大庆原油水溶物对方正银鲫胚胎及仔鱼的影响
叶剑雄,战培荣,黄晓丽,刘 伟,王 臣
(1.中国水产科学研究院黑龙江水产研究所,哈尔滨 150001;2.上海海洋大学水产与生命学院,上海 201306)
大庆原油水溶物对方正银鲫胚胎及仔鱼的影响
叶剑雄1,2,战培荣1,黄晓丽1,刘 伟1,王 臣1,2
(1.中国水产科学研究院黑龙江水产研究所,哈尔滨 150001;2.上海海洋大学水产与生命学院,上海 201306)
为了评价原油水溶物对水环境及鱼类的影响,以方正银鲫(Carassius auratus gibelio)为研究对象,研究了大庆原油水溶物对方正银鲫胚胎与仔鱼的影响。将以人工授精获得的方正银鲫受精卵(受精2 h)暴露于15%、33%、50%的大庆原油水溶物和对照溶液中培养,研究其孵化率、出膜时间及仔鱼发育状况等。结果表明,原油水溶物对仔鱼的影响大于对胚胎的影响。各浓度组之间胚胎死亡率总体上无明显差异,原油水溶性成分并不直接造成胚胎死亡;试验组仔鱼出膜时间相对于对照组有提前趋势;暴露在原油水溶物条件下,仔鱼出现发育缺陷、畸形、心率减缓、体色着色减弱、活动能力下降等问题,甚至造成死亡,且随着原油水溶物浓度的提高,上述现象的发生及死亡率逐渐增加。
原油水溶物;方正银鲫;胚胎;仔鱼
石油是一种重要的工业原料,成分复杂,包含有脂肪烃、单环芳烃、多环芳烃、苯酚、杂环化合物以及氮、硫和重金属元素。在其开采、运输、提炼的过程中进入环境的几率较大,历史上的石油污染事件层出不穷,对水生生态系统造成了严重的影响[1-5]。蒋闰兰等[6]就多环芳烃对水生动物的影响进行了总结,国内外学者关于石油污染对水生生态系统的污染也进行了多种研究[7-11],结果表明,石油成分对水生生物抗氧化系统产生影响,严重者鳃、肝脏、肾脏的器官会受到损伤。此外,在石油成分胁迫下,水生生物的基因也会出现损伤[8],导致营养行为和繁殖的改变[9]。研究证明生物化学、生理学和组织学等生物标志物可以用来确定石油中碳氢化合物对水生生物群落的影响[10-11]。
胚胎和仔鱼是鱼类生命发育的早期阶段,鱼体发育不完善,免疫和其它生理功能有待发育健全,这个时期对于各种污染物是最敏感的。黄辨非等[12]的研究表明,用硫酸铜与硫酸亚铁合剂(5∶2)、硫酸锌和重铬酸钾药物处理红龙睛金鱼(Carassius auratus)胚胎和受精卵,会导致金鱼受精卵孵化率降低,仔鱼畸形。田丽粉等[13]就胜利原油对褐牙鲆(Paralichthys olivaceus)仔稚鱼的影响进行研究,结果表明胜利原油会导致褐牙鲆仔鱼乏力、身体失去平衡、打旋和下沉死亡。陈民山等[14]研究了胜利原油对海洋鱼类胚胎和仔鱼的影响,结果表明胜利原油在鱼类胚胎发育过程中引起各种畸形,如弯体、脊柱扭曲、尾部弯曲呈“V”或“S”等。
方正银鲫(Carassius auratus gibelio)头小体大、背厚、出肉率高、耐寒冷、抗病力强、生长快,养殖经济性状十分优良[15]。国内对银鲫的研究较多,此鱼种几乎可以认为是模式种[16-19]。中俄原油管道经过黑龙江省,原油对方正银鲫的影响研究因此更有意义。本研究选用方正银鲫胚胎和仔鱼作为实验材料,以期为评价石油对水生生物的影响研究提供借鉴。
1 材料与方法
1.1 原油水溶物的制备
实验所用原油来自大庆油田,饱和烃含量为55%~72%,芳香烃含量为13%~23%,非烃化合物含量为10%~30%,沥青质含量为0.5%~2.0%[20]。参照ANDERSON等[21]方法配制原油水溶液,并以此作为100%饱和液,用全自动红外测油仪(EP3000,北京博海星源科技有限公司)测定其石油浓度,石油浓度值为8.6 mg·L-1。配制足量原油水溶液比例分别为15%、33%、50%的实验溶液,置于低温、阴暗条件下保存待用。
1.2 受精卵的获得
