HDAC2对豚鼠急性听力损失治疗中糖皮质激素抵抗的影响*
周琼琼 佘万东
·实验研究·
HDAC2对豚鼠急性听力损失治疗中糖皮质激素抵抗的影响*
周琼琼1佘万东1
【摘要】目的观察地塞米松(dexamethasone,DEX)加氨茶碱(amionophylline,AMI)治疗脂多糖(lipopolysaccharide,LPS)诱导的豚鼠急性听力损失的疗效及耳蜗组蛋白去乙酰化酶2(histone deacetylase 2,HDAC2)的表达,探讨治疗中HDAC2对糖皮质激素(glucocorticoid,GC)抵抗的影响。方法选取50只健康白色豚鼠,其中10只为对照组,仅双耳鼓阶注入5 μl 人工外淋巴液(artificial perilymph,AP)(AP组);其余40只鼓阶注入5 μl(5 mg/ml)的LPS后再随机分为4组,每组10只:模型组(LPS组),无其他处理;LPS+DEX组:术前30 min及术后24小时腹腔注射DEX 1 mg/kg;LPS+AMI组:术前30 min及术后24小时腹腔注射AMI 20 mg/kg;LPS+DEX+AMI组:术前30 min及术后24小时腹腔注射AMI 20 mg/kg及DEX 1 mg/kg。所有动物给药前及鼓阶注射术后48小时行ABR检测后取耳蜗,采用免疫荧光法,观察耳蜗基底膜铺片及耳蜗组织冰冻切片中HDAC2的分布及空间定位,用ELISA法测定耳蜗组织中HDAC2的含量。 结果LPS组鼓阶注射LPS后48小时全频听力损失,由低频向高频逐渐加重;LPS+DEX组及LPS+AMI组16、32 kHz ABR阈移较LPS组改善明显(<0.05),LPS+DEX+AMI组各频率ABR阈移均低于LPS+DEX组(P<0.05),以16 kHz处最显著(P<0.01)。耳蜗基底膜铺片及耳蜗冰冻切片免疫染色结果表明HDAC2广泛分布于耳蜗中,且主要存在于胞核内。8、16、32 kHz ABR阈移与耳蜗内HDAC2的含量呈负相关(P<0.05)。结论AMI可提高豚鼠耳蜗HDAC2的表达,HDAC2可改善治疗中GC抵抗,对提高GC治疗LPS导致的急性听力损失的敏感性有一定的作用。
【关键词】组蛋白去乙酰化酶2;糖皮质激素抵抗;听力损失;氨茶碱;豚鼠
糖皮质激素(glucocorticoid,GC)已被广泛用于治疗突发性聋、自身免疫性内耳疾病、梅尼埃病等内耳疾病及细菌性中耳炎造成的感音神经性聋[1]。临床研究提示部分患者使用GC治疗后效果不明显,存在对GC不敏感或抵抗[2,3]。目前认为GC抵抗的可能原因与遗传因素、核转运障碍、竞争性糖皮质激素受体β(glucocorticoid receptor-β,GRβ)表达增加及促炎相关因子,如:活化蛋白1(activator protein 1,AP1)、核转录因子-κB(Nuclear factor κB,NF-κB)过多激活等有关[4~6]。最近研究表明组蛋白去乙酰化酶2(histone deacetylase 2,HDAC2)的表达及活性下降是GC抵抗的重要机理[7,8]。
