不同碳源条件下PQQ对植物促生菌Rahnella aquatilis HX2溶解无机磷影响的研究
焦子伟,张相锋,张 娜,吾尔恩,郭岩彬
(1. 伊犁师范学院化学与生物科学学院,新疆伊宁 835000;2. 中国农业大学资源与环境学院,北京 100193)
不同碳源条件下PQQ对植物促生菌RahnellaaquatilisHX2溶解无机磷影响的研究
焦子伟1,张相锋1,张 娜1,吾尔恩1,郭岩彬2
(1. 伊犁师范学院化学与生物科学学院,新疆伊宁835000;2. 中国农业大学资源与环境学院,北京100193)
摘要:【目的】水生拉恩氏菌(Rahnella aquatilis)HX2能合成葡萄糖脱氢酶(glucose dehydrogenase, GDH)和吡咯喹啉醌(pyrroloquinoline quinone,PQQ),PQQ为GDH辅酶,与其共同参与葡萄糖溶磷代谢。揭示不同碳源条件下PQQ影响HX2菌株溶解无机磷作用的机理。【方法】以野生菌株HX2、突变体MH15及其互补菌株CMH15(pqq)为供试材料,在不同碳源条件下采用平板溶磷、钼锑抗比色法等方法对其进行溶解无机磷定性、定量以及pH相关分析。【结果】除D-山犁醇、D-果糖外,PQQ参与HX2菌株对木糖、葡萄糖、D-甘露糖、D-甘露醇、蔗糖、乳糖6种碳源的溶磷代谢,但其溶磷代谢因HX2菌株利用不同碳源的能力而不同,以乳糖利用最低,木糖利用最高。【结论】PQQ作为GDH的辅酶,两者共同参与HX2菌株的木糖、葡萄糖、D-甘露糖、D-甘露醇、蔗糖和乳糖溶解无机磷代谢,并起到重要调控作用。
关键词:吡咯喹啉醌;水生拉恩氏菌HX2;不同碳源;无机磷
0 引 言
【研究意义】RahnellaaquatilisHX2菌株已作为优良的溶磷促生菌应用于伊犁河谷绿色有机农业生产中[1],进一步明确其溶磷机理可对其促进植物生长提供理论依据和技术支持。【前人研究进展】 HX2菌株具有较强的溶磷能力,可利用不同碳源的培养基生长,产生PQQ和GDH,PQQ作为GDH的辅酶,两者共同参与其葡萄糖溶磷代谢[2]。焦子伟等[1]已明确在不同碳源溶磷培养基条件下,GDH参与了HX2菌株对木糖、萄萄糖、D-甘露糖、D-甘露醇、乳糖、蔗糖、D-果糖D-山梨醇溶磷代谢。【本研究切入点】Guo等[3]已通过Tn5插入突变pqq基因获得了突变菌株MH15,以及其互补菌株CMH15(pqq)。研究在已有基础上,采用平板溶磷、钼锑抗比色法等实验方法,分析研究不同碳源下PQQ对HX2菌株溶解无机磷能力的影响。【拟解决的关键问题】明确PQQ在不同碳源下对HX2菌株溶磷的作用与代谢,揭示PQQ作为GDH的辅酶共同参与对HX2菌株溶解无机磷作用影响机理。
1材料与方法
1.1 材 料
1.1.1 菌 株
RahnellaaquatilisHX2,Tn5插入突变pqq基因的突变体MH15,MH15的互补菌株CMH15(pqq),列出具体特性。表1
表 1 菌株及质粒特征
Table 1 Characteristis of bacteria strains and plasmids

名称Names特征Characteristics文献来源Literatureresources水生拉恩氏菌RahnellaaquatilisHX2Apr,WildtypeChen[2]MH15Apr,Kmr,HX2derivativecontainingaTn5insertioninpqqEgeneGuo[3]CMH15(pqq)Apr,Kmr,Tcr,MH15containingplasmidpCH15withthepqqgenesGuo[3]质粒PlasmidsCP465Tcr,pLAFR-5containingpqqgenes,cosmidGuo[3]pCH15Gmr,Tcr,pRK415Gcontainingapproximately8.0kbBamHIfragmentincludingpqqgenesfromcosmidCP465Guo[3]
注:Apr, Kmr, Gmrand Tcr分别表明抗氨苄青霉素、卡那霉素、庆大霉素和四环素
