玉米直链回生淀粉吸附含羟基红曲红色素的动力学
林旭辉,董世瑞,郭俊杰,连喜军※(. 天津商业大学天津市食品生物技术重点实验室,生物技术与食品科学学院,天津 30034;. 天津商业大学理学院化学系,天津 30034)
玉米直链回生淀粉吸附含羟基红曲红色素的动力学
林旭辉1,董世瑞1,郭俊杰2,连喜军1※
(1. 天津商业大学天津市食品生物技术重点实验室,生物技术与食品科学学院,天津 300134;2. 天津商业大学理学院化学系,天津 300134)
摘要:为了提高玉米回生淀粉通过吸附分离含羟基红曲红色素的效率,深入了解玉米直链回生淀粉吸附含羟基红曲红色素的机理,该文通过回生法制备分子量分布范围分别为267~4.6×107、589~9.7×104、565~6.2×104、794~6.0×104g/mol的玉米直链淀粉,进而利用这些淀粉吸附红曲红色素,研究不同淀粉在20、40、80 ℃温度下对红曲红色素的吸附量和吸附速度,采用Dubinin-Radushkevich等温吸附方程研究玉米直链回生淀粉吸附含羟基红曲红色素的动力学规律。结果表明,回生1~4次玉米直链回生淀粉在80℃、140 h吸附红曲红色素量最多,吸附量分别达到0.56、0.84、1.04、1.10 mg/g,直链回生淀粉分子量分布范围越窄,吸附温度越高,吸附速度越快。经Dubinin-Radushkevich等温吸附方程式计算所有样品平均吸附自由能低于8 J/g,玉米直链回生淀粉吸附红曲红色素方式为物理吸附。电子显微镜图结果表明,吸附红曲红色素的玉米直链淀粉干燥后结构更加蓬松。研究结果为玉米直链回生淀粉分离、纯化含羟基红曲红色素工艺条件的设计和探索提供理论参考。
关键词:淀粉;吸附;动力学; 红曲红色素
林旭辉,董世瑞,郭俊杰,连喜军. 玉米直链回生淀粉吸附含羟基红曲红色素的动力学[J]. 农业工程学报,2016,32(2):294-299.doi:10.11975/j.issn.1002-6819.2016.02.042http://www.tcsae.org
Lin Xuhui, Dong Shirui, Guo Junjie, Lian Xijun. Adsorption kinetics of monascus red pigments with hydroxyls by retrograded corn starch [J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(2): 294-299. (in Chinese with English abstract)doi:10.11975/j.issn.1002-6819.2016.02.042http://www.tcsae.org
Email:lianliu2002@163.com
0 引 言
红曲红色素是红曲色素中呈现红色的色素,是中国科技人员近年来利用现代生物技术液态发酵法制备出来的一种天然色素,具有色价高、着色力强、耐高温等特点,广泛用于火腿肠、腐乳、饮料等食品中[1-2]。液体发酵法生成的红曲红色素里面含有少量红曲霉发酵过程残留的糖类、氨基酸、水溶性蛋白、多肽等物质[3],这些物质的存在对色素的贮藏和加工稳定性带来不确定因素。吸附法是纯化色素常用的方法,采用的吸附材料有大孔吸附树脂、甲壳胺纤维、微孔淀粉、阴离子淀粉等[4-9]。这些方法可选择性吸附色素,一次性将大量杂质除去,较常规方法分离色素效率高[10]。大孔吸附树脂和甲壳胺纤维合成过程中使用了苯乙烯和甲壳胺,吸附过程中这些物质的残留给色素分离纯化带来干扰,给食品用色素带来安全隐患。甲壳胺纤维的价格比较高,大量使用成本太高,不能成为工业化生产中分离红曲红色素的可行方法。阴离子淀粉仅限于分离可进行离子交换的色素物质,成功用于红曲红色素大分子吸附分离尚未见报道。