydrazotriazolone can be produced by direct reduction of AZTO.These materials have potential for use as insensitive high explosives (IHE)by themselves,or might act as substrates for nitration to produce gas-generating solids.
They also proposed a mechanism for the reduction of nitrotriazoles,showing possible major reaction pathways.The mechanism of the reduction follows one of the standard pathways expected for nitrobenzenes.Note that there are very few examples of electrochemical azo/azoxy synthesis in 5-membered heterocycles.The f i nal product distribution is strongly inf l uenced by substrate concentration,reaction pH and temperature.In the case of NTO,the high yield ofAZTO is due partly to the low solubility of this species,which limits further reduction to hydrazo and azo derivatives.The experiments with NTr showed that the method can be applied to other triazoles,with a similar overall reaction mechanism being observed. However,it has been observed that the yield of the desired azo and azoxy products is highly dependent on the particular substrate used.
An eff i cient approach to generate a rather long catenated nitrogen atom chain is the oxidative azo coupling of the N—NH2moiety of the heteroaromatic ring to form a tetrazene structure (N—N=N—N)[35,65,66].Recently,1,1′-azobis-1,2,3-triazole (1)[26]and 2,2′-azobis(5-nitrotetrazole)(2)[25](an N8structure)and 1,1′-azobistetrazole (3)[27]with an N10structure were synthesized sequentially using sodium dichloroisocyanurate (SDIC)as an azo coupling reagent. Unfortunately,compounds 2 and 3 are thermally unstable,decomposing even at low temperatures and are even unstable in the solution.
The necessity of new energetic materials is dependent on economic and practical factors,as well as on performance. Nitrotriazolone (NTO)has been already in use in military explosives as an insensitive high explosive (IHE)replacement for RDX,but one issue with its manufacture has been the problem of treating the wastewater.The high aqueous solubility of NTO (up to 13 g L-1)means that the manufacturing waste cannot be treated with conventional means such as carbon scrubbers [67].
Wallace et al.reported azoxytriazolone (AZTO)(20)and azotriazolone (azoTO)(21)as insensitive high nitrogen compounds of energetic materials (Fig.9)[67].AZTO and azoTO were synthesized from NTO electrochemically,as mentioned above,and characterized by a range of techniques and compared to existing insensitive high explosives.AZTO,produced via electrolysis of aqueous NTO solutions,shows similar sensitiveness to NTO in most tests,but has better thermal stability. During the synthesisofAZTO, anothernew species,azotriazolone (azoTO),is also produced [42].AzoTO displays higher thermal and impact stability than AZTO but is more sensitive to electrostatic discharge.AZTO sensitiveness is not affected by the presence of azoTO (formed concomitantly during synthesis)at levels of <20%.Crude samples and recrystallized samples react similarly in the tests performed.The ease of synthesis,low sensitiveness and moderate energy output andthe structure and oxygen balance of AZTO suggest that AZTO may be an economical and environmentally-friendly addition to the existing range of IHE.Azoxytriazolone (AZTO)precipitates from solution in good yield [42].Thus,the formation of solidAZTO from waste NTO solutions could prove to be a very economical method of remediation.AzoTO is present at levels of 10-20%in typical batches of AZTO and cannot be removed by recrystallization.The amount of azoTO can be limited to<5%by use of lower cell temperature,which also increases the yield of AZTO from the reaction.AzoTO can be prepared in pure form via electrochemical reduction of AZTO [42].
They carried out small-scale tests on several different samples.Since typical samples of AZTO contain around 15% azoTO as a side product,it was crucial to determine the effect of azoTO on the behavior of AZTO.Tests were carried out on three sets of samples:typical AZTO samples with approximately 15%azoTO,AZTO samples with low azoTO content(<5%),and samples of pure azoTO.AZTO samples also might contain traces (<3%) of hydrazotriazolone and/or aminotriazolone [42](these can be removed by recrystallization from DMSO-water).The authors said that this process causes the proportion of azotriazolone to increase slightly and leaves traces of DMSO even after the samples have been washed many times with water and dried in vacuum at 100 °C for 48 h.The recrystallized samples in the study were also tested to assess the effect of minor impurities and residual DMSO on the sensitiveness of the samples.The samples were tested for sensitiveness to impact,friction,electrostatic discharge (ESD).The results are compared with data for established materials included for comparison.AZTO behaved similarly to NTO in impact and ESD tests and showed greater stability in the friction tests.On the other hand,AzoTO displayed greater sensitiveness to ESD than AZTO,but AZTO samples containing typical amounts of azoTO (<20%)were not found to display increased sensitiveness to ESD.However,the tests indicated that a higher azo content of 30%was suff i cient to increase the ESD threshold of AZTO samples.On the other hand,azoTO was found to be much less sensitive to impact than either NTO or AZTO.No measurable differences were observed in sensitiveness between AZTO samples synthesized at low temperature and synthesized at room temperature.There were also no observable differences between samples that had been recrystallized from DMSO-water and those that had simply been washed with water.
AZTO is soluble in DMSO but has very poor solubility in all other organic solvents,including DMF,acetone,methanol,ethanol and acetonitrile.Also the solubility in water is very low,measured at 20 ± 5 mg L-1at 18-19 °C.The investigation reveals that AZTO has good solubility (and stability)in neat sulfuric acid,from which it can be precipitated via addition of the solution into water.The material also dissolves readily in aqueous alkali due to deprotonation of the triazole ring(s).
When AZTO is prepared at room temperature and washed with water,small,elongated platelets of variable size are obtained,which agglomerate extensively to form rosette-like particulates up to around 30 mm in size.In the same way,low temperature synthesis generates similar aggregated platelets. On the other hand,AzoTO,precipitated by acidif i cation of the alkaline product solution and washed with water,gives small oval platelets resembling those of AZTO but more uniform in size,and less aggregation is seen.
In the same study,values for detonation pressure (P)and velocity of detonation (VoD)were calculated using CHEETAH 2.0 thermochemical code.Heats of formation,which are required input for CHEETAH,were estimated using the empirical method of Keshavarz [68]and compared with the data for NTO,TATB and RDX.The estimated ΔHfvalues follow the expected trend [69]:azo > azoxy > nitro (NTO),and the value already reported for NTO using this method was found to be -92 kJ mol-1[68],in good agreement with the experimental value [50].The calculation results suggest that the new materials obtained are less energetic than NTO,but slightly more powerful than TATB.