Note: Apr, Kmr, Gmrand Tcrindicate resistance to ampicillin, kanamycin, gentamicin and tetracycline, respectively
1.1.2 培养基
采用国际植物研究所磷酸盐生长培养基(NBRIP)、不同碳源NBRIP培养基和LB液体培养基参照焦子伟等[1]配方。
1.2 方 法
1.2.1碳源选择
根据前期已有研究结果[1],选用蔗糖、乳糖、D-甘露糖、D-甘露醇、D-山梨醇、木糖、D-果糖和葡萄糖(作为对照)8种碳源进行溶解无机磷的定性、定量分析。
1.2.2 溶磷定性检测
将HX2、MH15、CMH15(pqq)菌株活化、摇培后,将菌悬液接种于含有直径为0.5 cm的滤纸片的不同碳源NBRIP固体培养基平板上,并将各处理样品进行培养,具体操作与检测分析参照焦子伟等[1]溶磷平板定性检测方法。
1.2.3 有效磷定量、pH检测
将HX2、MH15、CMH15(pqq)菌株活化、摇培后,将菌悬液接种到不同碳源NBRIP液体培养基中进行培养,将所有处理样品于不同摇培时间分别抽取摇培液进行离心取上清液,进行有效磷定量和pH的检测,具体操作与检测参照焦子伟等[1]有效磷定量和pH值检测方法。
1.3 数据统计
采用EXCEL软件进行数据分析处理和SPSS软件方差分析(ANOVA)。
2结果与分析
2.1 不同碳源对HX2、MH15、CMH15菌株溶磷定性检测
在8种不同碳源作为NBRIP固体培养基的碳源,经7 d培养,HX2、MH15、CMH15(pqq)菌株都有溶磷现象,溶磷圈直径也各不相同。同一碳源溶磷培养基培养情况下,各菌株在D-果糖、D-山犁醇作为碳源情况下,其溶磷圈直径无显著差异;而在其它不同6种(木糖、蔗糖、乳糖、D-甘露糖、D-甘露醇和对照)碳源培养基情况下,其溶磷圈直径差异显著(P<0.05)。以D-山犁醇为例,其溶磷圈直径最小,HX2 、MH15、CMH15(pqq)菌株溶磷直径在1.35~1.43 cm;木糖、D-甘露糖和对照作为碳源,其溶磷圈直径较大,如在D-甘露糖作为碳源情况下,HX2、CMH15(pqq)溶磷圈直径分别为2.21、2.15 cm;突变体MH15溶磷圈直径1.10 cm,缺失pqq基因的菌株溶磷圈直径明显减小。图1,表2
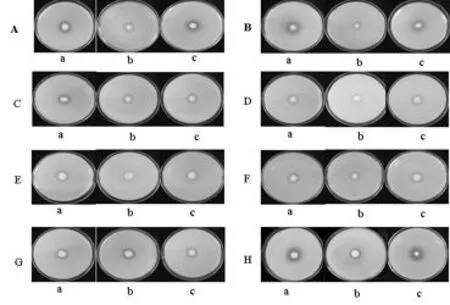
注:不同碳源分别为A,木糖; B, D-甘露糖; C, D-甘露醇; D, D-山犁醇; E, 乳糖; F, 蔗糖; G, D-果糖;H,对照(葡萄糖);a, HX2; b, MH15; c, CMH15(pqq) ;下同
Note: Different carbon resources: A, Xylose; B, D-Mannose; C, D-Mannitol; D, D-Sorbitol; E, lactose; F, Surcose; G, D-fructose; H, control (glucose). a, HX2; b, MH15; c, CMH15(pqq);the same as bolow
图1 不同处理和培养基下菌株溶磷圈
Fig.1 Clear halo of solubilization of tricalcium phosphate by strains under different treatment and medium
表2不同处理和培养基下菌株溶磷圈直径 (cm)
Table 2 Phosphate-solubilizing halo diameter (cm) produced by strains under different treatment and medium
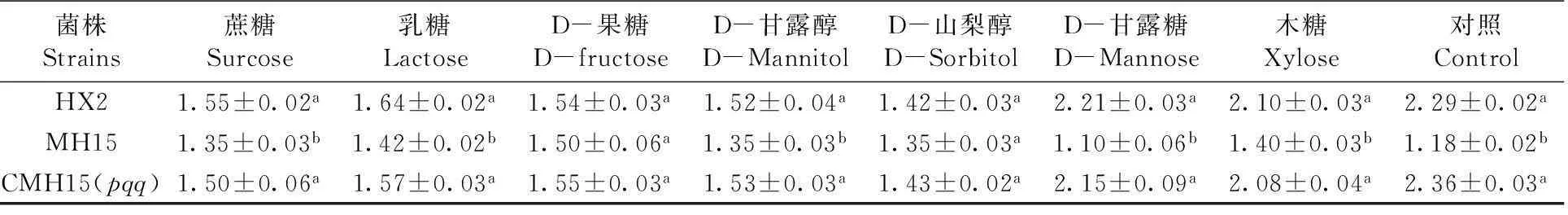
