Innovative boron nitride-doped propellants
Thelm MANNING*,Rihrd FIELDKenneth KLINGAMANMihel FAIRJohn BOLOGNINIRoin CROWNOVERCrlton P.ADAMVirl PANCHALEugene ROZUMOVHenry GRAUPul MATTER,Mihel BEACHY,Christopher HOLT,Smuel SOPOK
aUS Army RDECOM ARDEC,Picatinny Arsenal,NJ,USA
bpH Matter,LLC,Columbus,OH,USA
cBenet Laboratory,Watervliet,NY,USA
Innovative boron nitride-doped propellants
Thelma MANNINGa,*,Richard FIELDa,Kenneth KLINGAMANa,Michael FAIRa,John BOLOGNINIa,Robin CROWNOVERa,Carlton P.ADAMa,Viral PANCHALa,Eugene ROZUMOVa,Henry GRAUa,Paul MATTERb,Michael BEACHYb,Christopher HOLTb,Samuel SOPOKc
aUS Army RDECOM ARDEC,Picatinny Arsenal,NJ,USA
bpH Matter,LLC,Columbus,OH,USA
cBenet Laboratory,Watervliet,NY,USA
The U.S.military has a need for more powerful propellants with balanced/stoichiometric amounts of fuel and oxidants.However,balanced and more powerful propellants lead to accelerated gun barrel erosion and markedly shortened useful barrel life.Boron nitride (BN)is an interesting potential additive for propellants that could reduce gun wear effects in advanced propellants (US patent pending 2015-026P).Hexagonal boron nitride is a good lubricant that can provide wear resistance and lower f l ame temperatures for gun barrels.Further,boron can dope steel,which drastically improves its strength and wear resistance,and can block the formation of softer carbides.A scalable synthesis method for producing boron nitride nano-particles that can be readily dispersed into propellants has been developed.Even dispersion of the nano-particles in a double-base propellant has been demonstrated using a solvent-based processing approach.Stability of a composite propellant with the BN additive was verif i ed.In this paper,results from propellant testing of boron nitride nano-composite propellants are presented,including closed bomb and wear and erosion testing.Detailed characterization of the erosion tester substrates before and after f i ring was obtained by electron microscopy,inductively coupled plasma and x-ray photoelectron spectroscopy.This promising boron nitride additive shows the ability to improve gun wear and erosion resistance without any destabilizing effects to the propellant.Potential applications could include less erosive propellants in propellant ammunition for large,medium and small diameter f i re arms.
Nano-boron nitride;Additive;Lubricant;Gun barrel;Wear and erosion;Gun propellant
1.Introduction
The U.S.military has a need for more powerful propellants with balanced/stoichiometric amounts of fuel and oxidants to provide an advantage to its warf i ghters.The useful life of each gun is limited either by the effects of barrel erosion on its performance or metal fatigue.The enlargement of the origin of rif l ing or the down bore area can affect ammunition performance resulting in range and accuracy loss,fuze malfunctions,excessive torsional impulse and excessive muzzle f l ash and blast overpressure.With increased demands for guns that f i re faster,farther,and more accurately,barrel erosion has worsenedand become a major limitation in developing better guns [1-3]. For example,with advanced propellants 155 mm artillery barrels may only survive a couple hundred rounds before they must be replaced at a cost of over$70,000 [4].
Many low vulnerability (LOVA)propellant formulations contain RDX,and it has been convincingly shown by several investigators that RDX is highly chemically erosive.New,experimental low-erosivity LOVA propellants have been produced by reducing RDX content and introducing nitrogen-rich energetic binder or f i ller compounds.The resulting propellant combustion gases,rich in nitrogen,act to re-nitride bore surfaces during f i ring and inhibit erosive surface reactions.The result is increased bore hardness,increased resistance to melting,and reduced chemical erosion.The lowered hydrogen concentration in the combustion gas of some of these propellants may also reduce hydrogen-assisted cracking of the boresurface.Of the high-nitrogen propellants under development,the majority possess impetus and f l ame temperatures lower than RDX:a compromise between performance,sensitiveness and erosivity must be reached in these cases.

Fig.1.SEM image of double base powder and amorphous BN (1:1 by weight)dispersed with acetone/ethanol using a sonic horn,and deposited onto a glass slide(48,000× magnif i cation).
Signif i cant effort has recently been directed at understanding the erosion mechanisms for barrels coated with protective refractory metals.The most plausible mechanism is that micro-cracks in the coatings,present from the time of manufacture,propagate due to pressure and thermal stress cycling and eventually reach the gun steel substrate.Through numerical modeling and analysis of eroded barrels,a number of investigators have shown that once cracks reach the substrate,chemical erosion,gas wash,and high interfacial temperatures cause pitting of the substrate and eventually undermine the coating.Segments of coating are subsequently removed by the f l ow or engagement with the projectile,and at this point the erosion rate of coated barrels may exceed that of steel barrels.A number of ways to mitigate this erosion pathway have been suggested,including development of better coating techniques to avoid the initial micro-cracks,pre-nitriding the gun steel before coating to slow down substrate erosion,introducing a protective interlayer,and controlled barrel storage and post-f i ring treatment to prevent oxidation of exposed substrate.Modeling and experiments have additionally shown that,with the notable exception of chromium,the erosion resistance of refractory metal coatings varies among differentpropellantgaschemistryenvironments.Ceramicadditives to the propellant can theoretically reduce barrel deterioration by coating the inside of barrels,but implementation of composite propellants with conventional ceramics (i.e.alumina)has not resulted in improved wear resistance to date.Due to challenges with dispersing the particles in the propellant,and due to abrasion from incomplete sublimation,propellant and ceramic composites that produce regenerative wear-resistant coatings have not been demonstrated.Due to very good wear characteristics and thermal resistance,ceramic barrel liners have been identif i ed as a promising technology for some time.However,the susceptibility of ceramics to fracture,driven by stress induced bythedifferentthermalexpansionpropertiesofsteelandceramics,has prevented their widespread use.