The results show that AZTO and azoTO behave similarly to NTO in most sensitivity tests.Both of them have exceptionally high thermal stability,almost comparable to the level of TATB in the DSC test.AzoTO was found to be thermally slightly more stable than AZTO,but it was more sensitive to ESD.AZTO samples containing high enough amounts of azoTO (30%)also showed increased sensitiveness to ESD.However,the presence of azoTO (10-20%)had no measurable effect on the sensitivity ofAZTO.FTIR spectroscopy and DSC results indicated that the temperature of synthesis might affect the conformation or crystal structure of AZTO,but this did not have a signif i cant inf l uence on either the decomposition energy or the behavior of the material in standard sensitivity tests.AZTO has a higher energy of decomposition than azoTO as determined by DSC.It is expected as a result of its higher oxygen balance.The value of around 1600 J g-1forAZTO is comparable with the value of around 570 J g-1for NTO in the same test.Theoretical performance parameters indicate that AZTO and azoTO are highdensity,moderate power energetic materials.
5.Syntheses of azo-bridged triazoles via coupling reactions
Pang et al.reported the synthesis of 4,4′-azo-1,2,4-triazole(22)[27,35]via N—NH2coupling in 4-amino-1,2,4-triazole. The reaction of 4-amino-1,2,4-triazole with sodium dichloroisocyanurate (SDCI)afforded tetrazene(N—N=N—N)-linked bi(1,2,4-triazole)22 in excellent yield.Increasing the molar ratio of SDCI to 4-amino-1,2,4-triazole,a chlorinated product,1,5,5′-trichloro-4,4′-azo-1,2,4-triazole,was formed. These compounds were characterized by MS,1H NMR,13C NMR,and elemental analysis.They also obtained 2,5,2′-triazido-1,1′-azo-1,3,4-triazole (23) starting with the chlorinated product(see Fig.10)and characterized [27,36,37].
Later on they reported the effective synthesis and properties of 1,1′-azobis-1,2,3-triazole (24),which contains eight directly linked nitrogen atoms (N8structure).The larger the number of directly linked nitrogen atoms,the more diff i cult the compound is to synthesize.The diff i culties in synthesizing and handling polynitrogen compounds are a direct consequence of their high endothermicities;a further complication is the almost complete absence of methodology for preparing such compounds.
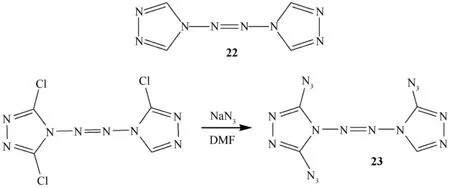
Fig.10.Synthesis of azido derivative (23)of compound 22.
Treatment of 1-amino-1,2,3-triazole with sodium dichloroisocyanurate (SDCI)in acetonitrile at low temperature for 30 min led to the isolation of 24 in 78%yield as a yellow solid (Fig.11)that was well characterized [27,32,36,37].Compound 24 is surprisingly stable.It undergoes thermal decomposition at 193.8 °C,which would be the highest decomposition temperature reported for compounds with eight-nitrogen chains.Its decomposition temperature is also much higher than those of hexazene (N6)ligand (140 °C)and N5+(70 °C),demonstrating the importance of the combined presence of acyclic and cyclic moieties in stabilizing the catenated nitrogen atoms. Probably the presence of the delocalized π-system is responsible for the remarkable stability of this type of compounds.
The crystal density of 24 was determined to be 1.640 g cm-3and was calculated to be 1.620 g cm-3.The heat of formation of 24 was predicted to be+962 kJ mol-1(+5869 kJ kg-1)by wellestablished methods.Compound 24 has higher density and heat of formation than 22 due to its longer nitrogen chain.The value of H50of 24 is 16.6 cm [less sensitive than TAAT (6.2 cm)and PETN (11 cm),but slightly more sensitive than RDX (28 cm)].
It is a photochromic molecule that undergoes a reversible color change when subjected to irradiation.In the natural state 24 is light yellow,and it becomes blue upon irradiation by sunlight or xenon light.Its unique photochromic feature is due to its trans-cis photoisomerization,which was conf i rmed by Raman spectroscopy.The UV-vis spectral changes of 24 in the solid state upon irradiation at room temperature in the 200-800 nm region.The characteristic absorption peak of 6 is located at 594.5 nm.The absorption peaks located at 234 and 346 nm are assignable to the π to π*electronic transition of the heterocycle and the n to π*electronic transition of the azo group,respectively,suggesting that many conjugated double bonds exist in 24 which cause redshifts compared with azobenzene-based compounds.
In conclusion,1,1′-azobis-1,2,3-triazole was reported as a novel high-nitrogen compound having the highest decomposition temperature for compounds with eight-nitrogen chains.It was designed and synthesized,and its photochromism was investigated.The authors did not investigate ballistic properties of the molecule except H50value.
Although the majority of investigations focus on the coupling of C—NH2heterocycles,compounds with the N—NH2moiety are also favorable precursors to diazo-bridged energetic materials [70].Compared with the former type,oxidative coupling of N—NH2substrates enables the formation of longer catenated nitrogen-atom chains,which contribute markedly to higher heats of formation.
Shreeve et al.synthesized some N-diazo-bridged azoles(nitroazoles,including nitropyrazoles,nitroimidazoles,and 3-nitro-1,2,4-triazole) based on oxidative coupling of N-aminoazoles [71].Incorporation of extended catenated nitrogen-atom chains with nitro groups led to compounds with favorable functional compatibilities.This combination of the groups gave rise to a series of high-density energetic materials(HEDMs)having high heats of formation,enhanced densities,positive oxygen balances,and good detonation properties while retaining excellent thermal stabilities and relatively low impact sensitivities.The calculated and experimental studies showedthat a delicate balance exists between the length of the nitrogen atom chain,energetic performance,and inherent stability hence,providing a promising strategy for some other advanced energetic materials.