实验用方正银鲫取自黑龙江水产研究所,采用人工催产的方式获得方正银鲫的精子和卵子。具体操作如下:选择性腺发育成熟、健康活泼的方正银鲫雌性鲫鱼[平均体质量(105.14±10.59)g]与雄性鲫鱼[平均体质量(92.46±6.32)g],注射促黄体素释放激素(LRH-A2)与地欧酮(DOM)进行人工催产,将所获精子、卵子除去杂质后进行人工授精,并在受精2 h后进行实验。
1.3 实验设计
实验用原油水溶液比例参照AKAISHI等[22]的浓度设置,把受精2 h后充分吸水膨胀的受精卵置于原油水溶液比例分别为0%、15%、33%、50%的实验溶液中,每组设置3个重复,每个重复放受精卵200 cell。浓度0%组为对照组,其余为试验组,孵化温度25~26℃,pH 7.0左右,溶解氧6~8mg·L-1。实验方法是半静水式,每24 h更换一次原油水溶液。实验过程中不投喂饲料。
实验期间保持观察,在仔鱼孵出前,每6 h清理一次死亡胚胎并计数;记录各组仔鱼孵出时间、孵化率;观察各组孵出仔鱼体色、眼睛发育状况、色素沉着状况,并记录尾部具有缺陷仔鱼数、畸形率;测定各组孵出仔鱼心率与活动能力。其中色素沉着的评定以对照组为标准,色素沉着弱于对照组者记为着色减弱;测定对照组的心率,得到心率范围是107~115次·min-1,平均值为110次·min-1,试验组心率小于110次·min-1且P<0.05则认为心率减缓;孵出仔鱼活动能力的测定参考KOCHHANN等[7]的研究方法,在烧杯底部画两条相互垂直的直径线,计算穿过直径线的仔鱼次数,然后算出100 ind仔鱼5 min的穿越次数,并以此评定各浓度组孵出仔鱼的活动能力。
1.4 数据处理
实验数据采用平均值±标准差表示,采用SPSS 19.0软件对数据进行单因素方差分析及Duncan多重比较。P<0.05为具有显著性差异。
2 结果与分析
2.1 死卵数与死亡仔鱼数
孵出前0%、15%、33%、50%浓度组死亡胚胎依次为(33.2±1.4)ind、(43.8±3.5)ind、(37.6±1.6)ind、(38.1±6.1)ind,其中15%浓度组与0%浓度组死亡胚胎数目间呈显著性差异(P<0.05),其它组之间无显著性差异(P>0.05)。各浓度组仔鱼孵出后每24 h死亡数如图1所示。由图1可知,对照组(0%浓度组)孵出仔鱼死亡率一直处于极低水平,无死亡高峰;而试验组均存在各自的死亡高峰,且50%浓度组死亡高峰出现时间早于15%、33%浓度组,出现于孵出后96~120 h时段,而15%、33%浓度组的孵出仔鱼死亡高峰均出现于孵出后120~144 h时段。就各组累计死亡仔鱼数而言,孵出144 h后,0%、15%、33%、50%浓度组累计死亡仔鱼依次为(2.0±1.0)ind、(22.5±2.1)ind、(50.4±4.4)ind、(96.3±3.8)ind,各组孵出仔鱼死亡率之间均呈显著性差异(P<0.05)。

图1 孵出后各浓度组每24 h死亡仔鱼数Fig.1 Death number of larvae every 24 h with different experimental concentrations after incubation
2.2 出膜时间
各浓度组首个仔鱼出膜均发生在受精53 h时,故在首个出膜时间点上各浓度组间无区别。受精后53~60 h间每小时出膜仔鱼数如图2所示。由图2可见,各浓度组均是在首个仔鱼出膜的第2~3小时出现孵出高峰,但试验组孵出仔鱼的绝对数量大于对照组;根据观察,在首个仔鱼出膜的第16小时,0%、15%、33%、50%依次孵出约70、90、87、84 ind,试验组均已孵出过半,而对照组尚未孵出过半。总体来看,试验组有比对照组孵化提前的趋势。
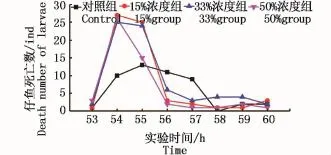
图2 受精后53~60 h的出膜数量Fig2 Number of hatched larvae from 53 h to 60 h after fertilization
2.3 仔鱼发育状况
各浓度组孵出仔鱼发育状况见表1。结果显示,随着原油水溶物浓度的升高,孵出仔鱼的心率、体色着色程度均表现出减弱趋势,各浓度组间呈显著性差异(P<0.05);尾部缺陷仔鱼数、畸形率随着原油水溶物浓度的增加而增加,各浓度组间呈显著性差异(P<0.05);但各浓度组均没有出现眼睛缺陷仔鱼。