The currently f i elded 155 mm artillery propelling charge,M232/M232A1,has exhibited spiral wear and erosion problems.This was due to either the wear reducing liner,containing titanium dioxide,talc and wax,or other contributing factors. This resulted from the propellant chemistry and interaction of the combustion products within the gun tube wall.Modeling and simulation studies performed by Dr Samuel Sopok from Benet Labs have determined that the reaction of titanium dioxide with the talc and wax produced a residue that was hard to remove [5].This product was an abrasive residue (number 80 ceramic grit)that built up in the gun barrel.This caused a spiral rif l ing imbalance and accelerated gun barrel erosion which markedly shortened gun barrel life.Boron nitride is an interesting potential additive to propellants that could reduce gun wear effects in advanced propellants.It has the properties of providing metal coating/lubricating,and steel hardening properties and nitrogen cooling effects.
On the other hand,boron nitride (in the form of crystalline hexagonal BN or amorphous BN)has interesting properties for a propellant additive (US patent pending).BN can form a lubricating coating on barrel walls.BN coated ammunition is currently used commercially for small arms to lubricate barrels and ammunition [2].Further,boron can be used to dope steel,which drastically improves its strength and wear resistance.Boron-doped steel is used to reduce wear in numerous industrial applications and is typically produced by annealing steel that has been packed in boron oxide [6-12].In this paper,we explore a new concept where BN is used as a propellant additive that can regeneratively coat and harden steel barrels.The BN is in the form of a nano-particle that can be evenly dispersed in the propellant without negative impact on its performance.Dispersion studies were performed to determine how easily the amorphous BN nano-particles could be dispersed in propellants.Scanning electron microscope(SEM)image of the BN in a commercial off-the-shelf double base propellant (1:1 by weight) dispersed with acetone/alcohol is shown in Fig.1.The BN nano-particles were evenly dispersed and measured 38 nm on average.
Further,the production of the nano-scale boron nitride is economical.An economic model was constructed to project the cost of producing BN nano-particles from raw materials at the anticipated commercial scale (50,000 kg/yr).Based on this analysis,the projected cost of BN at the 50,000 kg/yr scale was found to be$91.15 per kg.This cost is reasonable because we use such a small percentage in the propellant formulation.
2.Experimental section
2.1.X-ray photoelectron spectroscopy(XPS)
For XPS analysis,powder samples were pelletized by hand and loaded into a steel pellet sample holder.The samples were loaded into a Kratos axis ultra XPS and pumped down overnight to achieve ultra-high vacuum levels.All samples were f i rst analyzed in a survey scan from 1200 to 0 eV to determine the elements present on the surface.All samples analyzed contained B,N,and lower levels of C and O.The carbon and oxygen are typical from atmospheric contamination (dust and oils).Detailed scans were run for B 1s and N 1s regions to determine the oxidation state and ratio of species,with a charge neutralizer applied to the samples to prevent spectra shifting.
2.2.Differential scanning calorimetry(DSC)and vacuum thermal stability(VTS)tests
Testing was conducted per NATO PIP US/202.01.020 “differential scanning calorimetry (DSC)”which is also described in ASTM E537-07 “standard test method for the thermal stability of chemicals by differential scanning calorimetry”and MIL-STD 1751A method 1072 “differential scanning calorimetry (DSC).”A differential scanning calorimeter (TA instruments model 2910)was utilized to determine the ignition temperature (exothermic peak)and melting temperature (endothermic peak)of the material.The test was carried out in nitrogen gas.The temperature ranged from room temperature to 400 °C.The sample container (aluminum pan and cover)containing the material was placed into the measuring cell and heated at a rate of 10 °C and 20 °C per minute.The peak temperatures (corresponding to exothermic decomposition and endothermic melting)along with onset temperature were determined and recorded.
Vacuum stability testing is performed in accordance to STANAG 4556 ED.1 (Explosives:vacuum stability test).This standard testing procedure measures the stability of an explosive at an elevated temperature under vacuum.The stability of a candidate explosive is determined by the amount of gas evolved.To qualify as a chemically stable material,no explosive may produce more than 2 ml of gas per gram.The material is tested for 48 hours at 90 °C.
2.3.Closed bomb testing
One of the ways to assess propellant performance is through combustion testing.The closed bomb test is a standard device used to measure gasif i cation rates for energetic materials. Knowledge of propellant chemical formulation and geometry allows for calculation of a linear burn rate from the measured pressure versus time data.Performance is given in terms of relative quickness (RQ)and relative force (RF).Relative quickness applies to the speed with which the material burns and is a comparison of the pressurization rates (dP/dt).Relative force is a comparison of the peak pressure levels observed in the bomb(Pmax).There were two closed bomb tests conducted using the procedure P1-BPP MIL-STD-286C,Section 801.1.2 and guided by STANAG 4115.For the f i rst test,two shell-shaped inserts of heat-treated and polished 4340 steel were placed inside the chamber to determine if any reactivity occurs between the BN and the steel,and to determine if a boron-based coating forms.The second test was performed to determine the burn rates of the RPD-380 composite propellant with and without BN in preparation for the wear and erosion test.

Fig.2.RPD380 without BN-single perf grain used in erosion testing.
2.4.Composite propellant preparation for wear and erosion test
It is hypothesized that boron nitride (BN)in nano-particle size range incorporated into a propellant during mixing may reduce the erosion that propellant combustion gases cause to a gun bore.In order to provide an initial test of this proposal,two batches of nominal double base propellant composition RPD-380 were fabricated using a solvent mixing process.The two batches consisted of the baseline RPD-380 formulation and the same formulation with nano-scale boron nitride sample provided.The propellants were extruded in single perforation strand form and cut to grain length,as shown in Figs.2 and 3,respectively.Closed bomb testing was conducted on each of the materials and then analyzed for acceptable burning properties and burning rate.Both propellants are high energy propellants with high f l ame temperatures.Using the MCVEC [13]thermochemical equilibrium program,baseline RPD-380 calculated heat of explosion is 1156 cal/g,with a f l ame temperature of 3573 K at loading density of 0.13 g/cc;the baseline RPD-380 with BN composition has a calculated heat of explosion of 1100 cal/g and f l ame temperature of 3451 K at the same loading density.
2.5.Propellant wear and erosion testing

Fig.3.BN-RPD380 (US patent pending)single perf grains used in erosion testing.