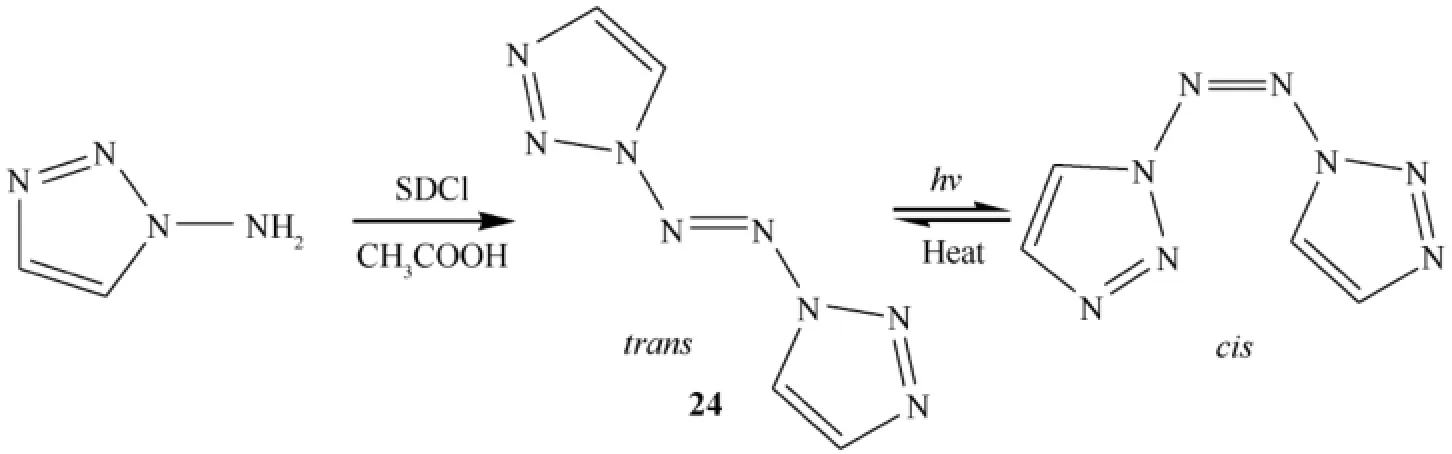
Fig.11.Synthesis and conversion of azo compound 24.
They prepared N-amino nitroazoles based on the literature procedure [11,72].Nitroazoles (1 mmol)in acetonitrile (5 mL)were cooled in an ice-salt bath.To this solution,a mixture of sodium dichloroisocyanurate (SDCl,0.5 mmol)and AcOH(0.2 mL)in water (10 mL)was added drop wise at-10 °C. After addition the reaction was stirred for 30 min at-10 °C. Then the reaction was neutralized to pH 7-8 with sodium bicarbonate solution.The precipitate was isolated by f i ltering the f i nal mixture and washed by cold water,dried in vacuum to give the desired product.
Characterization of the synthesized compounds was done by means of1H,13C NMR,IR spectra,elemental analyses (C,H,N)and X-ray crystallography.The melting and decomposition points were recorded on a differential scanning calorimeter(DSC,TA)at a scan rate of 5 °C min-1.Impact and frictionsensitivity measurements were determined.Densities were determined at RT by employing a gas pycnometer.Although many new compounds were reported in the article,the only triazole containing structure was 1,2-bis(3-nitro-1H-1,2,4-triazol-1-yl)diazene (25)(see Fig.12).
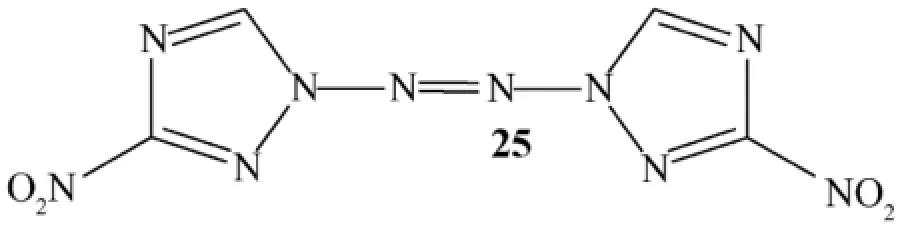
Fig.12.Structure of 1,2-bis(3-nitro-1H-1,2,4-triazol-1-yl)diazene (25).
It is a white solid and obtained in 70%yield.It decomposes at 247 °C.They also performed some computational study using density functional (B3LYP/6-31 G**) and MP2/6-311++G**level of approach.The X-ray analysis revealed that it is a planar structure.Its density was reported as 1.80 g cm-3,ΔHf [kJ g-1]:2.83, the calculated detonation pressure: 33.5 GPa,the calculated detonation velocity:8825 m s-1,impact sensitivity:10 J;and frictional sensitivity:160 N.
6.Azo-bridged triazole salts
On the other hand,the salts of nitrogen-rich energetic materials are also high-energy-density materials (HEDMs),and they have attracted considerable interest due to their lower vapor pressures,higher heats of formation,and enhanced thermal stabilities compared with their atomically similar nonionic analogs;additionally,they are environmentally friendly too[73-75].
The general methods for the preparation of energetic salts are by neutralization or metathesis reactions with N-protonated cations such as ammonium,hydrazinium,azolium,azinium,etc.,and C-,N-,or O-deprotonated anions such as nitroformate,azolate,or picrate [76-78].In order to get higher detonation performance and lower sensitivity,many energetic salts with nitrogen-rich cations ornitrogen-containing heterocyclic anions have been obtained through combination of carefully selected ion pairs.Their full characterization provides knowledge to enlighten the structure-property relationships in energetic salts [79-81].
Heterocyclic compounds with high nitrogen content are not only environmentally friendly but also have high heats of formation and endothermicity.The high nitrogen content of these compounds often leads to high crystal density;hence,it is associated with increased performance.The incorporation of a triazole ring into a compound is a known strategy for increasing thermal stability.Many triazole compounds show high thermal sensitivitycoupledwithlowsensitivitytoshockandimpact[54].