对孵出仔鱼形态进行观察,对照组形态正常,而试验组仔鱼多畸形(图3),主要表现为脊柱扭曲、肢体弯曲、尾部弯曲,其中尾部弯曲多呈现“S”、“V”形;早期畸形仔鱼主要表现为心包囊和卵黄囊水肿(图3)。

表1 不同原油水溶物浓度下孵出仔鱼发育状况Tab.1 Development state of larvae in crude oil water-soluble content with different concentrations(%)

图3 大庆原油水溶物对方正银鲫仔鱼的影响Fig.3 Effects of exposure to oil water-soluble content on fry of Carassius auratus gibelio
2.4 仔鱼活动能力
根据观察,试验组仔鱼有上下翻滚、冲撞跳跃、倒立、狂游、侧卧于烧杯底部等现象出现,而对照组仔鱼相对于试验组则活动平稳,较为舒缓。各组孵出仔鱼24、48、72、96、120 h活动能力如图4。结果显示,50%浓度组一开始表现出很强的运动能力,但随着时间的推移,其活动能力逐渐下降;15%、33%浓度组活动能力呈现为先上升后下降的特点,均是在孵出72 h后达到最大活动能力;而对照组仔鱼活动能力随着时间推移而逐渐增强。
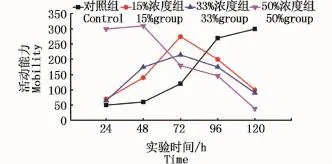
图4 仔鱼活动能力Fig.4 Mobility of larvae
3 讨论
石油对鱼类的毒性效应主要有滞缓发育、抑制孵化、生理功能衰退及畸形与死亡[14]。YASUNORI等[23]发现重油可导致圆斑星鲽(Verasper variegatus)胚胎早期阶段在体节的形成、头部发育、细胞增殖、胚胎分化、神经系统形成等发面出现异常,严重者直接死亡。而王振等[24]通过原油水溶性成分对斜带髭鲷(Hapalogenys nitens)受精卵毒性效应的研究,发现原油水溶性成分对斜带髭鲷受精卵的孵化存在明显的抑制、致畸甚至致死效应,且具有含量依赖性。本研究中,原油水溶物试验组胚胎死亡率与对照组间总体上无显著性差异,而孵出仔鱼的死亡率上表现出差异。可见,原油水溶物对胚胎的死亡并没有显著影响,但会显著降低孵出仔鱼的成活率;原油水溶物对仔鱼的损伤大于对胚胎的损伤。这与陈民山等[14]、王振等[24]的研究结果是一致的,仔鱼对于石油水溶性成分的敏感度高于胚胎,这可能是因为卵膜具有保护作用,出膜之后,仔鱼失去卵膜保护,直接接触石油水溶液,导致仔鱼死亡率随着浓度升高显著升高;石油水溶性成分虽未造成胚胎大量死亡,但是随着其浓度的升高孵出仔鱼发育缺陷、畸形、心率减缓、体色减弱等愈发明显,说明石油水溶性成分虽不会造成胚胎死亡,但会造成胚胎非正常发育,进而影响了孵出仔鱼的健康与存活。但刘在平等[25]以斑马鱼(Brachydanio rerio)为实验材料,用氯苯对其进行胁迫的研究结果表明,氯苯会造成其尾部缺陷、心跳减缓、色素沉着减弱等现象,仔鱼受到胁迫时产生明显的毒性效应,部分器官的功能受到不同程度的影响。所以,孵出仔鱼的不良发育状况起源于两个阶段,即原油水溶性成分对胚胎发育造成损害的阶段以及仔鱼孵出后原油水溶性成分对仔鱼直接损害的阶段。
PAUKA等[26]的研究显示,2%乙醇试验组的斑马鱼仔鱼出膜时间推后,甚至不能出膜;陈文利等[27]的研究中,2.16 mg·L-1二甲基联苯胺处理后,斑马鱼受精卵发育时间较对照组提前24 h。而本研究中原油水溶物造成了仔鱼出膜时间提前。楼允东[28]认为胚体运动并不是导致卵膜破裂唯一或主要因素,胚体在卵膜内的运动,只是促进已被变弱的膜的破裂,使孵出加速;至于卵膜的变弱,孵化酶的活动具有首要的作用。另外,张奇等[29]的实验表明,孵化酶的影响因子主要有温度、溶解氧、光照及其它因子等,提前出膜的仔鱼失去卵膜的保护,容易死亡和畸形,影响生产。总的来看,仔鱼出膜时间提前的主要原因可能是原油水溶性成分改变了孵化酶与卵膜之间原有的作用机理,如提前激发或增强了孵化酶活性,改变了卵膜(孵化酶底物)结构,造成酶促反应提前、加速等。