The goal of this testing is to determine whether the addition of the nano-scale BN to a propellant does reduce the erosion on typical gun steel,and to provide gun steel samples exposed to erosive gas f l ows for further analysis.The ARDEC erosion tester used produces the erosive environment by burning a known amount of propellant in a high pressure vented 200 cc bomb,and recording the weight of a metal insert sleeve before and after f i ring.The loss in weight of the insert sleeve is the erosive loss.The erosion tester is a modif i ed closed bomb that has a burst disk which breaks at a certain pressure (determined by the thickness and material of the disk)and then vents the bomb gases outward through the bore of the cylindrical steel test insert sleeve.
The two propellants were each f i red using two steel sleeves of different hardness.Prior to f i ring the baseline or propellant with BN,all sleeves had a shot of JA2 f i red through them with the intent to smooth out machining defects in the sleeves and have them be at a more uniform initial state prior to testing.The hardened steel sleeves had three shots each f i red in them;these were sleeves labeled 1 and 2,with the baseline propellant f i red in sleeve 1 and the propellant with BN in sleeve 2,as shown in Figs.4 and 5 respectively.The unhardened sleeves had four(baseline)or f i ve (with BN)shots each f i red in them,with the baseline propellant in sleeve 3 and the propellant with BN in sleeve 4.The f i nal shots in sleeves 3 and 4 were not cleaned and the sleeves were not weighed after those shots so that the residue could be retained for analysis.
The propellant with added BN burned at a lower rate than the baseline,so based on a closed bomb calculation an extra gram of that propellant was f i red in each of its shots to account for the pressure difference and thus provide a better pressure match with the shots generated by the baseline propellant.In order to get the best and most accurate results it is important to keep conditions in the erosion tester as similar as possible.In the present tests the maximum pressure range of 20,000-22,000 psi typically used for routine erosion tests was targeted.The thermo-chemistry,burning rate and form function of the propellant grains to be tested resulted in required propellant sample loading densities (grams of propellant per unit bomb volume)nearly 50%lower than is typically employed.Consideration of the propellant weight burned obviously affects the f l ow time of the combustion gases through the insert sleeve.In addition,in comparing individual tests,there are always some minor variations in the burning process and the peak pressure developed at burst disk rupture.

Fig.4.Hardened steel sleeves-cleaned after 3 shots.
The 26.4 g of baseline propellant and 27.3 g of propellant with BN were f i red.The propellants did not have the exact same weight due to variation in weights of individual grains.An extra shot was f i red in sleeve 4 due to having enough remaining material.All shots were ignited by using an electric match initiated 1-g sample of M38 ball powder.Using ball powder rather than black powder as an igniter reduces the sulfur and potassium compound content of the combustion products to a very low level.
The sleeves are marked with a number of small indentations equal to their sleeve number to identify them and to ensure that the sleeve was facing the same way on every shot.Each sleeve was cleaned and weighed before and after every shot(excluding the last shots on sleeves 3 and 4,as mentioned above)in order to measure the weight loss that each shot caused.Sleeves were cleaned with soap and water until no visible residue remained and then thoroughly dried prior to weighing.
3.Results and discussion
3.1.Boron nitride characteristics
Under this project,a proprietary process for production of dispersible boron nitride nano-particles to use as a propellant additive was developed.The process does not use a catalyst,and the boron nitride precursor is free (<1 ppm detection limit)of metal contamination.The process involves nucleation of aboron and nitrogen based precursor,so the product particle size can be controlled based on the reactant concentration.Typical bulk BET surface areas ranged from 20 m2/g to 80 m2/g,depending on process conditions,consistent with spherical particle diameters from 143 nm down to 37 nm.

Fig.5.Insert sleeve 2.
Electron microscopy of the BN product shows that the morphology of the BN is indeed nano-particle spheres.Fig.6 shows SEM images of typical particles.Although the particles agglomerate upon drying,it is clear from the images that the individual particles are spheres with diameters in the nanometer range.

Fig.6.Scanning electron micrographs of BN nano-particles (US patent pending)used for propellant additive testing.
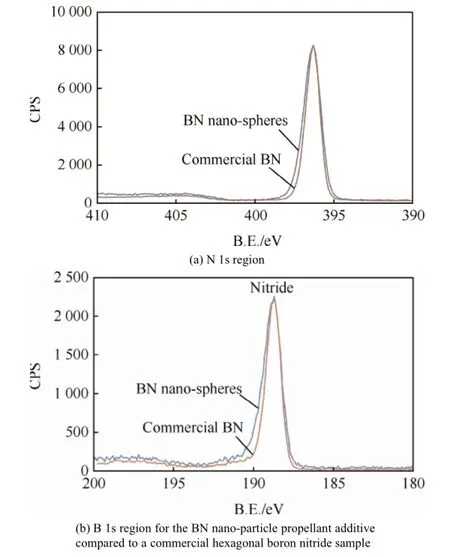
Fig.7.XPS analysis.
The surface of the BN nano-particles was characterized to verify the material composition and how it may interact with propellant.X-ray photo-electron spectroscopy (XPS)was used for this characterization.XPS analysis is sensitive to the f i rst few atomic layers of a material,so it can be considered a surface analysis tool.