HDAC2通过调控组蛋白的乙酰化水平,影响染色体重塑,抑制抗炎基因转录,在GC介导的抗炎过程中通过募集HDAC2抑制炎症相关因子的表达。Ito等[9]研究发现炎症可降低HDAC2的活性,从而降低靶细胞对GC的敏感性;炎症反应参与了各种因素导致的突发性聋发生发展过程[10];前期研究发现难治性突发性聋患者外周血单个核细胞HDAC2活性下降,鼓室灌注GC有效者,HDAC2活性增加[11]。最近研究表明低剂量氨茶碱 (aminophylline,AMI)有重要的抗炎作用,主要是通过提高HDAC2的活性,改善GC抵抗状态[12]。因此,本研究拟通过向豚鼠鼓阶内直接注射脂多糖(lipopolysaccharide,LPS),使其内耳产生炎症,导致急性听力损失,同时给予地塞米松(dexamethasone, DEX)治疗,并全身应用AMI,观察其疗效及耳蜗HDAC2的表达,探讨HDAC2对GC治疗急性听力损失的敏感性的影响。
1材料与方法
1.1实验动物分组及处理实验动物为健康成年白色红目豚鼠50只,体重250~350 g,雌雄不限(南京鼓楼医院实验中心购自南京市江宁区青龙山动物繁殖场)。随机选择10只,双耳均经鼓阶给予5 μl人工外淋巴液(artificial perilymph, AP)作为对照组(AP组);其余40只豚鼠行鼓阶微量注射术,注入5 μl(5 mg/ml)的LPS(Sigma,America),再随机分为4组,每组10只:模型组(LPS组),无其他处理;LPS+DEX组:术前30 min及术后24小时腹腔注射DEX 1 mg/kg;LPS+AMI组:术前30 min及术后24小时腹腔注射AMI 20 mg/kg;LPS+DEX+AMI组:术前30 min及术后24小时腹腔注射AMI 20 mg/kg及DEX 1 mg/kg。所有豚鼠实验前及术后48小时均行ABR检测,排除实验前听力下降者。
1.2实验方法
1.2.1急性内耳损伤造模方法[13]豚鼠麻醉后,切开耳后皮肤,打开听泡,在耳蜗底回靠近圆窗边缘打孔,用微量推进器缓慢注入5 μl AP[14]或等体积、浓度为5 mg/ml的LPS,用肌肉组织填塞,缝合皮肤,待其苏醒后,放回笼内自由喂养。
1.2.2听性脑干反应检测使用TDT-system3(RZ6,Tucker-Davis Technology)系统检测。声信号为10 ms短纯音,上升/下降时间为5 ms, 叠加1 024次,重复率21.1次/秒。刺激经MF1扬声器闭合声场给予。动物麻醉后置于屏蔽室恒温电热毯上,记录电极刺入颅顶皮下,参考及接地电极分别刺入左、右侧乳突皮下。测试频率为4、8、16、32 kHz,刺激声强度从90 dB SPL开始,5 dB下降一档,以刚刚诱发出可辨认波Ⅲ的最小声强值为ABR反应阈,将同一只动物双耳平均阈移作为该动物的ABR阈移。ABR检测完后即行以下实验。