Recently, Shreeve etal.reported the synthesis of trinitromethyl-substituted triazoles,5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-bis-(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole (26)[46,47].The salts of these compounds are likely to exhibit high density and detonation properties and to be insensitive energetic materials.Salts of trinitromethylsubstituted triazoles,5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole,form a new class of highly dense energetic materials.Single-crystal X-ray structuring supports the formation of the cocrystal of 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole (26) with 3,5-diamino-1,2,4-triazole,which was found to be remarkably less impact-sensitive than the azo precursor.Fig.13 shows the synthetic pathway for trinitromethyl substituted azo triazole[47,82,83],and Fig.14 displays the synthesis of some energetic salts of trinitromethyl-substituted azo triazole [47].
Reactions of 26 with 2 mol of 3-aminotriazole,3,4-oxadiazole,diamino urea,3,4,5-triamino 1,2,4-triazole,and bis(guanidinium)tetrazine resulted in the formation of dianionic salts 27-31 (Fig.14).
All of the salts mentioned in the article were found to be nonhygroscopic and stable in air,and were isolated as crystalline materials in good yields.The reaction of hexanitro triazole having azo compound (26)with hydrazine or hydroxylamine produced a mixture of compounds which were not identif i ed.
The compounds (27-31)were fully characterized using IR and multinuclear NMR spectroscopy,elemental analysis,and differential scanning calorimetry.In the1H NMR spectra,the hydrogen signals of the cations were observed and they were easily assigned since there are no protons associated with the anions of triazoles considered.In the IR spectra,several main absorption bands around 1540,1480,1420,and 1310 cm-1were attributed to the triazole anions.
The intense bands in the range of 1600-1630 cm-1were assigned to the trinitromethyl groups present in the structures. In the13C NMR spectra,resonance bands for the trinitromethyl group appeared between 120 and 130 ppm.
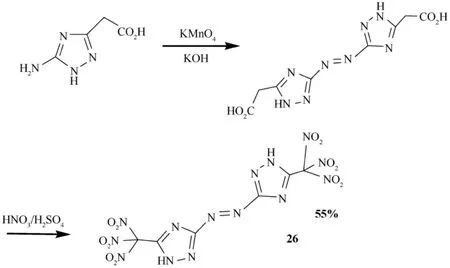
Fig.13.Synthetic pathway for trinitromethyl substituted azo triazole (26).
The preparation of crystals of 26 suitable for X-ray diffraction (XRD)analysis was unsuccessful.Therefore,an attempt by the authors was made to prepare cocrystals of 26 with aminesubstituted triazoles.Cocrystallization of different components represents supramolecular synthesis where hydrogen bonds link molecules.Thus,cocrystals are different from solid solutions or mixed crystals and can be considered as molecular complexes. The donor and acceptor functionalities can be brought together more easily than with single-component systems because the partners are more accessible to arrange themselves into an optimal geometry,leading to favorable intermolecular interactions [84-87].3-Amino-1,2,4-triazole,4-amino-1,2,4-triazole,and 3,5-diamino-1,2,4-triazole were used in the study in attempts to prepare cocrystals with hexanitrotriazole 26. Through N—H···O interactions,triazole 26 formed cocrystals with 3,5-diamino-1H-1,2,4-triazoleonly when equimolar amounts were dissolved in a mixture of 1:1 water:methanol solvent.
Based on the heats of formation calculated with Gaussian 03 and combined with experimentally determined densities,they calculated detonation properties of the energetic materials obtained in the study with the EXPLO5 program.The results identify them as potentially explosive compounds.
These salts all exhibit good physical and detonation properties,such as moderate thermal stabilities,high densities,moderate to high heats of formation,and high detonation pressures and velocities,as well as acceptable oxygen balance.Calculated detonation values for these compounds are comparable to those of explosives such asTNT and RDX.The salts of 26 are impact insensitive (9.0-15.0 J)compared to their molecular precursor(1.5 J).They are less sensitive than or comparable to RDX,which implies that they could be of interest for future applications because they are environmentally friendly and highperforming nitrogen-and oxygen-rich materials.Tables 2 and 3 show some physical and ballistic properties of azo-bridged polytrinitro triazole salts,respectively.
7.Epilogue
Simple triazole compounds often are not suff i ciently energetic.In that sense,tetrazoles are more effective.In azo-bridged triazoles this drawback has been tried to be eliminated. However,as presented,the azo-bridged triazoles,some of their derivatives and/or their salts are generally HEDMs having good thermal and ballistic properties.The salts show advantages over covalent analogs because of their lower vapor pressure,thus eliminating the inhalation toxicity.Furthermore,the salts usually have higher densities,higher thermal stabilities and larger critical diameters.Usually their water solubility and hygroscopic character are their disadvantages.The topic is still hot and in near future reports,more effective and interesting compounds of that sort are anticipated.
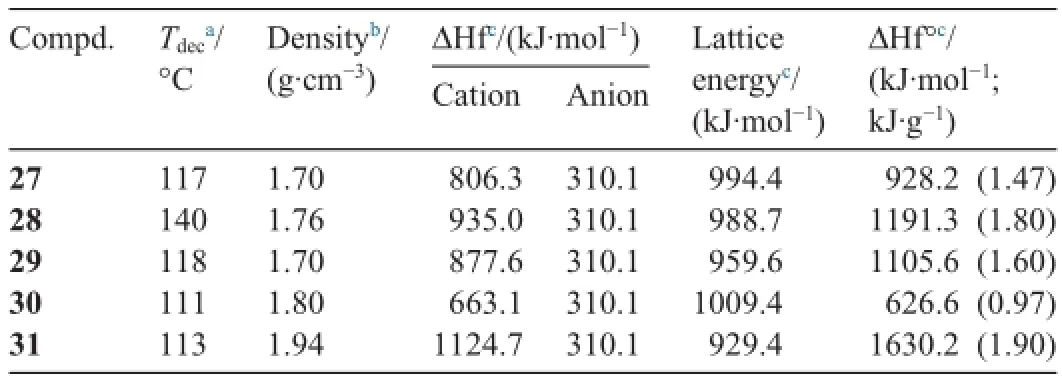
Table 2Some physical properties of azo-bridged polynitro triazole salts.