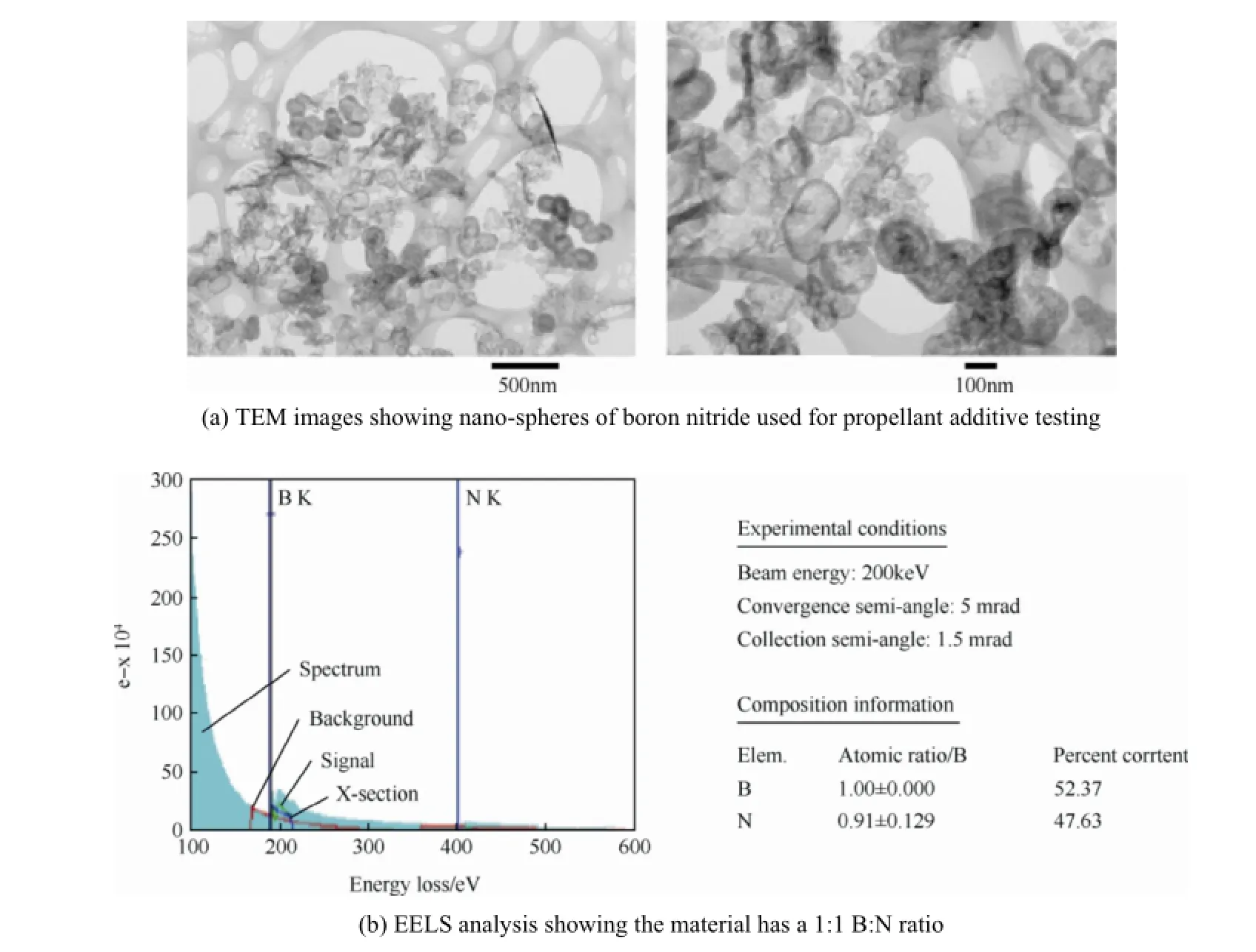
Fig.8.TEM images showing nano-spheres of boron nitride used for propellant additive testing (US patent pending)and EELS analysis showing the material has a 1:1 B:N ratio (US patent pending).
Figs.7(a)and (b)show the N 1s and B 1s regions for the samples respectively.The BN nano-particles prepared are compared to conventional commercial hexagonal boron nitride obtained fromAlfaAesar.As can be seen from these f i gures,the ratio of B:N is the same for both materials.Further the oxidation states of the boron and the nitrogen are the same in each sample.The binding energy (oxidation state)for boron is consistent with literature values for boron nitride.It should be noted that XPS analysis has been repeated on materials that were aged in air for 6 months and no change in oxidation states was observed.
Transmission electron microscopy (TEM)imaging in conjunction with electron energy loss spectroscopy (EELS)was also run on the material to characterize the BN particles. Fig.8(a)shows the TEM images of the particles,which appear to be amorphous spheres.Fig.8(b)shows the EELS analysis,within the accuracy of the measurement,which verif i ed that the material has a 1:1 B:N ratio,consistent with boron nitride.
3.2.Composite propellant testing
In the f i rst round of testing,a composite propellant was prepared using a commercially-available nitrocellulose double-base propellant,IMR-4198 (Hodgdon).Preparation of the composite propellantwasconducted undersolvent(acetone :ethanol 1:1)in a small rotating mixing chamber,with suff i cient solvent added to soften the propellant to a dough-like consistency.Two batches were prepared:one with additional B-wt%BNnano-particlesandtheotherwithoutadditionofBN. Both propellants were subjected to the same mixing conditions(~2daysinthemixingchamber)toprovideacontrolcomparison in testing.
RPD-380 nitrocellulose based propellant with and without BN were prepared using the conventional solvent process in a horizontal sigma blade mixer.The propellants were extruded into single perforated geometry.
3.2.1.Differential scanning calorimetry and vacuum thermal stability tests
DSC measures the temperatures and heat f l ows associated with transitions in materials as a function of time and temperature in a controlled atmosphere.These measurements provide quantitative and qualitative information about physical and chemical changes that involve endothermic or exothermic processes,or changes in heat capacity.This test method is recommended as an early screening test for detecting the thermal hazards of an uncharacterized substance.For explosives and energetic materials study or development,a DSC may be used to measure safely,the energy released by a small amount of a sample without any catastrophic consequences.
DSC tests were performed in triplicate on the materials (both IMR 4198 and IMR 4198 with B-wt%BN)to determine the combustion initiation temperatures.The onset temperature is indicated by examining any deviation in the reaction mass temperature from the heating rate.The peak height or area under the curve indicates the magnitude of the energetic activity.The DSC test results showed that the average onset exothermic reaction at heating rate of 10 °C/min was 161 °C and the average peak exothermic was 207 °C for both samples tested.It appears that the addition of BN (B wt%)did not have anysignif i cant effect on DSC thermal test results,indicating that the BN does not destabilize the propellant under the DSC conditions.The heat of reactions also remained unchanged within the measurement capabilities of the technique.