1.2.3耳蜗铺片及免疫染色每组随机取2只动物,迅速取出听泡,暴露耳蜗,于蜗尖打孔,刺破圆窗,灌注4%多聚甲醛固定过夜后,分离基底膜。5%羊血清封闭30分钟,加入一抗anti-HDAC2(Abcam, Cambridge, UK) 1:1 000,4 ℃过夜,用PBS漂洗,加入标记Alexa Fluor-555山羊抗兔IgG(Abcam, Cambridge, UK)1:1 000及FITC-鬼笔环肽(phalloidin)(cytoskeleton, USA)1:500及4',6-二脒基-2-苯基吲哚(4',6-diamidino-2-phenylindole,DAPI),避光孵育1小时,PBS漂洗。最后将标本平铺于载玻片上,封片,用荧光显镜观察HDAC2的分布,分析荧光强度,观察毛细胞的形态变化。
1.2.4耳蜗冰冻切片及免疫染色每组随机取3只动物,迅速取出双侧听泡,暴露耳蜗,蜗尖打孔,注入4%多聚甲醛固定过夜。PBS漂洗,10%的乙二胺四乙酸二钠 ( EDTA)中脱钙3~4周。30%蔗糖脱水后,用OCT胶包埋,平行于蜗轴行8 μm冰冻切片。免疫荧光染色方法同基底膜染色。用荧光显微镜观察HDAC2在耳蜗切片中的空间分布及各组荧光强度的变化。
1.2.5HDAC2蛋白含量检测每组随机取3只动物,迅速取出双侧听泡。解剖显微镜下取出耳蜗软组织放入1.5 ml EP管中,手动研磨充分后,用细胞核与细胞浆提取试剂盒(碧云天,北京)提取细胞核蛋白,按BCA法(碧云天,北京)对上述提取的核蛋白进行定量后,按照EPIQUIK HDAC2 Assay kit(Epigentek,Brooklyn,NY)说明操作,用ELISA法检测,检测波长450 nm,用吸光度(A)代表HDAC2蛋白含量。
1.3统计学方法所有数据经SPSS 19.0统计学软件处理,采用one way ANOVA Least-Significant Difference(LSD)检测,P<0.05为差异有统计学意义。
2结果
2.1各组ABR阈移由表1可见,AP组4、8、16 kHz平均阈移均小于10 dB,32 kHz阈移为11.50 ±11.56 dB;LPS组术后48 h全频听力损失,由低频到高频逐渐加重,32 kHz听力损失最严重,说明急性内耳损伤致听力损失模型造模成功。LPS+DEX组及LPS+AMI组16、32 kHz ABR阈移较LPS组改善明显(P<0.05),但LPS+DEX组和LPS+AMI组之间各频率ABR阈移差异均无统计学意义。LPS+DEX+AMI组各频率ABR阈移均低于LPS+DEX组(P<0.05),16 kHz处最显著(P<0.01)。

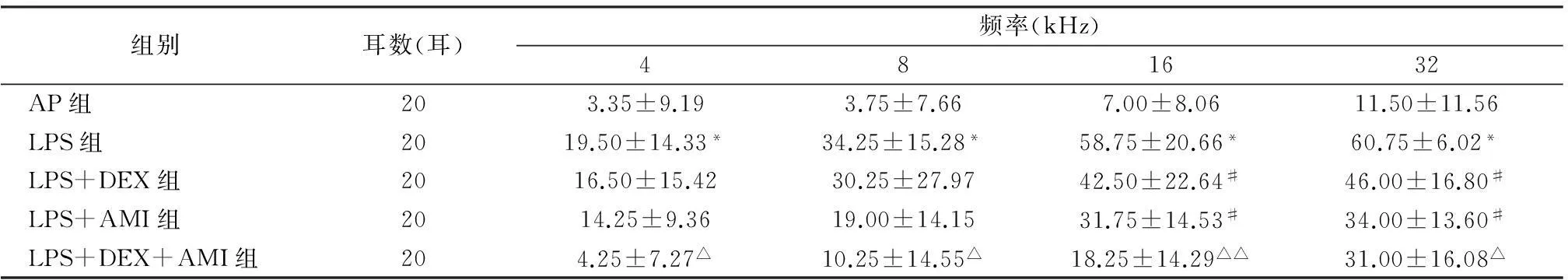