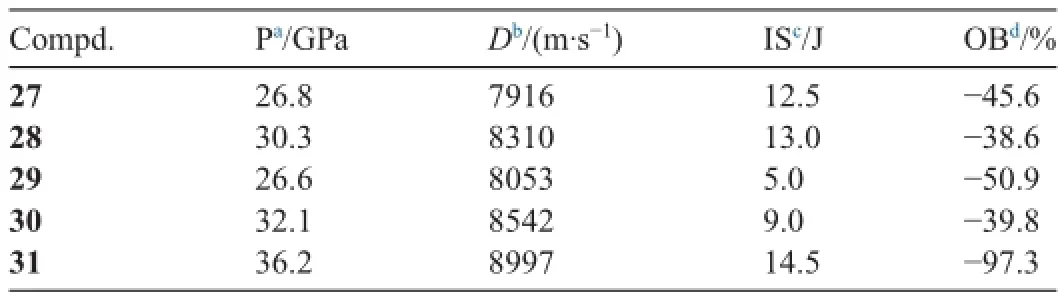
Table 3Some ballistic properties of azo-bridged polynitro triazole salts.
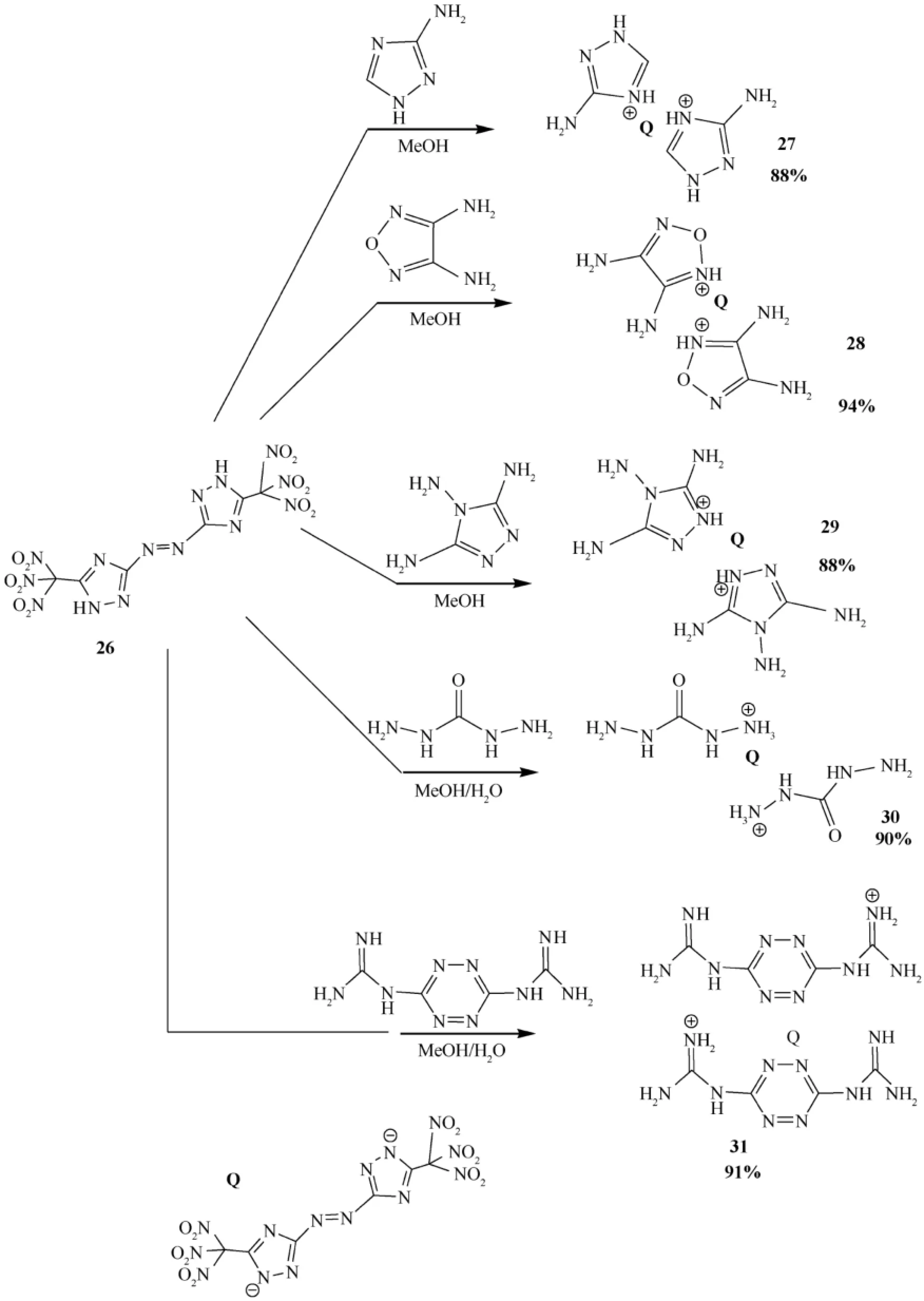
Fig.14.Synthesis of some energetic salts of trinitromethyl substituted azo-bridged triazole.
[1]Gao H, Shreeve JM.Azole-based energetic salts.Chem Rev 2011;111:7377-436.
[2]Klapötke TM.High energy density materials.Berlin:Springer;2007.
[3]Thottempudi V, Forohor F, Parrish DA, Shreeve JM. Tris(triazolo)benzene and its derivatives:high-density energetic materials. Angew Chem Int Ed 2012;51:9881-5.Angew Chem 2012;124: 10019-10023.
[4]HaigesR, Christe KO.Energetic high-nitrogen compounds:5-(trinitromethyl)-2h-tetrazole and -tetrazolates, preparation,characterization,and conversion into 5-(dinitromethyl)tetrazoles.Inorg Chem 2013;52:7249-60.
[5]Chavez DE,Hanson SK,Veauthier JM,Parrish DA.Electroactive explosives:nitrate ester-functionalized 1,2,4,5-tetrazines.Angew Chem Int Ed 2013;52:6876-9.Angew Chem 2013;125:7014-7017.