普通淀粉和微孔淀粉可用于红曲红色素的吸附[8, 11],但吸附后的色素洗脱比较难,因为有机溶剂不容易透过原淀粉材料[12],洗脱法脱色不容易实现。用碱液溶解淀粉时,普通淀粉和微孔淀粉都会因为分子量大而形成胶体[13],色素也无法溶解到水中。而回生淀粉吸附色素后,淀粉可在碱液中完全溶解,释放出色素,再通过乙醇沉淀淀粉即可得到纯化后的色素,具有潜在的开发前景。为了开发回生淀粉这种吸附色素的材料,本文选用分子量分布范围不同的4组玉米直链回生淀粉,分子量分布范围为267~4.6×107、589~9.7×104、565~6.2×104、794~6.0×104g/mol,选用课题组新发现的含羟基红曲红色素作为待吸附色素[14-15],在不同温度(20、40、80℃)下吸附纯化后的含羟基红曲红色素,测定不同色素吸附量,研究玉米回生淀粉吸附红曲红色素的规律及机制。论文的研究成果为开发回生淀粉作为色素吸附材料探索了新的途径,为高效分离发酵液中包括红曲红色素在内的含羟基有机物探寻一种新方法。同时,回生淀粉的水不溶性为清除水中含羟基污染物找到一种新的材料,这种材料可溶解于碱液中释放污染物,调节中性后干燥回生淀粉又能还原回来[16],可重复使用。
1 材料与方法
1.1材料与仪器
玉米淀粉,市售;红曲红(纯度86%,液体发酵法生产),山东中惠食品有限公司;红曲红色素标准品,上海一基实业有限公司;薄层层析硅胶板GF254 (20 cm×20 cm),青岛海洋化工有限公司;柱层析硅胶(200~300目),青岛海洋化工有限公司;甲醇、无水乙醇、二氯甲烷、正己烷、乙酸乙酯、四氯化碳(分析纯),天津市风船化学试剂科技有限公司。
YXQG02手提式电热压力蒸汽消毒器,山东安德医疗科技有限公司;HH-4数显恒温水浴锅,常州赛普实验仪器厂;DH-101-3BS电热恒温鼓风干燥箱,天津市中环实验电炉有限公司;BCD-229KB海尔冰箱,青岛海尔股份有限公司;LXJ-Ⅱ离心机,上海医用分析仪器厂;FA1104N电子天平、YD202N电子天平,上海精密科学仪器有限公司;旋转蒸发器RE-52AA型,上海亚荣生化仪器厂;Lambda25紫外分光光度计,美国PerkinElmer公司;KER3100-08S型精密恒温工作台,南京凯尔仪器有限公司;SCD 500离子溅射喷镀仪,LEO 1450 VP高分辨率扫描电镜(德国LEO公司)。
1.2试验方法
1.2.1红曲红色素提纯
参考文献[11]和[14],称取市售液体发酵法生产红曲红色素30 g装入纸包,放置于索式提取器中。分别用200 mL正己烷、乙酸乙酯、甲醇作为索氏提取溶剂,按先后顺序分别进行3次索氏提取。将正己烷和乙酸乙酯提取液弃掉,将所得甲醇提取液旋转蒸发浓缩(50℃、55 r/min)、干燥(65℃)至恒质量。将0.5 g色素溶解于5 mL甲醇中,吸取0.05 mL滴于薄层色谱板(20 cm×20 cm),用乙醇/石油醚(3∶7体积比)做展开剂展开10 cm后,层析板转90°后用甲醇/二氯甲烷(1∶1体积比)做展开剂继续展开10 cm。使用刮刀将红色色带刮下,甲醇溶解、过滤,自然干燥得纯红曲红色素,纯度为99%。 [17-19]的方法。取10 g淀粉溶于100 mL蒸馏水中,90℃糊化20 min,放入高压锅中120℃高压(4.62 Pa)30 min,取出冷却后放冰箱4℃老化48 h。取出老化后淀粉添加0.6 mL高温淀粉酶(200 000 U/mL)在95℃水解20 min后离心(×3 050 g)得到沉淀,加100 mL蒸馏水搅拌清洗后离心(×3 050 g),得到沉淀溶解于50 mL 4.0 mol/L KOH中,用6.0 mol/L HCl调节至中性,加入150 mL正丁醇离心(×3 050 g)沉淀,经水洗60℃干燥至恒质量后为第1次回生淀粉,粉碎过200目筛子备用。第2次、第3次、第4次分别以前一次所得回生淀粉为原料,重复以上试验操作。采用高效液相离子交换色谱法测定回生1~4次所得玉米直链淀粉分子量分布分别为267~4.6×107、589~9.7×104、565~6.2×104、794~6.0×104g/mol。