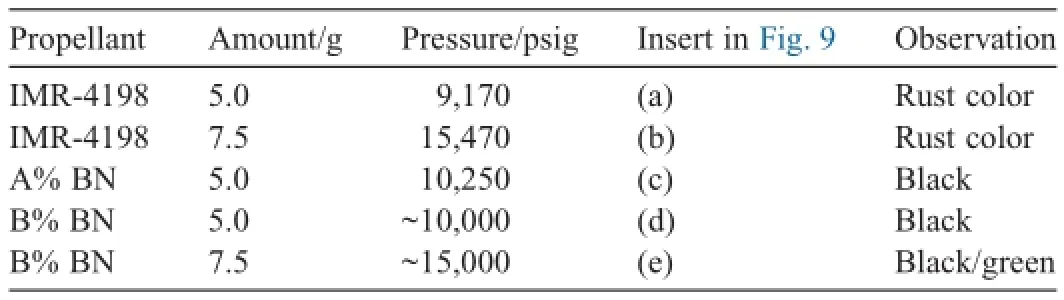
Table 1aOverview of bomb tests with steel inserts (A < B).
VTS is performed in accordance to STANAG 4556 ED.1(Explosives:vacuum stability test).This standard testing procedure measures the stability of an explosive at an elevated temperature under vacuum.The stability of a candidate explosiveisdeterminedbytheamountofgasevolved.Toqualify as a chemically stable material,no explosive may produce more than 2 ml of gas per gram.The material was tested for 48 hours at 90 °C.Based on the test results,which were conducted in accordance with the def i ning criteria of STANAG 4556 ED,the RDD24F-001T5 propellant lot with B%BN produced less than 2 mlofgaspergramforaf i vegramsampleandthereforepasses vacuum stability criteria according to military specif i cations.
3.2.2.Bomb testing with steel inserts
The f i rst closed bomb test was used to determine the relative quickness (RQ)/relative force (RF)to characterize propellant samples and the resulting coating formed on steel inserts.An overview of the results of the tests is given in Table 1(a).Pure IMR-4198 and the B%composite propellant were tested at different loadings.Additionally,a physical mixture of 1:1 IMR-4198 and the composite were tested to obtain B%BN composite.The maximum pressure was measured with a high speed DAQ system for the pure IMR-4198 and the B%BN composite.
A photograph of the steel inserts that resulted from this testing is shown in Fig.9.Stark differences in surface oxidation of the steel inserts were observed.Visually,there were some dramatic differences between steel inserts that were f i redwithout an additive versus inserts with BN additive.Samples(a)and (b)did not have any additive,and both samples looked oxidized with a distinctive orange rust color.The oxidation was worse for the higher propellant loading (higher chamber pressure).Samples (c)and (d)with B%BN respectively at 5 g loading each were darker after f i ring,but did not have an orange color indicative of steel oxidation.Sample (e)was f i red with 7.5 g of IMR-4198 with B%BN and had a green color.These initial results are promising,as it seems that BN may be preventing steel oxidation;however,more work remains to understand the nature of this coating and verify any effect on wear and erosion resistance.
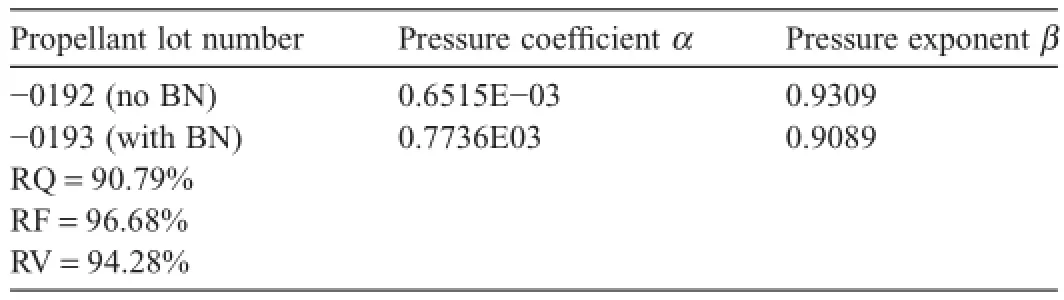
Table 1bClosed bomb test results for RPD-380 baseline propellant with and without BN.
A 200 cc high pressure closed bomb testing of the RPD-380 baseline propellant was also performed to determine the burn rates with and without BN added in the propellant,as shown in Table 1(b).The RQ,RF and relative vivacity (RV)values less than 100%value can be explained as due to the high percentage of BN added in the propellant formulation to simulate the worst case scenario of adding an inert additive.The propellants burned much better than their appearance might have indicated and followed the form function geometry of a single perforated grain geometry,as shown previously from Figs.2 and 3.The graphite was not incorporated.Using the data obtained from this test,the burn rate can be predicted using the Vielle’s burn rate law shown in Eq.(1),wherein P is the pressure in the chamber,α is the burn rate coeff i cient,and β is the burn rate pressure exponent [2,4]

3.2.3.Steel insert characterization

Fig.9.Photographs of steel inserts after closed bomb testing at~10,000 psi and ~15,000 psi;listed in Table 1.Steel inserts after bomb testing samples f i red with a composite propellant containing BN (A < B)had less oxidation than sample f i red with pure propellant.
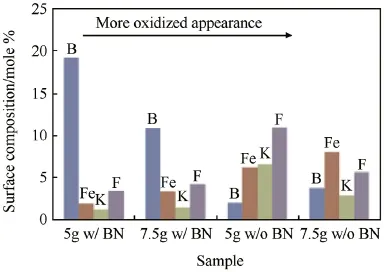
Fig.10.Surface elemental compositions for steel inserts after f i ring in bomb tests.
In order to better understand the f i rst closed bomb test results,the steel inserts were characterized by XPS and SEM. Insert samples were f i rst characterized by XPS to determine surface composition and element oxidation states after f i ring. All f i red samples contained B,C,N,O,F,K,and Fe on the surface.The fresh sample had only C,N,O,and Fe on the surface,indicating that some of the C,N,and O were in the steel or originated from atmospheric contamination.The f i rst sample f i red contained boron,so apparently boron in the chamber re-deposited on samples that were f i red in a non-boron propellant;however,the amount of boron in non-BN inserts was signif i cantly less,as will be discussed.The K and F likely originated from the propellant and remained on the inserts after f i ring and rinsing.Fig.10 shows the relative abundance of B,Fe,K,and F in the samples.It should be noted that all samples had a similar amount of C (33-44%)and O (33-41%).Clearly the samples f i red with the BN composite propellant had more boron,with the 5 g sample,which was the least oxidized,having the most boron coverage.The amount of iron on the surface increased steadily with the extent of apparent surface oxidation.Based on binding energies of these species,it was apparent that the BN additive is at least partially oxidized on the surface during propellant f i ring,and that the presence of boron does not seem to affect the iron oxidation state.However,ppm levels of boron doping in the steel would improve hardness and would not be detectable from XPS analysis of the iron.Samples f i red with a composite propellant containing BN exhibited less oxidation than samples f i red with pure propellant.Clearly,the less oxidized samples had less iron on the surface,which was generally displaced by boron.