菌株Strains蔗糖Surcose乳糖LactoseD-果糖D-fructoseD-甘露醇D-MannitolD-山梨醇D-SorbitolD-甘露糖D-Mannose木糖Xylose对照ControlHX21.55±0.02a1.64±0.02a1.54±0.03a1.52±0.04a1.42±0.03a2.21±0.03a2.10±0.03a2.29±0.02aMH151.35±0.03b1.42±0.02b1.50±0.06a1.35±0.03b1.35±0.03a1.10±0.06b1.40±0.03b1.18±0.02bCMH15(pqq)1.50±0.06a1.57±0.03a1.55±0.03a1.53±0.03a1.43±0.02a2.15±0.09a2.08±0.04a2.36±0.03a
数据为平均值和标准误差。(a-b),不同字母表示为显著性差异(P<0.05)
Data was shown by Mean (± standard error) values. (a-b), different letters indicated statistically significant (P< 0.05)
2.2 不同碳源对HX2、MH15、CMH15菌株的pH检测
8种碳源分别作为不同碳源NBRIP培养基,经7 d培养,HX2、MH15、CMH15(pqq)菌株在不同碳源NBRIP溶磷培养基上均能生长,但随着培养时间延长,pH均逐渐下降,但pH各不相同,最终相对趋于稳定。除D-果糖、D-山犁醇作为碳源溶磷培养基外,HX2、CMH15(pqq)与突变体MH15处理的pH值都有显著性差异(P<0.05);以D-甘露糖、对照(葡萄糖)和木糖作为碳源培养基,HX2及其衍生菌株pH值变化较大。如木糖作为碳源的NBRIP培养基,HX2、CMH15(pqq)的pH分别为3.28、3.26,MH15突变体的pH 4.53,明显增高。表3,图2
表3 不同处理和培养基下菌株pH值
Table 3 pH observed by strains under different treatment and medium

菌株Strains蔗糖Surcose乳糖LactoseD-果糖D-fructoseD-甘露醇D-MannitolD-山梨醇D-SorbitolD-甘露糖D-Mannose木糖Xylose对照ControlHX24.17±0.03b4.62±0.04b4.21±0.08a4.19±0.06b4.75±0.02a3.72±0.03b3.28±0.05b3.44±0.02bMH154.34±0.02a4.89±0.06a4.36±0.03a4.34±0.02a4.72±0.06a4.19±0.03a4.53±0.06a4.89±0.02aCMH15(pqq)4.14±0.07b4.63±0.05b4.22±0.09a4.18±0.03b4.80±0.08a3.64±0.12b3.26±0.30b3.56±0.03b

注:虚线部分代表有效磷浓度变化图;实线部分代表pH值变化
Note:pH of NBRIP after incubation was signed with the real line. Concentration of soluble-P was indicated by broken line
图2不同处理和培养基下菌株pH和产有效磷变化曲线
Fig. 2Change curve of pH and concentration of soluble-P produced by strains under different treatment and medium
2.3 不同碳源对HX2、MH15、CMH15菌株溶磷定量检测
HX2、MH15、CMH15(pqq)菌株在8种不同碳源溶磷培养基上,经7 d培养,随着培养时间延长,有效磷浓度逐渐上升,最终趋于相对稳定;各菌株均都能产生有效磷,但产生有效磷的浓度各不相同。同一碳源NBRIP培养情况下,D-果糖和D-山犁醇作为碳源下,各菌株之间产生有效磷浓度差异不显著;其余的如蔗糖、乳糖、D-甘露醇、D-甘露糖、木糖和对照作为碳源NBRIP培养基,野生菌株HX2、CMH15(pqq)与插入突变pqq基因的菌株MH15之间产生的有效磷浓度差异显著(P<0.05)。各菌株在D-山犁醇作为碳源溶磷培养基中,产生的有效磷浓度最小;在木糖中产生的最大,其次是葡萄糖和D-果糖。以木糖为例,HX2、CMH15(pqq)菌株产生的有效磷浓度分别为541.26和547.32 mg/L;MH15突变体产生的有效磷浓度为154.29 mg/L,产生的有效磷浓度最低。表4,图2