1.2.2玉米直链回生淀粉的制备
1.2.3玉米回生直链淀粉吸附红曲红色素
加0.5 mg左右纯化后色素于10 mL水中,使整个溶液中色素的吸光度为1.0左右。将0.5 mg回生1~4次的玉米直链淀粉样品加入浓度为0.09 mg/mL色素液,分别在20、40和80℃温度下吸附10、20、40、60、80、100、120和140 min,离心(×3 050 g)5 min后,取出淀粉样品溶于5 mL 4.0 mol/L KOH中(目的是打开淀粉之间及淀粉与含羟基红曲红色素之间的氢键,使色素溶于溶液中,准确测定淀粉吸附色素量),再用6.0 mol/L盐酸中和,去离子水定容到10 mL,分光光度法测定色素含量。具体测定方法参考文献[20]。准确称取50 mg分离纯化后红曲红色素溶于100 mL蒸馏水中,配置成浓度为500.0 μg/mL的色素液,分别准确吸取0、0.3、0.6、0.9、1.2、1.5、2.1、2.4、2.7、3.0、3.3、3.6、3.9、4.2、4.5、4.8、5.1、5.4、5.7mL,定容至25mL,配置成0、12.0、24.0、36.0、48.0、60.0、84.0、96.0、108.0、120.0、132.0、144.0、156.0、168.0、180.0、192.0、204.0、216.0、228.0 μg/mL色素液,在最大吸收波长490.0 nm处测定各自吸光度,绘制色素液浓度测定标准曲线。所得回归方程为Y(吸光度)=0.0022x(色素浓度)+0.0101,回归方程的决定系数(R2)为0.9994。淀粉吸附红曲红色素一段时间后,离心(×3 050 g)5 min后取出淀粉沉淀,上清液定容到10 mL,根据标准曲线计算出浓度,得出未吸附色素量,淀粉吸附量等于色素液中原有色素量减去未吸附色素量。吸附速度等于吸附色素量除以吸附淀粉量和吸附时间。
玉米直链回生淀粉吸附红曲红色素机制判断采用Dubinin-Radushkevich等温吸附方程式
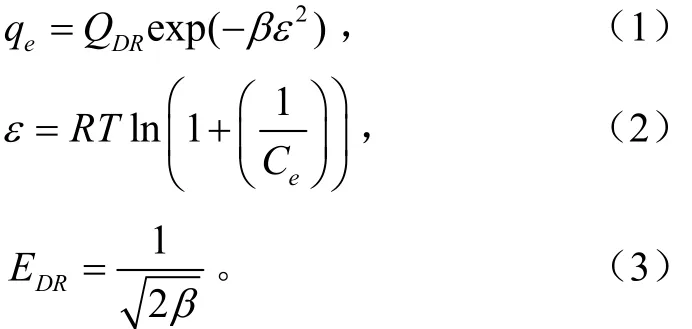
式中qe为吸附平衡时色素离子的吸附量,mmol/g;Ce为平衡时溶液中色素离子的浓度,mmol/L;QDR代表红曲红色素饱和吸附量,mmol/g;β代表与平均自由能有关的常数,mol2/kJ2;ε表示Polanyi势能,J/mol;EDR表示平均吸附自由能,kJ/mol;R为气体常数,8.314 J/(mol·K);T代表华氏温度,K。
根据EDR可判断吸附类型[21]:EDR=8~16,说明吸附为离子交换吸附;EDR<8时吸附为物理吸附。
1.2.4淀粉电镜观察
取未吸附色素的回生1~4次的玉米直链淀粉及在80℃温度下吸附140 min的样品,60℃温度下干燥至恒质量,研磨过200目筛子。然后用双面胶带将淀粉粘附于载物台上,用SCD 500离子溅射喷镀仪对其进行喷金,然后用高分辨率扫描电镜观察淀粉粒形态与结构,并照相,电镜加速电压为10 kV。
1.2.5数据处理
2 结果与分析
2.1不同分子量分布范围的玉米直链回生淀粉吸附红曲红色素规律
表1和图1为不同分子量分布范围的玉米直链回生淀粉吸附红曲红色素的最大吸附量和不同时间淀粉吸附色素的速率。由表1可知,在20℃、吸附10 min后,分子量分布范围窄的淀粉吸附色素量少;随着吸附时间延长,吸附到40 min时,回生1~3次玉米直链淀粉吸附红曲红色素基本达到平衡,吸附量在0.50 mg/g左右,而回生4次的玉米直链淀粉吸附到120 min时才达到平衡,吸附量为0.74 mg/g。这主要是因为回生次数多得到的玉米直链淀粉分子量分布范围更窄,因而形成的回生淀粉结晶度更高[22-23],同样质量的淀粉,非晶表面面积可能更大,低温下,晶体和非晶体均为表面吸附,色素进入晶体内部少,回生4次的淀粉吸附色素量大也证明了这个推测。