Fig.11.SEM images of steel insert.
SEM with energy dispersive x-ray (EDX)spectroscopy analysis of sample surfaces was performed to characterize the morphology of any coatings formed during propellant f i ring tests.For reference,an unf i red steel insert was imaged,as seen in Fig.11(a).The steel insert showed few features at 2500× magnif i cation,as only straight grooves were visible.Figs.11(b)and (c)compare steel inserts f i red without and with BN additive respectively.Both samples seemed to have rougher surfaces compared to unf i red steel.At 2500× major differences were visible in the surface morphology.The surface of the sample f i red with BN had what appeared to be micron sized platelets covering the surface.These platelets are consistent with the shape of hexagonal BN.The sample f i red without BN had spheres and pits,and what appears to be octahedral crystals consistent with Fe3O4.EDX elemental analysis conf i rmed that the samples f i red with BN additive were mostly boron,oxygen,and carbon on the surface.The samples f i red without BN did not have any B detectable by EDX and were mostly Fe,K,and F.This analysis supports the hypothesis that the inserts f i red with BN-containing propellant formed a boron-based coating that apparently covers the iron and reduces the extent of oxidation.It is not clear if the crystals are partially oxidized hexagonal BN or mostly oxidized boron.
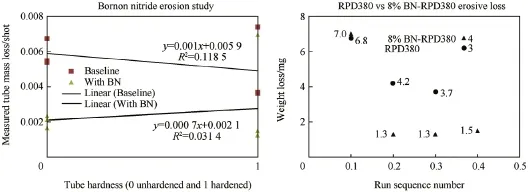
Fig.12.Wear and erosion test results for hard and unhardened sleeves (US patent pending).
3.2.4.Wear and erosion test results
The two types of insert sleeves used for these tests were prepared much earlier for another test.The initial bore surface roughness in both sets of inserts used were of a lower quality than ideal.Based on prior experience with high f l ame temperature propellants,imperfections in the bore surfaces are usually rapidly removed due to much higher mass loss rates than were observed in tests with highly energetic propellants.From the photos shown in Figs.4 and 5,it can be seen that the hardened inserts had rough surfaces with extensive machining features,even after f i ring.With these features,and the corresponding higher surface area,the mass loss for the hardened inserts is higher than the annealed inserts despite the higher hardness.In both cases with the hardened inserts the shot to shot variation in mass loss is reasonable.The unhardened inserts apparently had less severe initial machining roughness,which apparently accounts for the lower mass loss values despite the lower hardness.From the data shown in Fig.12,it appears that the f i rst shot in each group of unhardened inserts experienced a much larger weight loss than the following shots.The small number of shots limits the ability to demonstrate statistically supportive conclusions.
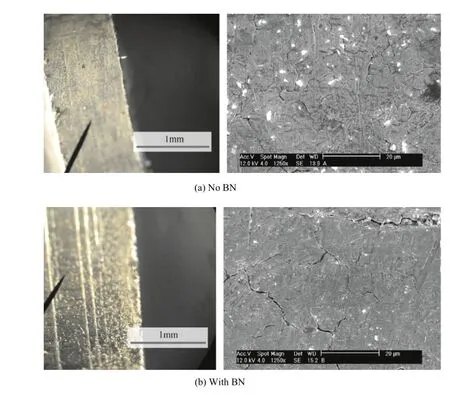
Fig.13.Hardened steel f i red.
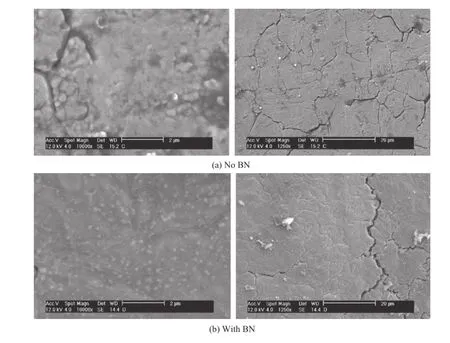
Fig.14.Un-hardened steel f i red.
The effect of the BN propellant additive (US patent pending)suggests an apparently signif i cant reduction in the mass loss for both hardened and unhardened insert sleeves.The results look compelling at 2.8 and 1.8 times life increase for hard and unhardened insert sleeves shown in Figs.13 and 14,respectively.These results must be considered in light of the less than ideal test insert bore surface conditions mentioned above. However,since the propellant formulation with B%BN has a theoretical f l ame temperature only approximately 100 K less than the baseline composition,mechanisms other than thermal(bore heat transfer)leading to the reduced mass loss must be considered.The presence of particles in the wall boundary layer during f l ow typically relates to heat transfer alteration to the substrate.The un-cleaned insert sleeves shown in Fig.15 following BN-propellant f i ring show deposits collected as a result of the entire blow-down process of the bomb gas emptying process.Due to the limited number of exposures of the inserts to the combustion products containing BN derived materials,alteration of the steel would seem to be minimal.The very limited number of shots with the BN propellant does not show a progressive reduction in mass loss on subsequent shots after the initial shot.Probing of the steel surface layers and the coating residue may provide added information.