对于孵出仔鱼的运动能力而言,试验组仔鱼有上下翻滚、冲撞跳跃、倒立、狂游、侧卧于烧杯底部等现象出现,而对照组仔鱼相对于试验组活动平稳、较为舒缓,这与刘在平等[25]、田丽粉等[13]的观察结果一致。另一方面,试验组仔鱼的活动能力总体上呈随着时间推移而逐渐下降趋势,仔鱼的活力在逐渐减弱。其中,15%及33%浓度组仔鱼活力呈现先上升后下降趋势,且起始运动能力与对照组接近;而50%浓度组仔鱼起始运动能力很高,而后呈逐步下降趋势。原因可能是50%浓度过高,对仔鱼形成强刺激,仔鱼表现出强烈的应激反应,而仔鱼短时间内可以承受15%与33%浓度原油水溶物的刺激,尔后才表现出应激反应。对照组仔鱼活力随时间的推移逐渐增加,符合其正常发育状况。自然环境下,仔鱼摄食之前的营养由卵黄提供,开口后逐步由外源营养替代卵黄。本研究表明,原油水溶性成分会对仔鱼形成刺激,造成仔鱼运动异常频繁,仔鱼既缺乏良好的水环境,又不能正常摄食,最终在毒性物质侵害下,畸形发育,营养不良,衰竭而死。
综上所述,原油水溶性成分对方正银鲫受精卵的影响主要是致其不正常发育,进而造成孵化异常。主要表现为出膜时间提前,孵出仔鱼心率减缓、体色着色减弱、发育缺陷及畸形。同时,孵出仔鱼受到原油水溶性成分刺激,产生应激反应,活动异常,正常的生命活动受到影响,最终死亡。原油水溶性成分浓度越大,对水生动物的危害越大。
[1]KATSUMITI A,DOMINGOS F,AZEVEDO M,et al.An assessment of acute biomarker responses in the demersal catfish Cathorops spixii after the Vicuña Oil Spill in a harbour estuarine area in Southern Brazil[J].Environmental Monitoring and Assessment,2009,152(1):209-222.
[3]THEODORAKIS C,BICKHAM J,DONNELLY K,et al.DNA damage in cichlids from an oil production facility in Guatemala[J].Ecotoxicology,2012,21(2):496-511.
[4]BARBOUR E,SHAIB H,YAGHI R,et al.Regression of the level of different heavy metals to size of marine organisms harvested from the“Jiyeh”oil spill zone of the Eastern Mediterranean Sea[J].Bulletin of Environmental Contamination and Toxicology,2009,83(2):219-222.
[5]BIRPINAR M,TALU G,GÖNENÇGIL B,et al.Environmental effects of maritime traffic on thestanbul Strait[J].Environmental Monitoring and Assessment,2009,152(1):13-23.
[6]蒋闰兰,肖佰财,禹 娜,等.多环芳烃对水生动物毒性效应的研究进展[J].海洋渔业,2014,36(4):372-384.
JIANG R L,XIAO B C,YU N,et al.Research advance in toxic effects of PAHs on aquatic animals[J].Marine Fisheries,2014,36(4):372-384.