[6]Chavez DE,Tappan BC,Mason BA,Parrish DA.Synthesis and energetic properties of bis-(triaminoguanidinium) 3,3′-dinitro-5,5′-azo-1,2,4-triazolate (TAGDNAT):a new high-nitrogen material.Propellants Explos Pyrotech 2009;34:475-9.
[7]Yin P,Zhang J,Parrish DA,Shreeve JM.Energetic N,N′-ethylene-bridged bis(nitropyrazoles):diversif i ed functionalities and properties.Chem Eur J 2014;20:16529-36.
[8]Zhang Y,Parrish DA,Shreeve JM.Synthesis and properties of 3,4,5-trinitropyrazole-1-oland itsenergeticsalts.JMaterChem 2012;22:12659-65.
[9]Zhang Y, Parrish DA, Shreeve JM. 4-Nitramino-3,5-dinitropyrazole-based energetic salts.Chem Eur J 2012;18:987-94.
[10]Zhang Y, HuangY, Parrish DA, ShreeveJM.4-Amino-3,5-dinitropyrazolate salts-highly insensitive energetic materials.J Mater Chem 2011;21:6891-7.
[11]He C,Zhang J,Parrish DA,Shreeve JM.4-Chloro-3,5-dinitropyrazole: a precursor for promising insensitive energetic compounds.J Mater Chem A 2013;1:2863-8.
[12]Herv G, RousselC, Graindorge H.Selective preparation of 3,4,5-trinitro-1H-pyrazole:a stable all-carbon-nitrated arene.Angew Chem Int Ed 2010;49:3177-81.Angew Chem 2010;122:3245-3249.
[13]Sabatini JJ,Raab JM,Hann RK,Damavarapu R Jr,Klapötke TM. High-nitrogen-based pyrotechnics:developmentofperchlorate-free green-light illuminants for military and civilian applications.ChemAsian J 2012;7:1657-63.
[14]Sabatini JJ,Nagori AV,Chen G,Chu P,Damavarapu R,Klapötke TM. High-nitrogen-based pyrotechnics: longer- and brighter-burning,perchlorate-free, red-light illuminants for military and civilian applications.Chem Eur J 2012;18:628-31.
[15]Tao GH, Parrish DA, Shreeve JM. Nitrogen-rich 5-(1-methylhydrazinyl)tetrazole and its copper and silver complexes.Inorg Chem 2012;51:5305-12.
[16]Steinhauser G,Giester G,Wagner C,Weinberger P,Zachhuber B,Ramer G,et al.Nitrogen-rich compounds of the actinoids:dioxouranium(vi)5,5′-azobis[tetrazolide]pentahydrate and its unusually small uranyl angle. Inorg Chem 2012;51:6739-45.
[17]Joo YH,Gao HX,Parrish DA,Cho SG,Goh EM,Shreeve JM.Energetic salts based on nitroiminotetrazole-containing acetic acid.J Mater Chem 2012;22:6123-30.
[18]Fu Z,Su R,Wang Y,Wang YF,Zeng W,Xiao N,et al.Synthesis and characterization of energetic 3-nitro-1,2,4-oxadiazoles.Chem Eur J 2012;18:1886-9.
[19]Klapotke TM,Leroux M,Schmid PC,Stierstorfer J.Energetic materials based on 5,5′-diamino-4,4′-dinitramino-3,3′-bi-1,2,4-triazole.Chem Asian J 2015;doi:10.1002/asia.201500701.
[20]Tang Y,He C,Gao H,Shreeve JM.Energized nitro-substituted azoles through ether bridges.Chem Eur J 2012;18:4051-62.
[21]Huynh MHV,Hiskey MA,Chavez DE,Naud DL,Gilardi RD. Synthesis, characterization, and energetic properties ofdiazido heteroaromatichigh-nitrogen C-N compound.J Am Chem Soc 2005;127:12537-43.
[22]Abe T,Tao GH,Joo YH,Huang YG,Twamley B,Shreeve JM.Activation of the C-F bond:transformation of CF3N=N-into 5-azidotetrazoles. Angew Chem 2008;120:7195-8.
[23]KlapötkeTM,MayerP, SchulzA,Weigand JJ.1,5-Diamino-4-methyltetrazolium dinitramide.J Am Chem Soc 2005;127:2032-3.
[24]Sato T, NarazakiA, KawaguchiY, Niino H, Bucher G. Dicyanocarbodiimide and trinitreno-s-triazine generated by consecutive photolysis of triazido-s-triazine in a low-temperature nitrogen matrix. Angew Chem Int Ed 2003;42:5206-9.
[25]Klapötke TM,Piercey DG,Stierstorfer J.Amination of energetic anions:high-performing energetic materials.DaltonTrans 2012;41:9451-9.
[26]Klapötke TM,Piercey DG.1,1′-Azobis(tetrazole):A highly energetic nitrogen-rich compound with a N10chain.Inorg Chem 2011;50:2732-4.
[27]LiYC, QiC, LiSH,ZhangHJ,SunCH,YuYZ,etal. 1,1′-Azobis-1,2,3-triazole:a high-nitrogen compound with stable N8structure and photochromism.J Am Chem Soc 2010;132:12172-3.
[28]Tang YX,Yang HW,Shen JH,Wu B,Ju XH X.,Lu CX,et al.Synthesis and characterization of 1,1′-azobis (5-methyltetrazole).New J Chem 2012;36:2447-50.
[29]Chavez DE, Hiskey MA, Gilardi RD.3,3′-Azobis(6-amino-1,2,4,5-tetrazine):a novel high-nitrogen energetic material.Angew Chem 2000;112:1861-3.Angew Chem Int Ed 2000;39:1791-1793.
[30]Huynh MHV,Hiskey MA,Hartline EL,Montoya DP,Gilardi RD. Polyazido high-nitrogen compounds:hydrazo-and azo-1,3,5-triazine. Angew Chem 2004;116:5032-6.
[31]Evans RA,Hanley TL,Skidmore MA,Davis TP,Such GK,Yee LH,et al. The generic enhancement of photochromic dye switching speeds in a rigid polymer matrix.Nat Mater 2005;4:249-53.
[32]Gorostiza P,Isacoff EY.Optical switches for remote and noninvasive control of cell signaling.Science 2008;322:395-9.