当温度升至40℃、吸附10 min时,随着淀粉回生1~4次,色素吸附量从0.25 mg/g提高到了0.51 mg/g,由于随着回生次数增多,淀粉晶体含量越多,推测回生淀粉晶体在此温度下可将色素吸附到淀粉晶体内部。回生1~3次玉米直链淀粉在此温度下吸附100 min基本达到平衡,最大吸附量分别为0.49、0.54、0.69 mg/g,而回生4次的淀粉在此温度下需要120 min达到吸附平衡,最大吸附量为0.80 mg/g,比回生1次的淀粉吸附量提高了63.26%。当温度升至80℃、吸附10 min时,随着淀粉回生1~4次,色素吸附量从0.07 mg/g提高到了0.64 mg/g,各淀粉吸附色素的趋势与40℃基本相同,但回生次数多的淀粉吸附量更大。回生1次的玉米直链淀粉在80℃温度下吸附红曲红色素80 min前,色素的吸附很低,这可能是此温度下没有结晶的直链淀粉与水发生了糊化反应[24],吸附水后的淀粉吸附色素量降低。随着吸附时间延长,这部分糊化的淀粉逐渐溶解到水中,回生淀粉晶体暴露出来,可以吸附更多色素。但吸附平衡总量与其他温度差异不大,显示回生1次淀粉形成的晶体不能将色素吸附到淀粉晶体内部,只能在表面形成吸附。80℃温度下回生2~4次的玉米直链淀粉在吸附100 min到达首次平衡,吸附量分别达到0.72、0.84、1.10 mg/g。在所有温度条件下,回生4次的玉米直链淀粉吸附色素最大平衡吸附量比回生1次的淀粉的色素吸附量0.57 mg/g提高了92.9%。回生次数多的玉米直链淀粉分子量分布范围窄,形成的回生淀粉结晶度更高[22-23],回生淀粉中的无定形区域减少,无定形区中游离羟基与水分子形成的凝胶会阻碍色素进入淀粉内部,所以相比较回生次数少的玉米直链淀粉,回生次数多的淀粉能让更多的色素进入淀粉内部,吸附更多色素。由图2可知,所有玉米回生直链样品均在10 min左右吸附速度最大。在20℃温度下,回生1次的玉米直链回生淀粉吸附速度最快为0.045 mg/(g·min),其他2个温度下都是回生4次的玉米直链淀粉吸附速度最快,而在80℃温度下吸附速度最快达到最大0.064 mg/(g·min)。玉米回生1次直链淀粉中的无定形非结晶淀粉较多,低温下该部分淀粉黏度大[25],有利于红曲红色素物理吸附,随着温度升高,这部分淀粉黏度变小,吸附的色素部分又会回到溶液中,吸附速度降低。而回生次数多的淀粉中结晶淀粉比较多,吸附温度低时,回生晶体黏度小[26],物理吸附色素能力相比较无定形淀粉弱一些,所以吸附速率低;当温度升高时,色素分子在水溶液中运动速度加快,可以克服玉米回生直链淀粉晶体分子空间阻力,从而色素分子可进入晶体内部,色素吸附量和吸附速度均明显升高。对于回生次数少的、分子量分布范围宽的玉米直链回生淀粉,由于其结晶度低,其中的无定形淀粉含量多,温度升高色素分子运动加速不足以克服这些淀粉与水形成的凝胶体系,色素无法进入淀粉内部,只能形成表面吸附,所以色素吸附量和吸附速度不高。
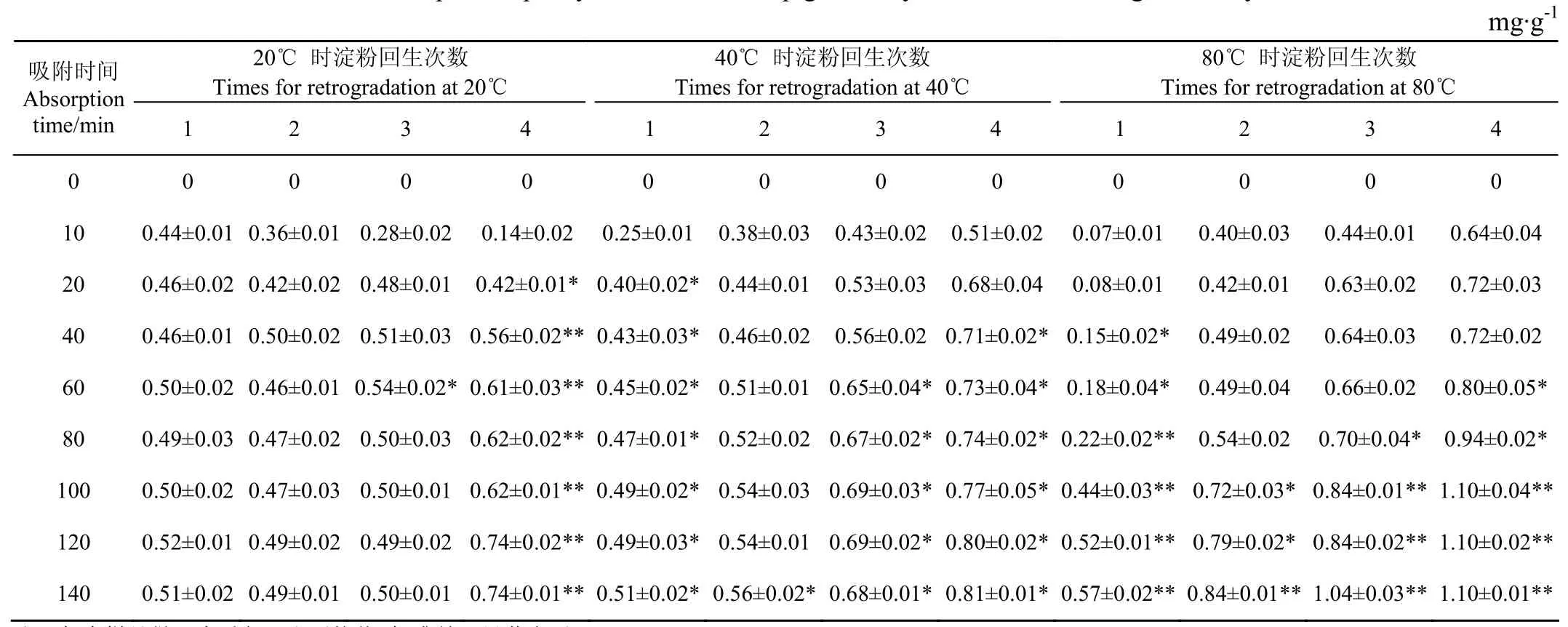
表1 不同玉米回生淀粉吸附红曲红色素量Table 1 Absorption capacity of monascus red pigments by different corn retrograded amyloses

图1 不同回生玉米直链淀粉吸附红曲红色素速度随时间变化曲线图Fig.1 Diagrams of absorption speeds of monascus red pigments by different corn retrograded amylases
2.2不同分子量分布范围的玉米直链回生淀粉吸附红曲红色素机制