[7]KOCHHANN D,AZEVEDO BRUST S,DOMMINGOS F,et al.Linking hematological,biochemical,genotoxic,and behavioral responses to crude oil in the Amazon fish Colossoma Macropomum(Cuvier,1816)[J].Archives of Environmental Contamination and Toxicology,2013,65(2):266-275.
[8]AMAT A,BURGEOT T,CASTEGNARO M,et al.DNA adducts in fish following an oil spill exposure[J].Environmental Chemistry Letters,2006,4(2):93-99.
[9]BILLIARD S,TIMME-LARAGY A,WASSENBERG D,et al.The role of the aryl hydrocarbon receptor pathway in mediating synergistic development toxicity of polycyclic aromatic hydrocarbon to zebrafish[J].Toxicological Sciences,2006,92(2):526-536.
[10]SIMONATO J,GUEDES C,MARTINEZ C.Biochemical,physiological and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil[J].Ecotoxicology and Environmental Safety,2008,69(2):112-120.
[11]SILVA C,OLIVEIRA RIBEIRO C,KATSUMITIA,et al.Evaluation of waterborne exposure to oil spill 5 years after an accident in Southern Brazil[J].Ecotoxicology and Environmental Safety.2009,72(2):400-409.
[12]黄辨非,王晓娟,罗静波,等.3种重金属药物对金鱼胚胎—仔鱼的毒性试验[J].水利渔业,2006,26(1):92-94.
HUANG B F,WANG X J,LUO J B,et al.The toxicity test of 3 kinds of heavy metals on the embryos and larvae of goldfish[J].Reservoir Fisheries,2006,26(1):92-94.
[13]田丽粉,任 仲,崔 毅,等.胜利原油对褐牙鲆仔稚鱼的急性毒性和幼鱼碱性磷酸酶的影响[J].海洋水产研究,2008,29(6):95-100.
TIAN L F,REN Z,CUIY,et al.Acute toxicity of Shengli crude oil and its impact on AKP activity of Paralichthys olivaceus[J].Marine Fisheries Research,2008,29(6):95-100.
[14]陈民山,范贵旗.胜利原油对海洋鱼类胚胎及仔鱼的毒性效应[J].海洋环境科学,1991,10(2):1-5.
CHEN M S,FAN G Q.The toxic effect of Shengli crude oil on embryos and larvae of marine fish[J].Marine Environmental Science,1991,10(2):1-5.
[15]曹顶臣,贾智英,鲁翠云,等.同源与异源精子对方正银鲫子代存活、生长及性别的影响[J].水产学杂志,2012,25(3):11-14.
CAO D C,JIA Z Y,LU C Y,et al.Effects of spermatozoa of different species on survival and growth of offsprings in fangzheng silver crucian carp(Carassius auratus gibelio)[J].Chinese Journal of Fisheries,2012,25(3):11-14.
[16]陈家林,韩 冬,朱晓鸣,等.不同脂肪源对异育银鲫的生长、体组成和肌肉脂肪酸的影响[J].水生生物学报,2011,35(6):988-997.
CHEN JL,HAN D,ZHU X M,et al.Dietary lipid sources for gibel carpcarassius auratus gibelio:growth performance,tissue composition and muscle fatty acid profiles[J].Acta Hydrobiologica Sinica,2011,35(6):988-997.
[17]张媛媛,刘 波,戈贤平,等.不同脂肪源对异育银鲫生长性能、机体成分、血清生化指标、体组织脂肪酸组成及脂质代谢的影响[J].水产学报,2012,36(7):1111-1118.
ZHANG Y Y,LIU B,GE X P,et al.Effect of dietary oil sources on growth performance,body composition,the serum biochemical indices,fatty acids composition and lipid metabolism ofCarassius auratus gibelio[J].Journal of Fisheries of China,2012,36(7):1111-1118.
[18]缪凌鸿、刘 波,戈贤平,等.高碳水化合物水平日粮对异育银鲫生长、生理、免疫和肝脏超微结构的影响[J].水产学报,2011,35(2):221-230.
MIAO L H,LIU B,GE X P,et al.Effcet of high carbohydrate levels in the dietary on growth performance,immunity and transmission electron microscopy(TEM)on hepatic cell ofallogynogenetic crucian carp(Carassius auratus gibelio)[J].Journal of Fisheries of China,2011,35(2):221-230.