4.Characterization of steel inserts after wear and erosion testing
4.1.Composition analysis
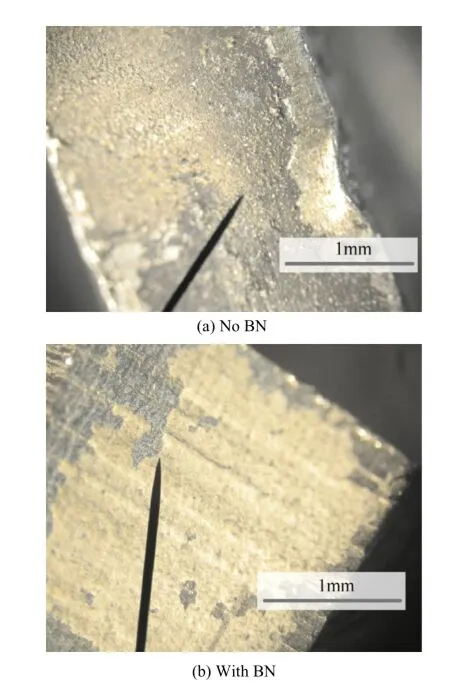
Fig.15.Un-hardened un-cleaned steel.
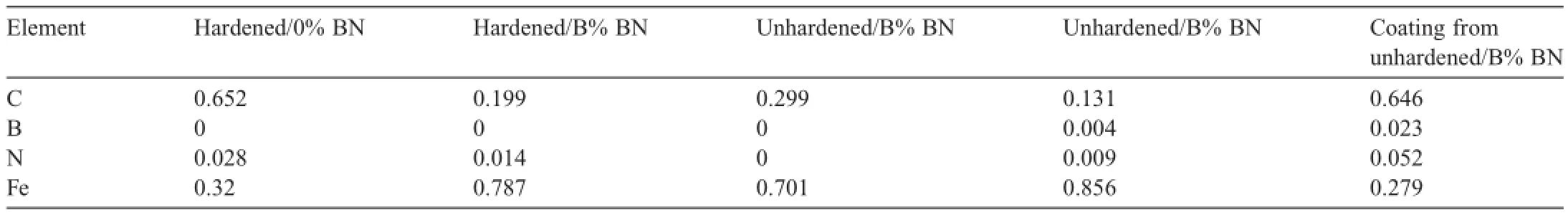
Table 2Relative surface composition for samples f i red in wear and erosion testing.
After f i ring,the samples were analyzed by XPS to determine surface composition,and inductively coupled plasma (ICP)analysis to determine the bulk composition.A number of elements were detected on the surface,including Pb,Fe,Cu,Zn,Sn,Si,Al,S,F,O,N,and B.Since many of the elements may only be surface contamination from the test,and not signif i cant to erosion effects,we focused the analysis on Fe,N,C,and B. A breakdown of the surface composition is given in Table 2. After cleaning,very little boron remained on the surface.Only the unhardened sample f i red with B%boron contained a detectable amount of boron (0.4%);the detectable limit is about 0.1%.Boron was detected in the coating that was scraped from the surface,but it was lower than the amount present during closed bomb testing,and less than the amount of iron removed with the coating.The bulk ICP analysis showed less than 0.01% B in all samples,and the remaining composition is consistent with the respective steel specif i cation.This low boron content in the 1 mm thick sample indicates that the weight loss differences are real and not due to build-up of boron on the steel.
A large amount of carbon was found on the surface of all samples,but less was present in samples f i red with boron.It is not clear if this surface carbon is related to erosion,but iron carbides are softer and melt at a lower temperature than iron.It is possible that boron,apparently in small amounts,could dope(or coat)the steel and block carbide formation.It is also possible,as will be discussed below,that boron dopes the steel in small amounts,resulting in hardened steel.Again,similar to closed bomb tests,based on the oxidation state of boron in the XPS analysis (data not shown)the boron nitride is at least partially oxidized.The iron oxidation state (data not shown)indicates that the iron is mostly 3+on the surface with a small amount of reduced iron as well.
4.2.SEM imaging
SEM images of the cleaned and uncleaned samples f i red in the erosion test stand are shown below.The steel had a number of surface cracks,but the crack density appeared to be less,or cracks were f i lled in,for the samples f i red in boron nitride shown in Figs.13-15.The surface also appeared to be smoother and less pitted for the samples f i red with boron.
4.3.Hardness testing
A simple Moh’s hardness test was performed on the samples after erosion testing to determine if the boron is playing a role in hardening the steel.Table 3 shows the results of this analysis. A reference unhardened steel sample was measured to have a hardness of 5.5,typical for steel.Surprisingly,the unhardened steel samples showed an increase in hardness up to 7.5 after being f i red with boron in the propellant.The sample f i red without any boron remained at 5.5.The hardened sample f i red with boron was also 7.5,and the hardened sample f i red without boron was approximately 7.0.Based on these results,it is possible that boron doping could regeneratively harden the steel,thus reducing erosion.However,more quantitative testing,such as Rockwell Hardness testing,after extended f i ring tests would be benef i cial to verify this possible mechanism.Further,improved characterization of how the boron may or may not be inf i ltrating the steel in small amounts would be benef i cial to determine if the reduced erosion results from a chemical mechanism (i.e.increased hardness from B doping)and/or a more physical mechanism (i.e.protection of the steel surface or cracks through a coating).
5.Conclusion
A scalable and economical proprietary process for production of BN nano-particles has been developed.An economic model was constructed to project the cost of producing BN nano-particles from raw materials at the anticipated commercial scale (50,000 kg/yr).Based on this analysis,the projected cost of BN at the 50,000 kg/yr scale was found to be$91.15 per kg.This cost is reasonable because we use such a small percentage in the propellant formulation.
These particles were con fi rmed to be spherical,with an average size less than 100 nm,and can be dispersed in propellants using the conventional solvent approaches.The particles were con fi rmed to not destabilize the nitro-cellulose based propellants such as the RPD-380 and IMR 4198.To simulate the interaction of BN nano-particles with gun barrels under combustion environments,steel inserts were fi red in a closed bomb test chamber in the presence of propellant compositions with and without the BN additive.Samples fi red with BN additive in the propellant showed signs of less oxidation in this testing. XPS showed that boron oxide coated the surface of samples fi red with BN additive,and less iron was present on the surface in samples that were less oxidized.SEM and EDX analyses showed stark differences in surface morphology and composition for samples fi red with or without BN additive.Samplesfi red with BN had a boron oxide surface coating of f l at platelets and seemed to lack signif i cant iron oxide (less than 10%). Samples f i red without BN were covered with pits,bumps,and octahedral crystals indicative of Fe3O4.Hardness testing of the insert surfaces was performed to quantify any differences between samples,but the results for these samples were inconclusive.