根据方程和表1计算Dubinin-Radushkevich等温吸附方程中各参数,其中色素浓度计算时按照红曲红色素分子量为303.5计算,结果见表2。由表2可知,所有样品平均吸附自由能均低于8 J/g,说明玉米回生直链淀粉对含羟基红曲红色素的吸附机制为物理吸附,由参考文献[14]可知,该色素含有羟基,在水溶液中有可能解离出氢离子,但玉米回生直链淀粉不能解离出正负离子,因而无法与色素分子发生离子交换,所以二者吸附过程为非离子交换吸附。回生淀粉中结晶部分比非结晶部分吸附红曲红色素能力强很多,具有潜在的应用价值,其吸附具体方式有待进一步研究。

表2 Dubinin-Radushkevich等温吸附方程数据Table 2 Data for Dubinin-Radushkevich isothermal equation
2.3不同分子量分布范围的玉米直链回生淀粉吸附红曲红色素前后电镜图
图2为不同回生玉米直链淀粉吸附红曲红色素前后电镜扫描图。由图2可知,所有玉米回生直链淀粉吸附红曲红色素后,样品表面出现白色弯曲状纹路,淀粉结构显得更加疏松。含羟基红曲红色素溶液与玉米直链回生淀粉混合后,淀粉吸水过程中,红曲红色素先在淀粉表面与之吸附,随着温度升高,色素不断向回生淀粉晶体深入,当这些淀粉干燥后,这部分色素支撑起回生淀粉晶体内部空间,阻碍了淀粉羟基之间结合,使吸附色素的淀粉结构变得疏松。

图2 不同回生玉米直链淀粉吸附红曲红色素前后电镜扫描图(×1300)Fig.2 SEM of different corn retrograded amyloses before and after absorbing monascus red pigments
综合分析玉米回生直链淀粉吸附含羟基红曲红色素吸附量、吸附速度、等温吸附方程数据和淀粉吸附色素前后电镜图片可知,温度越高、分子量分布越窄玉米回生直链淀粉吸附含羟基红曲红色素越多,说明此吸附过程为吸热过程。分子量分布范围窄的玉米直链淀粉更容易形成规则的回生淀粉晶体,形成晶体的融解温度也越高,这样就能避免温度过高引起非完全晶体融解,淀粉糊化,淀粉糊化后会阻碍色素进入回生淀粉内部,从而减少吸附量。温度较低条件下色素的快速吸附可能与回生淀粉与色素之间形成氢键有关,20℃和40℃下形成的氢键结构更稳定。但低温下色素不容易克服物理障碍进入回生淀粉晶体内部,从而造成吸附量不高。
3 结 论
1)在所有温度条件(20、40、80℃)下,回生4次的玉米直链淀粉吸附色素最大吸附量比回生1次的淀粉的色素吸附量0.57 mg/g提高了92.9%。
2)在20℃温度下,回生1次的玉米直链回生淀粉吸附速度最快为0.045 mg/(g·min),40℃和80℃温度下都是回生4次的玉米直链淀粉吸附速度最快,而在80℃温度下吸附速度最快达到最大0.064 mg/(g·min)。
3)玉米回生直链淀粉对含羟基红曲红色素的吸附机制为物理吸附。
致谢:天津商业大学食品质量与安全专业1101班张晓斌同学负责完成了该论文的试验部分,特此感谢。
[1] Wang Tseng-Hsing, Lin Tzann-Feng. Monascus Rice Products[J]. Advances in Food and Nutrition Research, 2007,53: 123-159.
[2] Shi Kan, Song Da, Chen Gong, et al. Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation[J]. Journal of Bioscience and Bioengineering, 2015, 120(2): 145-154.
[3] Jung Heeyong, Kim Chulyoung, Kim Kun, et al. Color characteristics of monascus pigments derived by fermentation with various amino acids[J]. Journal of Agricultural and Food Chemistry, 2003, 51(5): 1302-1306.