[19]王爱民,吕 富,杨文平,等.饲料脂肪水平对异育银鲫生长性能、体脂沉积、肌肉成分及消化酶活性的影响[J].动物营养学报,2010,22(3):625-633.
WANG A M,LV F,YANG W P,et al.Effects of dietary lipid levels on growth performance,dody fat deposition,muscle composition and activities of digestive enzymes of gibel carp(Carassius auratus gibelio)[J].Chinese Journal of Animal Nutrition, 2010,22(3):625-633.
[20]黄福堂.大庆油田原油的物理化学性质、组成与特征[J].大庆石油学院学报,1983(2):54-66.
HUANG F T.Physical,chemical properties,composition and characteristics of Daqing crude oil[J].Journal of Daqing Petroleum Institute,1983(2):54-66.
[21]ANDERSON J,NEFF J,COX B,et al.Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish[J].Marine Biology Research,1974,27(1):75-88.
[22]AKAISHIF,SILVA A,JAKOBIS,et al.Morphological and neurotoxicological findings in tropical freshwater fish(Astyanaxsp.)after waterborne and acute exposure to water soluble fraction(WSF)of crude oil[J].Archives of Environmental Contamination and Toxicology,2004,46(2):244-253.
[23]YASUNORI M,SHIN-ICHIK,KEIN,et al.Effects of heavy oil in the developing spotted halibut,Verasper variegates[J].Marine Pollution Bulletin,2008,57(6):524-528.
[24]王 振,郑森林,刘文华,等.原油水溶性成分对斜带髭鲷受精卵及仔鱼的急性毒性效应[J].台湾海峡,2010,29(3):367-372.
WANG Z,ZHENG SL,LIUW H,et al.Acute toxic effects of the water accommodated fraction of crude oil on hapalogenys nitens zygotes and larvae.[J].Journal of Oceanography in Taiwan strait,2010,29(3):367-372.
[25]刘在平,张松林,杨敬辉,等.氯苯对斑马鱼胚胎发育和仔鱼的毒性效应研究[J].环境科学与技术,2012,35(7):25-28.
LIU Z P,ZHANG S L,YANG J H,et al.Toxic effects of chlorobenzene on embryonic development and larva of zebrafish[J].Environmental Science&Technology,2012,35(7):25-28.
[26]PAUKA L,MACENO M,ROSSI S,et al.Embryotoxicity and biotransformation responses in zebrafish exposed to water-soluble fraction of crude oil[J].Bulletin of Environmental Contamination and Toxicology,2011,86(4):389-393.
[27]陈文利,赵 梅,杨丽莉,等.二甲基联苯胺对斑马鱼胚胎发育及成活率的影响[J].河北医药,2011,33(17):2610-2612
CHENW L,ZHAOM,YANG L L,et al.The effect of dimethyl benzidine on embryonic development and the survival rate of zebrafish[J].Hebei MedicalJournal,2011,33(17):2610-2612
[28]楼允东.鱼类的孵化酶[J].动物学杂志,1965,7(3):97-101.
LOU Y D.Hatching enzyme of fish[J].Chinese Journal of Zoology,1965,7(3):97-101.
[29]张 奇,赵红雪,吴旭东,等.鱼类孵化酶及鱼类人工孵化提前脱膜问题探讨[J].内陆水产,2005,30(5):19-20.
ZHANG Q,ZHAO H X,WU X D,et al.Exploration of hatching enzyme and why artificial hatched eggs out of the membrane earlier[J].Inland Fisheries,2005,30(5):19-20.