组别耳数(耳)频率(kHz)481632AP组203.35±9.193.75±7.667.00±8.0611.50±11.56LPS组2019.50±14.33*34.25±15.28*58.75±20.66*60.75±6.02*LPS+DEX组2016.50±15.4230.25±27.9742.50±22.64#46.00±16.80#LPS+AMI组2014.25±9.3619.00±14.1531.75±14.53#34.00±13.60#LPS+DEX+AMI组204.25±7.27△10.25±14.55△18.25±14.29△△31.00±16.08△
注:*与AP组相比,P<0.01;#与LPS组相比,P<0.05;与LPS+DEX组相比,△P<0.05,△△P<0.01
2.2HDAC2在各组耳蜗的表达及定位耳蜗基底膜铺片显示,各组毛细胞无明显缺失,三排外毛细胞纤毛排列整齐,结构完整,无缺失(图1)。HDAC2在耳蜗基底膜广泛表达,包括内外毛细胞、内外支持细胞等,与DAPI分布范围一致,提示HDAC2在耳蜗组织中主要定位于细胞核内。耳蜗冰冻切片显示,HDAC2在蜗轴、螺旋韧带、血管纹区域均有广泛表达(图2)。
2.3各组耳蜗HDAC2蛋白表达LPS组HDAC2的表达量(0.354±0.039)显著低于AP组(0.442±0.047)(t=2.49,P<0.05),LPS+DEX组HDAC2蛋白表达量(0.376±0.051)较LPS组(0.354±0.039)未明显增加(t=0.61,P>0.05),与LPS组相比,LPS+DEX+AMI组耳蜗中HDAC2表达量(0.439±0.054)增加,差异有统计学意义(t=2.53,P<0.05)。
2.4ABR阈移与耳蜗组织中HDAC2表达的相关性将各组耳蜗HDAC2的蛋白表达量(用吸光度A表示)与各频率ABR阈移进行相关性分析发现,在4 kHz处两者间无相关性(r=-0.38,P>0.05),而在8、16、32 kHz处两者呈负相关(r分别为-0.56、-0.68、-0.62;P<0.05)。
3讨论
很多内耳疾病中存在炎症反应过程,如突发性聋、自身免疫性内耳病、年龄相关性聋等。Ulu等[15]研究发现突发性聋患者血清中中性粒细胞与淋巴细胞比率较健康对照组显著增高。LPS为细菌内毒素,众多动物实验研究[16,17]发现内耳局部应用LPS可引起耳蜗炎性细胞浸润,损伤血管纹,造成内外毛细胞肿胀、变性甚至凋亡,导致急性听力损失。本研究将LPS注射到豚鼠耳蜗底回中,造成动物急性听力损失,高频区听力损失最严重,与文献报道一致[17],可能为药物直接作用于耳蜗底回,使该区域药物浓度短时间内达最大,因此高频区听力损伤最严重。本研究中对照组(AP组),除32 kHz外其余各频率术后阈移均在10 dB以内,因此,可忽略鼓阶注射术造成的损伤。