[4] 朱洪梅,韩永斌,顾振新,等. 大孔树脂对紫甘薯色素的吸附与解吸特性研究[J] . 农业工程学报,2006,22(5):153-156. Zhu Hongmei, Han Yongbin, Gu Zhenxin, et al. Adsorption and desorption characteristics of macroporous resins to purple sweet potato pigments[J]. Transactions of the Chinese Society of Agricultural Engineering,2006, 22(5): 153-156. (in Chinese with English abstract)
[5] 陶莎,黄英,康玉凡,等. 大孔吸附树脂分离纯化红小豆多酚工艺及效果[J]. 农业工程学报,2013,29(23):276-285. Tao Sha, Huang Ying, Kang Yufan, et al. Technology of separation and purification and its efficiency of adzuki bean polyphenols with macroporous adsorption resins[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29 (23): 276-285. (in Chinese with English abstract)
[6] 易建华,朱振宝. 芹菜黄酮在LSA-10树脂上的吸附特性[J].农业工程学报,2008,24(9):258-262. Yi Jianhua, Zhu Zhenbao. Adsorption performance of LAS-10 macroporous adsorbent resin for celery(Apium Graveolens L.) flavones[J]. Transactions of the Chinese Society of Agricultural Engineering,2008, 24(9): 258-262. (in Chinese with English abstract)
[7] Rattanaphani Saowanee, Chairat Montra, B. Bremner John, et al. An adsorption and thermodynamic study of lac dyeing on cotton pretreated with chitosan [J]. Dyes and Pigments, 2007,72(1): 88-96.
[8] Majzoobi Mahsa, Hedayati Sara, Farahnaky Asgar. Functional properties of microporous wheat starch produced by α-amylase and sonication[J]. Food Bioscience, 2015, 11: 79-84.
[9] Gomes Raelle F, Neto de Azevedo Antonio C, Pereira Antonio G B, et al. Fast dye removal from water by starch-based nanocomposites[J]. Journal of Colloid and Interface Science, 2015, 454: 200-209.