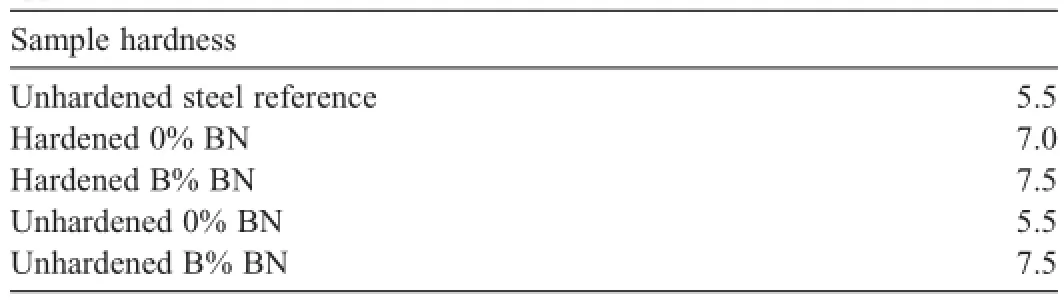
Table 3Hardness testing results for insert sleeves f i red in wear and erosion test apparatus.
While at f i rst glance,the results do show that the propellant with the added BN propellant shows less erosion than the baseline propellant,the sample size is clearly too small for the results to be considered proof that the BN does reduce erosion. Further testing of the propellants is recommended.Other differences in the two propellants or side effects from the addition of the BN could also be the cause of the lower erosion seen,for example,the lower f l ame temperature that the propellant with BN generates.Better control of the insert bore surface roughness is needed in future tests.Only a small amount of boron remained on the surface after f i ring and cleaning,but ppm levels of boron doping can harden steel,and an increased hardness was observed in unhardened steel f i red with boron nitride additive.SEM imaging showed less surface crack density in the samples f i red with boron nitride.Important considerations for any further tests are an alternate grain form to allow larger bomb loading density,and the corresponding larger amount of propellant necessary for that condition,as well as to support a suff i cient number of f i rings to generate supportable statistical conclusions.More quantitative hardness testing after extended f i ring would be useful to verify a hardening mechanism,and better characterization of the boron possibly in or on the steel surface would also be benef i cial.
Further wear and erosion testing of the propellant additive is planned in a projectile test stand that will simulate the conditions of the 155 mm artillery.
Acknowledgments
This work was supported by the USARMY RDECOMTech Base Program and Small Business Innovation Research (SBIR)Army Contract Number W15QKN-12-C-0041.
[1]Lawton B.1984.Thermal and chemical effects of gun barrel wear.In:8th International Symposium on Ballistics,Orlando,United States.
[2]Bracuti AJ.Wear-reducing additives-role of the propellant.In:Stiefel L,editor.Gun propulsion technology,vol.109 of Progress in Astronautics andAeronautics.Washington,DC:AIAA;1988.p.377-411 [Chapter 12].
[3]Sopok S,O’Hara P,Pegl G,Dunn S,Coats D,Nickerson G.Unif i ed computer model for predicting thermochemical erosion in gun barrels.J Propul Power 1999;15(4):601-12.
[4]Johnston IA.Understanding and predicting gun barrelerosions,DSTO-TR-1757,August 2005,Edinburg,SouthAustralia,Australia 5111.
[5]Manning TG.Spiral wear discussion with Dr Samuel Sopok and Duncan Park,Bennet Labs and ARDEC-Picatiny Arsenal,May 11,2015,respectively.
[6]Hasenbein RG.Wear and erosion in large caliber gun barrels,Unclassif i ed Army Report,Williamsburg,USA,RTO-MP-AVT-109,<http://www .dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD =ADA440980>;2003 [accessed 17.06.11].
[7]Tubb D.Tubb precision blended boron nitride bullet coating kit,<http:// www.davidtubb.com/boron-nitride-coating-bullets>; 2013 [accessed 01.12.15].
[8]Calik A,Duzgun A,Ekinci AE,Karakas S,Ucar N.Comparison of hardness and wear behavior of boronized and carburizedAISI 8620 steels. Acta Phys Pol A 2009;116:1029-32.
[9]Marucci M,Lawley A,Causton R,Saritas S.Effect of small additions of boron on the mechanical properties and hardenability of sintered P/M steels,<http://www.hoeganaes.com/TechPapersv2/113.pdf>;2011[accessed 17.06.11].
[10]Suwattananont N,Petrova R,Zunino J,Schmidt D.Surface treatment with boron forcorrosion protection.In: Tri-Service Corrosion Conference, <https://www.corrdefense.org/Academia%20Government %20and%20Industry/06T041.pdf>;2005 [accessed 17.06.11].
[11]Qian L,Stone GA.A study of the behavior of boron diffusion in plain carbon steels.J Mater Eng Perform 1995;4:59-62.
[12]FichtlW.Boronizing and itspracticalapplications.MaterDes 1981;2:276-86.
[13]Vladimiroff T,Carignan YP,Chiu DS,Macpherson AK.Flame temperature calculations at high temperature and pressure.Propellants Explos Pyrotech 1994;19(6):281-5.
Received 30 June 2015;revised 1 October 2015;accepted 8 October 2015 Available online 4 November 2015
Peer review under responsibility of China Ordnance Society.
*Corresponding author.Tel.:+1 9737243015.
E-mail address:thelma.g.manning.civ@mail.mil (T.MANNING).
http://dx.doi.org/10.1016/j.dt.2015.10.001
2214-9147/Production and hosting by Elsevier B.V.on behalf of China Ordnance Society.
Production and hosting by Elsevier B.V.on behalf of China Ordnance Society.
- Defence Technology的其它文章
- The ballistic performance of the bombard Mons Meg
- Application of transient burning rate model of solid propellant in electrothermal-chemical launch simulation
- Calculation of propellant gas pressure by simple extended corresponding state principle
- 3D numerical simulation and analysis of railgun gouging mechanism
- Mechanism of plasma ignition in electrothermal-chemical launcher