Marwick等[18]研究表明炎症及氧化应激可激活磷脂酰肌醇-3激酶(PI3K),磷酸化HDAC2丝氨酸残基,可导致HDAC2活性下降,并增加其降解;Ni等[19]向羊膜腔内注射LPS诱导支气管肺发育不良动物模型,发现LPS可以降低HDAC2的表达及活性。本研究结果显示在豚鼠内耳组织中注射LPS可产生急性炎症,可观察到耳蜗内HDAC2表达下降;基底膜铺片及冰冻切片结果均表明HDAC2在内耳组织中广泛表达,且存在于细胞核内,荧光染色显示各组耳蜗中均无明显的毛细胞缺失,可能的原因为LPS造模后两天,时间较短,毛细胞形态保留(鬼笔环肽标记),但功能已部分丧失。
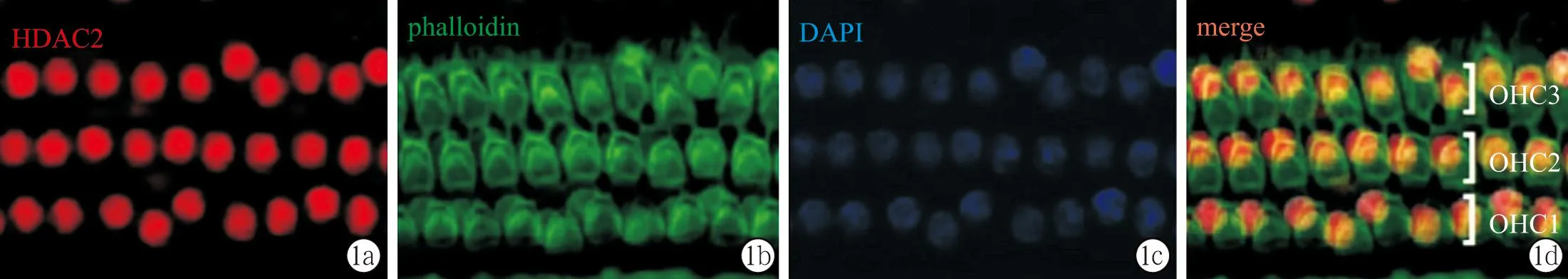
图1 耳蜗基底膜铺片
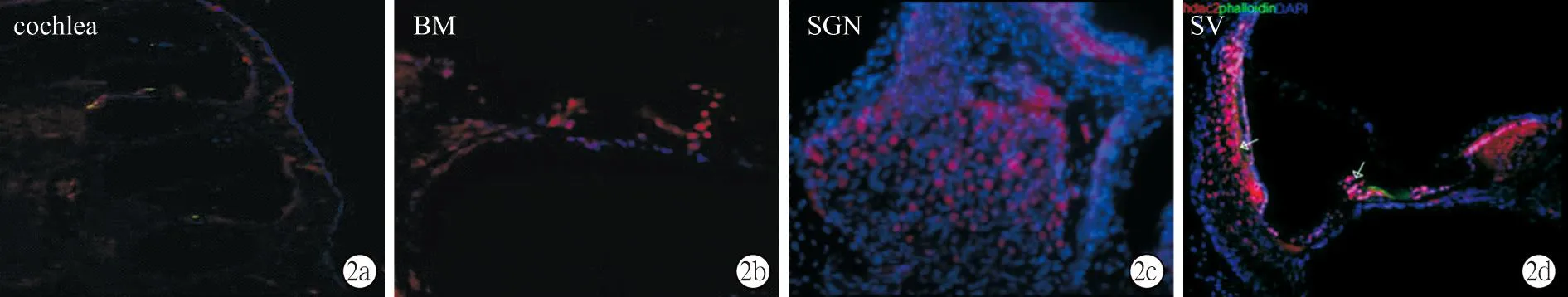
图2 耳蜗冰冻切片
近年来HDAC2与GC抵抗的分子机制相关性研究成为热点。GC通过募集HDAC2至促炎启动子区,使靶基因组蛋白去乙酰化,DNA由疏松态转为紧密态,抑制NF-κB激活的促炎基因的转录[9]。Barnes等[6]研究发现组蛋白的乙酰化及去乙酰化受炎症影响。炎症及氧化应激可激活并稳定低氧诱导因子1α亚基(HIF-1α),激活的HIF-1α可降低核因子-E2相关因子2(NF-E2-related factor 2,Nrf2)的表达[20],从而通过Nrf2-HDAC2轴来影响HDAC2活性及表达[21],使得GC治疗不敏感及抵抗。本实验中LPS+DEX组豚鼠鼓阶注射LPS前后全身应用DEX,结果与LPS组相比,其听功能改善并不显著,提示存在GC不敏感及抵抗;ABR阈移与耳蜗组织中HDAC2表达的相关性分析表明耳蜗组织中HDAC2含量越高,DEX的疗效越好,提示HDAC2对提高GC敏感性有一定作用。
近年来有研究表明,茶碱在低剂量时,能发挥抗炎效应,同时提高哮喘患者对GC的疗效,这种抗炎作用主要是通过抑制PI3Kδ,提高HDAC2的活性,改善GC抵抗状态[22]。AMI为茶碱与乙二胺复盐,水溶性更强;本实验中LPS+DEX+AMI组动物鼓阶注射LPS前后给予AMI联合DEX治疗后,豚鼠耳蜗内HDAC2表达较LPS组增强,提示AMI可提高HDAC2的含量,改善GC抵抗状态。另外LPS+AMI组动物的急性听力损失亦有一定的改善,可能是因为AMI恢复HDAC2的活性,抑制了炎症相关基因的激活,其具体机制有待进一步探讨。
综上所述,本研究表明AMI可提高豚鼠耳蜗HDAC2的表达,从而提高GC对LPS诱导的急性听力损失的疗效。通过提高HDAC2的活性或/和含量,改善治疗中GC抵抗,为GC治疗众多内耳疾病提供了新思路。由于GC不敏感的相关机制很多,而且动物模型还需要临床研究的支持,希望在将来的实验中,进一步研究内耳中GC不敏感的相关分子机制以及HDAC2下降的机制,以指导GC抵抗患者的临床治疗。
(致谢 :本实验得到了东南大学王坚、刘莉洁、柴人杰教授,美国Hough Ear Institute杜小平研究员的指导,在此表示衷心感谢!)