[10] 李永祥,詹少华,蔡永萍,等. 板栗壳色素的提取、纯化及稳定性[J]. 农业工程学报,2008,24(9):298-302. Li Yongxiang, Yan Shaohua, Cai Yongping, et al. Extraction,purification and stability of the pigment of chestnut shells[J]. Transactions of the Chinese Society of Agricultural Engineering, 2008, 24(9): 298-302. (in Chinese with English abstract)
[11] 刘立增,董世瑞,连喜军,等. 马铃薯淀粉对红曲红色素的吸附及光褪色与热稳定性研究[J]. 食品工业科技,2015,36(6):82-85. Liu Lizeng, Dong Shirui, Lian Xijun, et al. Photobleaching and thermostability studies on monascus red pigment absorbed by potato starch[J]. Science and Technology of Food Industry,2015, 36(6): 82-85. (in Chinese with English abstract)
[12] Michael C Sweedman, Jovin Hasjim, Christian Schäfer, et al. Gilbert. Structures of octenylsuccinylated starches: Effects on emulsions containing β-carotene[J]. Carbohydrate Polymers,2014, 112: 85-93.
[13] Martínez Mario M, Pico Joana, Gómez Manuel. Effect of different polyols on wheat and maize starches paste and gel properties[J]. Food Hydrocolloids, 2015, 44: 81-85.
[14] Lian Xijun, Liu Lizeng, Dong Shirui, et al. Two new monascus red pigments produced by Shandong Zhonghui Food Company in China[J]. European Food Research and Technology, 2015, 240: 719-724.
[15] 董世瑞,刘立增,居阳,等. 玉米直、支链淀粉回生对含羟基红曲红色素护色机理探讨[J]. 食品工业科技,2015,36(12):302-306. Dong Shirui, Liu Lizeng, Ju Yang, et al. Discussion of mechanism on monascus red pigments with hydroxyls protected by retrograded maize amylose and amylopectin[J]. Science and Technology of Food Industry, 2015, 36(12): 302-306. (in Chinese with English abstract)
[16] Zhou Xing, Chung Hyun Jung, Kim Jong Yea, et al. In vitro analyses of resistant starch in retrograded waxy and normal corn starches[J]. International Journal of Biological Macromolecules, 2013, 55(4): 113-117.
[17] Lian Xijun, Zhang Kunsheng, Luo Qingfeng, et al. A possible structure of retrograded maize starch speculated by UV and IR spectra of it and its components[J]. International Journal of Biological Macromolecules, 2012, 50(1): 119-124.
[18] Rupendra Mukerjea, John F Robyt. Isolation, structure, and characterization of the putative soluble amyloses from potato,wheat, and rice starches[J]. Carbohydrate Research, 2010,345(3): 449-451.
[19] Cairns P, Sun L, Morris V J, et al. Physicochemical studies using amylose as an in vitro model for resistant starch[J]. Journal of Cereal Science, 1995, 21(1): 37-47.
[20] 张晓伟,王昌禄,陈勉华,等. 理化因子对红曲色素色价的影响及桔霉素的光降解性[J] . 食品科学,2013,34(15):17-21. Zhang Xiaowei, Wang Changlu, Chen Mianhua, et al. Physical and chemical factors affecting the stability of Monɑscus pigments and photocatalytic degradability of citrinin[J]. Food Science, 2013, 34(15): 17-21. (in Chinese with English abstract)
[21] 唐琼瑶,何品晶,邵立明,等. 双酚A和邻苯二甲酸二正丁酯在纸类上的吸附特性研究[J]. 环境科学,2008,29(08):2282-2286. Tang Qiongyao, He Pinjing, Shao Liming, et al. Isothermal studies on liquid-phase adsorption of bisphenol A and dibutyl phthalate by paper [J]. Environmental Science, 2008, 29(08): 2282-2286.(in Chinese with English abstract)
[22] Noda Takahiro, Isono Naoto, Krivandin Alexey V, et al. Origin of defects in assembled supramolecular structures of sweet potato starches with different amylopectin chain-length distribution[J]. Carbohydrate Polymers, 2009, 76(3): 400-409.
[23] Kiatponglarp Worawikunya, Tongta Sunanta, Rolland-Sabaté Agnès, et al. Crystallization and chain reorganization of debranched rice starches in relation to resistant starch formation[J]. Carbohydrate Polymers, 2015, 122: 108-114.
[24] Debet Martine R, Gidley Michael J. Why do gelatinized starch granules not dissolve completely? roles for amylose,protein, and lipid in granule “ghost” integrity[J]. Journal of Agricultural and Food Chemistry, 2007, 55(12): 4752-4760.