[33]Cheben P,del Monte F,Worsfold DJ,Carlsson DJ,Grover CP,Mackenzie JD.A photorefractive organically modif i ed silica glass with high optical gain.Nature 2000;408:64-7.
[34]Koshima H,Ojima N,Uchimoto H.Mechanical motion of azobenzene crystals upon photoirradiation.J Am Chem Soc 2009;131:6890-1.
[35]Li S-H,Pang S-P,Li X-T,Yu Y-Z,Zhao X-Q.Synthesis of new tetrazene(N-N/N-N)-linked bi(1,2,4-triazole). Chin Chem Lett 2007;18:1176-8.
[36]Li S-H,Pang S-P,Li X-T,Yu Y-Z,Zhao X-Q.Synthesis and crystal structure of novel highly nitrogen-containing compound of polyazidotriazole.Chin J Org Chem 2008;4:727-31.
[37]Li S-H,Shi H-G,Sun C-H,Li X-T,Pang S-P,Yu Y-Z,et al.Q,Synthesis and crystal structure of nitrogen structure of nitrogen-rich compound: 2.5,2′-triazido-1,1′-azo-1,3,4-triazole.JChem Crystallogr2009;39: 13-16.
[38]Tang Y,Yang H,Wu B,Ju X,Lu C,Cheng G.Synthesis and characterization of a stable,catenated N11energetic salt.Angew Int Ed 2013;52:4875-7.
[39]Zhang J,Shreeve JM.3,3′-Dinitroamino-4,4′-azoxyfurazan and its derivatives:an assembly of diverse N-O building blocks for highperformance.Energ Mater J Am Chem Soc 2014;136:4437-45.
[40]Bian C,Wang K,Liang L,Zhang M,Li C,Zhaüou Z.Nitrogen-rich energetic salts of bis-heterocycle-substituted 1,2,3-triazole (HTANFT). Eur J Inorg Chem 2014;2014:6022-30.
[41]Guo Y,Tao G,Zeng Z,Gao H,Parrish DA,Shreeve JM.Energetic salts based on monoanionsofn,n-bis(1h-tetrazol-5-yl)amineand 5,5′-bis(tetrazole).Chem Eur J 2010;16:3753-62.
[42]Wallace L,Underwood CJ,Day AI,Buck DP.Electrochemical reduction of nitrotriazoles in aqueous media as an approach to the synthesis of new green energetic materials.New J Chem 2011;35:2894-901.
[43]Zhang J,He C,Parrish DA,Shreeve JM.Nitramines with varying sensitivities: functionalized dipyrazolyl-N-nitromethanamines as energetic materials.Chem Eur J 2013;19:8929-36.
[44]Yin P,Parrish DA,Shreeve JM.Bis(nitroamino-1,2,4-triazolates): N-Bridging strategy toward insensitive energetic materials.Angew Chem Int Ed 2014;53:12889-92.
[45]Joo YH,Shreeve JM.Synthesis,characterization,and energetic properties of 6-amino-tetrazolo[1,5-b]-1,2,4,5-tetrazine-7-N-oxide:a nitrogen-rich material with high density.Chem Asian J 2015;10:1130-2.
[46]Thottempudi V,Gao H,Shreeve JM.Trinitromethyl-substituted 5-nitroor3-azo-1,2,4-triazoles:synthesis, characterization, and energetic properties.J Am Chem Soc 2011;133:6464-71.
[47]Thottempudi V,Shreeve JM.Synthesis and promising properties of a new family ofhigh-density energeticsaltsof5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-Bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole. J Am Chem Soc 2011;133:19981-92.
[48]Tang Y,Gao H,Parrish DA,Shreeve JM.1,2,4-Triazole links and azo bridges yield energetic compounds.Chem A Euro J 2015;27:11401-7.
[49]Suceska M.EXPLO5,version 6.01,2013.
[50]Meyer R,Köhler J,Homburg A.Explosives.6th ed.Weinheim: Wiley-VCH;2007.
[51]Bulusu S,Damavarapu R,Autera JR,Behrens R Jr,Minier LM,Villanueva J,et al.Thermal rearrangement of 1,4-dinitroimidazole to 2,4-dinitroimidazole:characterization and investigation of the mechanism by mass spectrometry and isotope labeling.Phys Chem 1995;99:5009-15.
[52]Cho G, Park BS. Some high nitrogen derivatives of nitrotetrazolylimidazole as new high performance energetic compounds. Propellants Explos Pyrotech 1999;24:343-8.
[53]Cho JR,Kim KJ,Cho SG,Kim JK.Synthesis and characterization of 1-methyl-2,4,5-trinitroimidazole (MTNI). J Heterocycl Chem 2002;39:141-7.
[54]Agrawal JP,Hodgson RD.Organic chemistry of explosives.West Sussex: John Wiley&Sons;2007.
[55]BMA.Bundesanstalt für Materialforschung und-prüfung. <http://www .chilworth.co.uk/media/35390/bam_fallhammer_3.12.pdf>;2014.
[56]Zhang CJ.Review of the establishment of nitro group charge method and its applications.Hazard Mater 2009;161:21-8.
[57]Frontana-Uribe BA,Little RD,Ibanez JG,Palma A,Vasquez-Medrano R. Organic electrosynthesis:a promising green methodology in organic chemistry.Green Chem 2010;12:2099-119.
[58]Chen JP,Chang S-Y,Hung Y-T.Electrolysis,In:Wang LK,Hung Y,Shammas NK,editors.Handbook of environmental engineering,vol.3. Humana Press;2005.p.359-78.
[59]Scott K.Electrochemistry and sustainability,In:Clark JH,Macquarrie DJ,editors.Handbook of green science and technology.Wiley-Blackwell;2002.p.433-65.
[60]Cronin M,Day A,Wallace L.Electrochemical remediation produces a new high-nitrogen compound from NTO wastewaters.J Hazard Mater 2007;149:527-31.
[61]Smith MW,Cliff MD.NTO-based explosives formulations:a technology review.DSTO Technical Report DSTO-TR-0796. <http://www.dsto .defence.gov.au/corporate/reports/DSTOTR-0796.pdf>; 1999 [accessed 22.11.06].