表4不同处理和培养基下菌株产有效磷量
Table 4 Concentration of soluble-P produced by strains under different treatment and medium(mg/L)

菌株Strains蔗糖Surcose乳糖LactoseD-果糖D-fructoseD-甘露醇D-MannitolD-山梨醇D-SorbitolD-甘露糖D-Mannose木糖Xylose对照ControlHX2332.76±28.35b109.12±12.19a316.23±9.61a247.81±16.25a75.39±10.04a429.18±26.76a541.26±30.18a431.44±15.27aMH15249.02±14.61c86.90±6.49b306.90±7.81a212.92±12.36b81.24±16.22a252.49±13.37b154.29±4.32b91.19±2.98bCMH15(pqq)391.61±41.40a111.30±2.35a319.32±8.02a246.03±14.14a77.86±4.42a448.77±25.64a547.32±88.73a427.20±11.72a
3讨 论
许多研究表明一些革兰氏阴性菌如Pseudomonascepacia[4]、Rahnellaaquatilis[5]和Enterobacterintermedium60-2G[6]的PQQ 合成酶的pqq基因决定其溶解无机磷的能力[7]。野生菌株HX2在以葡萄糖、D-甘露醇、乳糖、D-甘露糖等为碳源的培养基中均可正常生长[2]。研究以野生菌株HX2为供试研究对象,除葡萄糖作为NBRIP的碳源之外,把乳糖、蔗糖、木糖、D-甘露醇、D-甘露糖、D-山梨醇、D-果糖来作为NBRIP不同的7种碳源,进行其溶磷定性、定量分析,HX2、CMH15(pqq)与MH15菌株之间在蔗糖、乳糖、D-甘露糖、D-甘露醇、木糖和葡萄糖6种碳源NBRIP培养基上产生的有效磷浓度差异显著,这说明插入突变pqq的突变体很难利用不同碳源溶解难溶性的无机磷,也得出了类似的结果。
PQQ作为GDH等脱氢酶的辅酶,对GDH的功能发挥起关键控制作用,溶磷细菌通过GDH-PQQ 全酶作用能溶解土壤无机或有机磷酸盐,促进植物对营养的摄入和植物生长[4,6,8,9]。Rodríguez等[10]研究报道导入pqq基因的BurkholderiacepaciaIS-16 和假单孢菌属(Pseudomonassp)两个菌株能增强矿质磷酸盐溶解表型,促进对磷的溶解。野生菌株HX2既能合成GDH和PQQ,在HX2菌株中,GDH都参与木糖、萄萄糖、D-甘露醇、D-甘露糖、乳糖、蔗糖、D-山梨醇、D-果糖溶磷代谢[1];PQQ参与木糖、葡萄糖、D-甘露醇、D-甘露糖、蔗糖、乳糖溶磷代谢,不参与D-山梨醇、D-果糖溶磷代谢;这说明PQQ作为GDH的辅酶参与了HX2菌株的木糖、葡萄糖、D-甘露糖、D-甘露醇、蔗糖、乳糖的溶磷代谢。这也可能说明了GDH有更好的底物特性,PQQ作为GDH重要的辅酶,但不是唯一的,PQQ的结构类似物也可作为GDH氧化还原酶的辅酶或辅助因子参与溶磷代谢[11]。
MH16、MH15突变体在不同碳源溶磷培养基条件下也能产生有效磷,说明除葡萄糖溶磷主要机制外,可能还有其它的溶磷机制也参与HX2菌株的溶磷代谢。关于PQQ作为GDH的辅酶参与其溶磷产酸的调控机理,以及该菌株涉及的其它相关溶磷机理还有待进一步深入研究。
4结 论
从PQQ参与不同碳源条件下对HX2菌株溶解无机磷作用影响结果分析,HX2菌株在8种不同碳源上具有不同的溶磷效果,PQQ除D-山梨醇、D-果糖外均参与该菌株对木糖、葡萄糖、D-甘露糖、D-甘露醇、蔗糖和乳糖溶磷代谢,以乳糖利用最低,木糖利用最高。PQQ作为GDH的辅酶,共同参与HX2菌株的木糖、葡萄糖、D-甘露糖、D-甘露醇、蔗糖和乳糖溶磷代谢,并起到重要调控作用。
参考文献(References)