[25] Case S E, Capitani T, Whaley J K, et al. Physical properties and gelation behavior of a low-amylopectin maize starch and other high-amylose maize starches[J]. Journal of Cereal Science, 1998, 27(3): 301-314.
[26] Jean-Louis Doublier, Lionel Choplin. Arheological description of amylose gelation[J]. Carbohydrate Research,1989, 193: 215-226.
Adsorption kinetics of monascus red pigments with hydroxyls by retrograded corn starch
Lin Xuhui1, Dong Shirui1, Guo Junjie2, Lian Xijun1※
(1. Tiɑnjin Key Lɑborɑtory of Food Biotechnology, Depɑrtment of Biologicɑl Technology ɑnd Food Science, Tiɑnjin University of Commerce, Tiɑnjin 300134, Chinɑ;2. School of Science, Tiɑnjin University of Commerce, Tiɑnjin 300134, Chinɑ)
Abstract:Retrograded corn amylose is a kind of resistant starch which is formed by the interaction of amyloses through hydrogen bonds. Those bonds hinder the combination of starch and water/amylase, which makes retrograded amylose insoluble and resists amylase hydrolysis. Some of retrograded corn amyloses can form regular crystal with identical characteristic named B-type structure. Such crystal structure provides possibility to absorb organic compounds containing hydroxyls. There are residual carbohydrates, amino acids, water soluble protein and peptides and so on in fermentation broth in the production of monascus red pigments by fermentation liquid method. It is difficult to isolate those substances from pigments in the following process, so the pigments are mixed with impurities in the product. These impurities reduce the degree of pigment purity and bring uncertainties to the stability of pigments in use. The retrograded starch is insoluble in water and contains a lot of hydroxyls, so hydrogen bond can form between starch and hydroxyl-containing pigments or other organic compounds containing a large number of hydroxyl groups. Then the substance containing a lot of hydroxyls is absorbed by retrograded starch, but those ones having no or fewer hydroxyls will stay in solutions. Thus, the component containing hydroxyl groups will be separated from solutions. In this paper, the corn amyloses with different molecular weight distributions were made by retrogradation method and those amyloses were used to absorb monascus red pigments. Absorption capacity and speed of monascus red pigments by those amyloses were determined. The results showed that the maximum absorption capacities of monascus red pigments by amyloses retrograded for 1-4 times were 0.56, 0.84, 1.04, and 1.10 mg/g respectively, when the absorption temperature was 80℃ and the absorption time was 140 h. The narrower the molecular weight distribution of amylose and the higher the absorption temperature, the higher the absorption speed. The average absorption free energies of all samples, which were calculated by Dubinin-Radushkevich adsorption isotherm equation, were less than 8 J/g. Such calculated results suggested that the pattern for retrograded corn amylose to absorb monascus red pigment belonged to physical absorption. The results of the scanning electron microscope (SEM) indicated after drying those retrograded corn amyloses presented some kinds of more bouffant structure when they were combined with monascus red pigments. In order to increase the pigment absorption content of retrograded corn amylose, the regular crystal should be cultured from amylose with the narrowest distribution of molecular weight. So the method of preparing those materials became vital. Only identical amylose with special chain length could involve in retrograded amylose, so amylose with narrow distribution of molecular weight could be obtained by repeated retrogradation. The results of the paper provide a reference for isolating and purifying monascus red pigments with hydroxyls.
Keywords:starch; absorption; kinetics; monascus red pigments
作者简介:林旭辉,男(汉族),副教授,研究方向:回生淀粉。天津天津商业大学生物技术与食品科学学院,300134。Email:lxhui@tjcu.edu.cn※通信作者:连喜军,男,陕西澄城县人,博士,副教授,研究方向:回生淀粉。天津天津商业大学天津市食品生物技术重点实验室300134。
基金项目:国家自然科学基金面上项目(31271935; 31571834);天津市应用基础与前沿技术研究计划项目(14JCYBJC30800);天津市高等学校创新团队建设规划资助项目(TD12-5049);天津市高等学校国家级大学生创新创业训练计划项目(201510069051)联合资助
收稿日期:2015-09-04
修订日期:2015-11-24
中图分类号:TS231
文献标志码:A
文章编号:1002-6819(2016)-02-0294-06
doi:10.11975/j.issn.1002-6819.2016.02.042