[62]Underwood C,Wall C,Day AI,Provatas A,Wallace L.Electrolytic treatment of nitroazole solutions in the synthesis of new high-nitrogen compounds.Presented at PARARI 2009:9th Australian Explosives Ordnance Symposium,Adelaide,Australia,9-11 November 2009.
[63]Smith MW,Cliff MD,Childs D.Development of low vulnerability NTO/TNT explosives for artillery and mortar projectiles meeting insensitive munitions requirements,DSTO-TR-1338.Edinburgh,South Australia:Weapons Systems Division DSTO Defence Science and Technology Organisation;2002.
[64]Oxley JC,Smith JL,Moran JS.Decomposition of azo-and hydrazo-linked bis triazines.J Energ Mater 2009;27:63-93.
[65]Qi C,Li SH,Li YC,Yuan Y,Chen XK,Pang SP.A novel stable high-nitrogen energetic material:4,4′-azobis(1,2,4-triazole).J Mater Chem 2011;21:3221-5.
[66]Heppekausen J,Klapötke TM,Sproll SM.Synthesis of functionalized tetrazenes as energetic compounds.J Org Chem 2009;74:2460-6.
[67]Underwood CJ,Wall C,Provatas A,Wallace L.New high nitrogen compounds azoxytriazolone (AZTO)and azotriazolone (azoTO)as insensitive energetic materials.New J Chem 2012;36:2613-17.
[68]Keshavarz MH.Prediction of the condensed phase heat of formation of energetic compounds.J Hazard Mater 2011;190:330-44.
[69]Cohen N, Benson SW.Estimation ofheatsofformation of organic compounds by additivity methods.Chem Rev 1993;93:2419-38.
[70]Zhang Q, Shreeve JM. Wachsende ketten aus catenierten stickstoffatomen.Angew Chem 2013;125:8954-6.
[71]Yin P,Parrish DA,Shreeve JM.N-Diazo-bridged nitroazoles:catenated nitrogen-atom chains compatible with nitro functionalities.ChemA Eur J 2014;20:6707-12.
[72]Yin P,Zhang Q,Zhang J,Parrish DA,Shreeve JM.N-Trinitroethylamino functionalization of nitroimidazoles:a new strategy for high performance energetic materials.J Mater Chem A 2013;1:7500-10.
[73]Christe KO,Wilson WW,Sheehy JA,Boatz JA.N5+:ein neuartiges homoleptisches polystickstoff-ion als substanz mit hoher energiedichte. Angew Chem 1999;111:2112-18.
[74]Giles J.Green explosives:collateral damage.Nature 2004;427:580-1.
[75]Singh RP,Verma RD,Meshri DT,Shreeve JM.Energetic nitrogen-rich salts and ionic liquids.Angew Chem 2006;118:3664-82.Angew.Chem. Int.Ed.2006,45,3584-3601.
[76]Zeng Z,Gao HX,Twamley B,Shreeve JM.Energetic mono and dibasic 5-dinitromethyltetrazolates:synthesis,properties,and particle processing. J Mater Chem 2007;17:3819-26.
[77]Darwich C,Klapötke TM,Sabate CM.1,2,4-Triazolium-cation-based energetic salts.Chem Eur J 2008;14:5756-71.
[78]Gao HX,Zeng Z,Twamley B,Shreeve JM.Polycyano-anion-based energetic salts.Chem Eur J 2008;14:1282-90.
[79]Gao HX,Joo YH,Twamley B,Zhou ZQ,Shreeve JM.Hypergolic ionic liquids with the 2,2-dialkyltriazanium action. Angew Chem 2009;121:2830-3.
[80]Huang Y,Gao Y,Twamley B,Shreeve JM.Highly dense nitranilatescontaining nitrogen-rich cations.Chem Eur J 2009;15:917-23.
[81]Sabate CM,Klapötke TM.New energetic compounds based on the nitrogen-rich 5,5′-azotetrazolateanion ([C2N10]2-).New J Chem 2009;33:1605-17.
[82]Abdel-Megeed AM,Abdul-Rahaman HM,Alkaramany G-H,El-Gendy MA.Design,synthesis and molecular modeling study of acylated 1,2,4-triazole-3-acetates with potential anti-inf l ammatory activity.Eur J Med Chem 2009;44:117-23.
[83]Kofman TP,Uvarova TA,Kartseva GY.Synthesis and some reactions of 3-r-1,2,4-triazol-5-ylacetic acids.Zh Org Khim 1995;31:270-5.
[84]Desiraju GR.Crystal and co-crystal.Cryst Eng Comm 2003;5:466-7.
[85]Smolka T, Sustmann R, Boese R.Phenazine and meso-1,2-diphenyl-1,2-ethanediol-partners in photochromic cocrystals.J Prakt Chem 1999;34:378-83.
[86]Landenberger KB,Matzger A.Cocrystal engineering of a prototype energetic material:supramolecular chemistry of 2,4,6-trinitrotoluene.J Cryst Growth Des 2010;10:5341-7.
[87]Bolton O, MatzgerAJ.Improved stability and smart-material functionality realized in an energetic cocrystal.Angew Chem Int Ed 2011;50:8960-3.
Received 12 November 2015;accepted 13 November 2015 Available online 4 December 2015
Peer review under responsibility of China Ordnance Society.
*Corresponding author.Tel.:+903122103244.
E-mail address:lturker@metu.edu.tr (L.TÜRKER).
http://dx.doi.org/10.1016/j.dt.2015.11.002
2214-9147/© 2015 China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
© 2015 China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
- Defence Technology的其它文章
- Friction assisted solid state lap seam welding and additive manufacturing method
- Ballistic behavior of boron carbide reinforced AA7075 aluminium alloy using friction stir processing-An experimental study and analytical approach
- Optimization of three-loop missile autopilot gainunder crossover frequency constraint
- Enhancement of seal life through carbon composite back-up rings under shock loading conditions in defence applications
- Studies on impact sensitivity of nanosized trinitrotoluene (TNT)conf i ned in silica processed by sol-gel method
- Effect of f i bre orientations on the mechanical properties of kenaf-aramid hybrid composites for spall-liner application