4参考文献
1Simpson SA, Lewis R, van der Voort J, et al. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children[J]. Cochrane Database Syst Rev, 2011, 5: Cd001935.
2Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss[J]. Cochrane Database Syst Rev, 2013, 7:Cd003998.
3Broughton SS, Meyerhoff WE, Cohen SB. Immune-mediated inner ear disease:10-year experience[J]. Seminars In Arthritis And Rheumatism, 2013, 34: 544.
4Lamberts SW. Hereditary glucocorticoid resistance[J]. Ann Endocrinol (Paris), 2001, 62:164.
5Irusen E, Matthews JG, Takahashi A, et al. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma[J]. J Allergy Clin Immunol, 2002, 109: 649.
6Barnes PJ, Adcock IM .Glucocorticoid resistance in inflammatory diseases[J]. Lancet, 2009, 373: 1905.
7Ito K, Hanazawa T, Tomita K, et al. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration[J]. Biochemical and Biophysical Research Communications, 2004, 315: 240.
8Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation[J]. J Steroid Biochem Mol Biol, 2010, 120: 76.
9Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappa B suppression[J]. Journal of Experimental Medicine, 2006, 203: 7.
10Capaccio P, Pignataro L, Gaini LM, et al. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss[J]. European Archives Of Oto-Rhino-Laryngology, 2012, 269: 449.
11后婕, 戴艳红, 谢利生,等.难治性突发性聋患者外周血单个核细胞HDAC2表达与其预后的相关性分析[J]. 听力学及言语疾病杂志,2014,22:559.
12Sun X, Li Q, Gong Y, et al. Low-dose theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammation[J]. Canadian Journal of Physiology and Pharmacology, 2012,90(7):895.
13Konishi M, Kawamoto K, Izumikawa M, et al. Gene transfer into guinea pig cochlea using adeno-associated virus vectors[J]. The Journal of Gene Medicine, 2008,10:610.
14Kawamoto K, Yagi M, Stover T, et al. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF[J]. Molecular Therapy : the Journal of the American Society of Gene Therapy,2003, 7:484.
15Ulu S, Ulu MS, Bucak A, et al. Neutrophil-to-lymphocyte ratio as a new, quick, and reliable indicator for predicting diagnosis and prognosis of idiopathic sudden sensorineural hearing loss[J]. Otology & Neurotology, 2013, 34:1400.
16Watanabe K, Jinnouchi K, Hess A, et al. Detection of apoptotic change in the lipopolysaccharide (LPS)-treated cochlea of guinea pigs[J]. Hear Res, 2001,158: 116.
17Jang CH, Cho YB, Choi CH, et al. Effect of topical dexamethasone on sensorineural hearing loss in endotoxin-induced otitis media[J]. In Vivo, 2007,21:1043.
18Marwick JA, Caramori G, Stevenson CS, et al. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice[J]. Am J Respir Crit Care Med , 2009, 179:542.
19Ni W, Lin N, He H,et al. Lipopolysaccharide induces up-regulation of TGF-alpha through HDAC2 in a rat model of bronchopulmonary dysplasia[J]. PloS One, 2014, 9:e91083.
20Loboda A, Stachurska A, Florczyk U, et al. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells[J]. Antioxid Redox Signal, 2009, 11: 1501.
21Adenuga D, Caito S, Yao H,et al. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction[J]. Biochem Biophys Res Commun, 2010, 403: 452.