[1]焦子伟, 吴文良, 郭岩彬. 不同碳源条件下GDH对植物促生菌HX2溶解无机磷影响的研究[J]. 新疆农业科学, 2015,52(2):268-274.
JIAO Zi-wei, WU Wen-liang, GUO Yan-bin. (2015). Effect of glucose dehydrogenase on mineral phosphate solubilization with different carbon sources in Rahnella aquatilis HX2 [J].XinjiangAgriculturalSciences, 52(2):268-274. (in Chinese)
[2]陈凡. 水生拉恩氏菌HX2菌株防治葡萄根癌病的初步研究[D]. 北京:中国农业大学博士论文,2007.
CHEN Fan. (2007).PrimarystudiesonbiologicalcontrolofgrapevinecrowngallbyRahnellaaquatilisHX2 [D]. PhD Dissertation. China Agriuclture University, Beijing. (in Chinese)
[3]Yan Bin, G., Jinyun, L., Lei, L., Fan, C., Wenliang, W., & Jianhui, W., et al. (2009). Mutations that disrupt either the pqq or the gdh gene of rahnella aquatilis abolish the production of an antibacterial substance and result in reduced biological control of grapevine crown gall.Applied&EnvironmentalMicrobiology, 75(21):6,792-6,803.
[4]Babukhan, S., Yeo TCMartin, W. L., Duron, M. R., Rogers, R. D., & Goldstein, A. H. (1995). Cloning of a mineral phosphate-solubilizing gene from pseudomonas cepacia.Applied&EnvironmentalMicrobiology, 61(3):61--972.
[5]Kim, K. Y., Nald, G. A., & Jordan, D. (1997). Solubilization of hydroxyapatite by enterobacter agglomerans and cloned escherichia coli in culture medium.Biology&FertilityofSoils, 24(4):347-352.
[6]Kim, C. H., Song, H. H., Kim, K. Y., Cho, B. H., Yong, H. K., & Koo, B. S., et al. (2003). Cloning and expression of pyrroloquinoline quinone (pqq) genes from a phosphate-solubilizing bacterium enterobacter intermedium.CurrentMicrobiology, 47(6):457-461.
[7]Vikram, A., Alagawadi, A. R., Krishnaraj, P. U., & Kumar, K. S. M. (2007). Transconjugation studies in azospirillum sp. negative to mineral phosphate solubilization.WorldJournalofMicrobiology&Biotechnology, 23(9):1,333-1,337.