Effects of water-soluble content of Daqing crude oil on Carassius auratus gibelio embryo and larvae
YE Jian-xiong1,2,ZHAN Pei-rong1,HUANG Xiao-li1,LIU Wei1,WANG Chen1,2
(1.Heilongjiang River Fisheries Research Institute,Chinese Academy of Fishery Science,Heilongjiang150001,China;2.College of Fisheries and Life,Shanghai Ocean University,Shanghai201306,China)
As an important complex raw material for chemical industry,oil is mainly composed by alkane,arene,phenol,heterocyclic compound,nitrogen,sulphur and heavy metals,which will considerably go into the environment and cause pollution in oil exploration,transportation and refining processes.Oil pollution events can cause serious influences on the local ecological system,especially for water environment and aquatic organisms.To assess the effects of water soluble content of crude oil on the aquatic environment and fish,Carassius auratus gibeliowas chosen as a test animal,and the embryo and larvae damage after exposure to water soluble content of crude oil was studied.The conclusion can provide scientific basis for the study of fish culture environmental toxicology and the monitoring of spilled oil pollution.The oil used was from Daqing oil field.The crude oil contained 13%-23%of aromatic hydrocarbons,55%-72%of saturated hydrocarbons,10%-30%of non-hydrocarbons and 0.5%-2%of asphaltene.The water-soluble content of Daqing crude oil was prepared by 1 part of oil plus 9 parts of water in a Pyrex bottle and stirred by ultrasound wave for 4 h at(20.0±2.0)℃.The bottle was capped by plastic foil,and then covered with black plastic to avoid the interference of light.After mixing,the water-soluble content of crude oil was separated,which was designated as the 100%soluble content.The gained 100%soluble content was as the stock solution,and different concentrations(15%,33%,and 50%)of soluble content were prepared.Carassius auratus gibelio used was from Heilongjiang River Fisheries Research Institute.Healthy and motivatedCarassius auratus gibeliowere selected.The average weight of the female was(105.14±10.59)g,and the average weight of the male was(92.64±6.32)g.Drugs(Luteinizing hormone releasing hormone A2 and domperidone)were injected into the fishes to get eggs and sperms.After removing the impurities in eggs and sperm,the artificial insemination process was performed.The eggs fertilized after 2 h and full swell were utilized for tests.The group without any petroleum was used as the control group.The experimental temperature range was from 25 to 26℃and the pH of solution was kept around 7.The dissolved oxygen range was from 6 to 8 mg·L-1.The water-soluble content of different concentrations(0.15%,33%and 50%)was replaced every 24 h,there was no feed through the experiment.The main items were egg death number(recorded every 6 hours),number of hatched larvae(recorded every hour),pigmentation,tail deformities,eye defects,physical abnormality,heart rate variation and athletic ability.Results showed that the effect of water-soluble content of crude oil on larvae was more significant thanembryo.It did not cause the death of embryos directly,and embryos cultured in different concentrations of oil water-soluble content showed no significant difference in death rates.The number of dead embryos in different concentrations of oil water-soluble content(0%,15%,33%and 50%)was33.2±1.4,43.8±3.5,37.6 ±1.6,38.1±6.1 respectively,whereas the number of dead larvae was 2.0±1.0,22.5±2.1,50.4± 4.4,96.3±3.8 respectively.The incubation time of experimental groups was shorter than the control group.Pigmentation was inversely related to the concentrations of oil water soluble content.At 15%concentration,approximately 57%of embryos showed weak pigmentation.However,at 33%and 50%concentrations,the percentages of embryos showing weak pigmentation were 77%and 93%,respectively.Tail deformities were positively related to concentration of oil water soluble content.At15%concentration,approximately 7.5%of embryos showed tail deformities.At 33%and 50%concentrations,it increased to 16.4%and 25.4%,respectively.At high concentration(50%),the embryos showed significant physical abnormality and low heart rates,but no eye defects.The mobility of larvae was also affected by the oil water-soluble content.The mobility of larvae was steadily worse with time passing.At 50%concentration,larvae showed strong activity at the first 48 hours,and then the mobility weakened.However,at 15%and 33%concentrations,the mobility enhanced before 72 h,and gradually weakened subsequently.In the control group,the mobility enhanced in the entire experiment period.Results showed that water-soluble content of crude oil caused damage,malformation and even death on the embryo and larvae ofCarassius auratus gibelio.The oil spill would affect the reproduction and growth of fish and destroy the population structure.Consequently,to study the fish culture environmental toxicology and find methods in pollution control and spilled oil pollution monitoring is significant.
water-soluble constituents of crude oil;Carassius auratus gibelio;embryo;larvae
S 917.4
A
1004-2490(2016)02-0182-08
2015-09-10
农业部应对溢油关键技术专项(2013-2015)
叶剑雄(1989-),男,硕士研究生,研究方向为生态毒理。E-mail:15180603499@163.com
战培荣,男,研究员,zhanpr@163.com;刘伟,女,研究员,liuwei_1020@aliyun.com