22To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease[J]. Am J Respir Crit Care Med, 2010, 182:897.
(2015-09-16收稿)
(本文编辑李翠娥)
HDAC2 is Associated with Glucocorticoid Sensitivity in LPS-induced Sudden Hearing Loss in Guinea Pig
Zhou Qiongqing, She Wandong
(Department of Otolaryngology-Head and Neck Surgery, the Affiliated Drum Tower Hospital Medical School of Nanjing University, Nanjing, 210008, China)
【Abstract】ObjectiveTo investigate the effect of histone deacetylase 2(HDAC2) expression by aminophylline on glucocorticoid sensitivity of guinea pigs with lipopolysaccharide-induced sudden hearing loss.MethodsFifty guinea pigs were randomly divided into five groups : control/ artificial perilymph(AP) group (n=10) in which both the ears were administrated 5 μl sterile artificial perilymph fluid by means of drilling the scala tympani of the cochlear basal turn; whereas 5 μl of 5 mg/ml LPS was transferred into the cochlea of both the ears of other groups in the same way,which were model(LPS) group(n=10), lipopolysaccharide+ dexamethasone(LPS+DEX) group(n=10), lipopolysaccharide+ aminophylline(LPS+AMI) group(n=10), and lipopolysaccharide+ dexamethasone+ aminophylline(LPS+DEX+AMI) group(n=10). Guinea pigs with normal hearing tested by auditory brain stem response (ABR) before treatment were included in this study. ABRs were recorded in all guinea pigs 48 hours after surgery. The immunofluorescence staining was used to detect for the HDAC2 in the inner ear. The HDAC2 levels in the cochlea of guinea pigs were detected by ELISA test. ResultsABR results showsed that hearing loss in AP group was mild, the threshold shifts were less than 10 dB at 4 kHz,8 kHz,16 kHz frequencies, at 32 kHz the threshold shift was 11.50 dB, respectively. However, the hearing loss was obvious in LPS group, especially at the high frequency (the threshold shift was 60.75±6.02 dB SPL). Compared to AP group, hearing loss in LPS group was statistically significant at all frequencies (P<0.01). The hearing improvement was obvious at frequeniies of 16 kHz and 32 kHz in group of LPS+DEX and LPS+AMI (P<0.05). The LPS+DEX+AMI treatment for LPS-induced acute hearing loss was the most remarkable at all frequencies compared with glucocorticoid or aminophylline treatment alone, especially at 16 kHz (P<0.05). The immunofluorescence staining showed positive expression of HDAC2 in the cochlea in the inner and outer hair cells, stria vascularis, spiral ganglion and spiral ligament. The correlation analysis showed negative correlations between the expression of HDAC2 and threshold shift of ABR at 8 kHz, 16 kHz, and 32 kHz (P<0.05).ConclusionIt is effective for dexamethasone and aminophylline treatment in induced hearing loss in guinea pigs. Aminophylline can elevate HDAC2 expression and improve the effect of glucocorticoid. In conclusion, HDAC2 plays a critical role in restoring glucocorticoid sensitivity in the inner ear.
【Key words】HDAC2;Glucocorticoid insensitivity;Hearing loss;Aminophylline;Guinea pig
【中图分类号】R764.43+7
【文献标识码】A
【文章编号】1006-7299(2016)02-0157-05
DOI:10.3969/j.issn.1006-7299.2016.02.012
作者简介:周琼琼,女,安徽人,研究生,主要研究方向为听力学基础与临床。通讯作者:佘万东(Email:shewandong@163.com)
网络出版时间:http://www.cnki.net/kcms/detail/42.1391.r.20151230.1119.008.html
网络出版地址:2015-12-3011:19
*国家自然科学基金项目(81271074)、江苏省第十批“六大人才”高峰项目(WSN-009)、江苏省临床医学科技专项(BL2014002)、南京市国际联合研究项目(2012sd311038)联合资助
1南京大学医学院附属鼓楼医院耳鼻咽喉科(南京210008)