[8]Han, S. H., Kim, C. H., Lee, J. H., Ju, Y. P., Song, M. C., & Park, S. K., et al. (2008). Inactivation of pqq genes of enterobacter intermedium 60-2g reduces antifungal activity and induction of systemic resistance.FemsMicrobiologyLetters, 282(1):140-146.
[9]Liu, S. T., Lee, L. Y., Tai, C. Y., Hung, C. H., Chang, Y. S., & Wolfram, J. H., et al. (1992). Cloning of an erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in escherichia coli hb101.JournalofBacteriology, 174(18):5,814-5,819.
[10]Rodríguez, H., Gonzalez, T., & Selman, G. (2000). Expression of a mineral phosphate solubilizing gene from erwinia herbicola in two rhizobacterial strains.JournalofBiotechnology, 84(2):155-161.
[11]周怡雯,陈建华. 新辅酶吡咯喹啉醌研究进展. 中国生化药物杂志,2008,29(4):279-282.
ZHOU Yi-wen, CHEN Jian-hua. (2008).ProgressintheresearchofpyrroloquinolinequinoneChineseJournalofBiochemicalPharmaceutics, 29(4):279-282. (in Chinese).
Fund project:Supported by University Scientific Research Projects of Xinjiang Uygur Autonomous Region (XJEDU2014I041) and NSFC (31200386)
Influences of Pyrroloquinoline Quinone on Inorganic Phosphate
Solubilization under Different Carbon Sources in
RahnellaaquatilisHX2
JIAO Zi - wei1, ZHANG Xiang - feng1, ZHANG Na1, Wueren1, GUO Yan- bin2
(1.CollegeofChemistryandBiologicalSciences,YiliNormalUniversity,YiningXinjiang835000,China; 2.DepartmentofEcologyandEcologicalEngineering,CollegeofResourcesandEnvironmentalSciences,ChinaAgriculturalUniversity,Beijing100193,China)
Abstract:【Objective】 Rahnella aquatilis strain HX2 can produce glucose dehydrogenase (GDH) and pyrroloquinoline quinone (PQQ). PQQ as coenzyme of GDH jointly take part in metabolism of phosphate solubilization under glucose source in HX2 strain. Based on prior researches, this paper further revealed effects of PQQ on inorganic phosphate solubilization under different carbon source conditions in HX2 strain. 【Method】 HX2 wild strains, mutant MH15 and its complementary strains were used as test material. The methods of solubilzing phospate on plate, molybdenum-blue method and so on were used to analyze the qualitative and quantitative ability of phosphate solubilization under different carbon sources. 【Result】Except D-sorbitol and D-fructose, PQQ played a key role in HX2 phosphate solubilization with glucose, xylose, D-mannose, D-mannitol, sucrose and lactose as a sole carbon sources. However, its ability of phosphate solubilization was different with different carbon sources, which was the lowest for lactose and the highest for xylose as carbon sources. 【Conclusion】 It has been clear that PQQ and GDH as holoenzyme were involved in metabolism of phospate solubilization under different carbon sources such as glucose, xylose, D-mannose, D-mannitol, sucrose and lactose, and played an important regulatory role in HX2 strain.
Key words:PQQ; Rahnella aquatilis HX2; Carbon sources; Inorganic phosphate
通讯作者:郭岩彬(1978-),男,博士,副教授,研究方向为植生物生态学、土壤微生物、有机农业,(E-mail)guoyb@cau.edu.cn
作者简介:焦子伟(1973-),男,博士,副教授,研究方向为微生物生态及绿色有机农业有害生物综合防控,(E-mail)741285332@qq.com
基金项目:自治区高校科研计划项目(XJEDU2014I041);国家自然科学基金项目(31200386)
收稿日期:2015-09-09
中图分类号:S188+.4
文献标识码:A
文章编号:1001-4330(2016)02-0295-07
doi:10.6048/j.issn.1001-4330.2016.02.015

