毒死蜱对中国石龙子胚胎发育及孵出幼体表型的影响
徐纯夏, 徐 卫, 陆洪良, 党 伟
(杭州师范大学生命与环境科学学院,浙江 杭州 310036)
毒死蜱对中国石龙子胚胎发育及孵出幼体表型的影响
徐纯夏, 徐卫, 陆洪良, 党伟
(杭州师范大学生命与环境科学学院,浙江 杭州 310036)
摘要:采用中国石龙子作为动物模型,研究有机磷农药毒死蜱对其胚胎发育及幼体生长的影响.结果表明,1.2 mg/g的毒死蜱完全抑制石龙子胚胎的发育,0.12 mg/g处理组卵孵化成功率显著低于对照组.相对于对照组,0.12 mg/g处理组卵孵化期无显著影响,孵出幼体个体显著较小、运动能力较差,但饲养一个月的存活率和日生长速率无显著差异.
关键词:卵孵化;幼体形态;运动表现;幼体发育
随着现代农业的发展,为了追求高产,大剂量的农药被用于农业生产,其中胆碱酯酶抑制剂类的化合物是最为常见的农药有效成分.毒死蜱作为一种广泛使用的胆碱酯酶抑制剂的有机磷脂类农药,自20世纪60年代被广泛使用后,便在人类健康和环境领域得到了广泛的关注.毒死蜱在水产养殖鱼类鲤鱼体内的累积会导致解毒器官肝脏的衰退[1],在家畜猪体内的累积会导致肺等器官的病变,严重的将导致动物死亡[2].这类农药在动物体内的代谢与肝脏细胞色素P450有关[3].在以小鼠为模型的研究中发现,毒死蜱的累积会导致多个器官如肝、肾、脾中胆碱酯酶的活性显著降低[4].同时,毒死蜱在水产养殖动物体内的累积刺激氧化应激的发生,导致体内大量氧自由基和其他活性氧物质的累积,而清除这些活性氧物质的酶类如超氧化物歧化酶、过氧化氢酶及过氧化物酶的表达量则显著降低[5-6].动物体内氧化应激的发生,会对免疫器官造成巨大的伤害,同时抑制免疫系统一些重要酶类的表达,进而影响体液免疫或细胞免疫的进程[7].
胚胎发育期和幼体生长期对成年后的动物发育有着重要的影响,近年来有一些关于农药残留对于动物胚胎及幼体影响的文献报道.对于胎生动物如小鼠而言,母体暴露于毒死蜱污染的环境中对幼体T淋巴细胞的分化有长期的影响[8],同时也会影响胚胎发育过程中一些基因的甲基化,进而影响相关基因的表达[9].对于卵生动物而言,由于胚胎发育过程是脱离母体的,现在的研究多集中在农药污染对于胚胎发育、卵孵化以及幼体特征的影响.在一些鱼类中的研究结果表明胚胎发育过程中基因和蛋白的表达有着显著的变化[10],同时胚胎也会产生氧化应激,但乙酰胆碱酯酶的活性并未受到影响,其他标记分子的表达量却发生改变[11-12].乙酰胆碱酯酶抑制剂类农药如氯氰菊酯、溴氰菊酯、丙线磷和氯代芳烃类农药污染也可以导致卵生动物胚胎发育畸形,孵化率下降以及幼体存活率的降低[13-16].
中国石龙子(Plestiodonduinensis)广泛分布于中国华东、华南及沿海地区,是低地田野草丛或灌木丛中常见的蜥蜴种类.本文以中国石龙子卵为实验对象,在其孵化基质中加入不同浓度的有机磷农药毒死蜱,以检测毒死蜱对石龙子卵孵化成功率、孵出幼体表型的影响,评估有机磷农药对卵生爬行动物胚胎发育的毒害效应.
1材料与方法
1.1 卵收集与孵化
2013年4月、5月上旬在浙江丽水采集实验用的中国石龙子成体,部分捕获个体运至杭州师范大学两栖爬行动物实验室.石龙子经测量、称重、性别鉴定后,关养在户外水泥围栏内.围栏底部铺有厚约15 cm的沙土,表面覆上草皮以模拟野外生境条件,提供足量黄粉虫幼虫和饮水.5月下旬将怀卵母体移入产卵缸中,6月初石龙子开始产卵,产卵期间每天至少检查产卵缸2次,确保新生卵能在产后短时间内被收集以免因其从环境中吸收水分而导致重量发生变化,新生卵及时称重、测量长径和短径.产后雌体经称重和测量后放回水泥饲养池.至6月中旬有10条母体产卵,窝卵数9~20枚,每窝选取5~6枚(共计52枚)用于本实验.剩余新生卵在其它处理下孵化,另文报道.
将购买的20%毒死蜱乳油兑水稀释至0.12和1.20 mg/g,蒸馏水为对照.以蛭石为孵化基质,农药溶液或蒸馏水与干蛭石按1∶1混合成潮湿基质,分配至3个大小一致的塑料盒中.来自同窝的新生卵随机分配到3个不同处理.卵半埋于潮湿基质中,胚胎面朝上.孵化盒用穿孔的塑料薄膜覆盖,放置于温度预先设置为(28.0±1.0) ℃的人工气候箱(宁波莱福科技有限公司)内,每3日补充水分以保持基质湿度恒定.
1.2 幼体形态、功能表现和生长
待幼体开始出壳,每天检查孵化盒至少两次,收集新生幼体,随即测定其体重、体长和尾长.孵化期为卵产出时间到幼体破壳时间的间隔.1.20 mg/g处理下无幼体成功孵出,但多数胚胎已发育完整,其体重、体长、尾长数据以剖出卵内死胎后测量获得.孵出幼体运动表现在温度为(28.0±1.0) ℃的恒温室内进行.实验开始前,预先将动物置于恒温室内适应2 h.测定时将石龙子放入2 m长的木质跑道一端,用毛刷驱赶使之奔跑,用SONY DCR-SR220E数码摄像机记录动物的运动过程;每只石龙子测定一个来回.磁带中的数据用MGI Video Wave III软件读出.动物若在3 min内拒绝跑动,对应数据将不用于统计分析.
运动表现测定后,幼体剪指编号饲养于铺设约15 cm沙土的整理箱内,并覆盖草皮模拟野外生境.整理箱放置在(28.0±1.0) ℃的恒温室中,一端悬挂1只60 W灯泡(光周期为早上07:00自动开启,晚上19:00关闭)使动物在箱内能自行调节体温,每天投喂足量小的黄粉虫幼虫和蟋蟀.饲养约1个月后测定动物体重、记录幼体死亡情况.幼体生长速率以每日增重量表示.
1.3 统计分析
用Statistica 6.0软件包进行统计分析.对数据进行参数统计分析前,分别检验其正态性(Kolmogorov-Smirnov检验)和方差同质性(Bartlett检验).用单因子方差分析检测毒死蜱浓度对卵孵化期、幼体表型特征(形态、运动表现、生长速率)的影响;Tukey多重比较检验多样本处理间的差异.文中涉及的非参数统计为G检验.描述性统计值用平均值±标准误表示,显著性水平设置为α=0.05.
2结果
不同处理组的入孵卵重无显著差异(F2,48=1.91,P=0.159).1.20 mg/g毒死蜱处理下,入孵卵全部无法成功孵出(表1);0.12 mg/g处理组卵孵化成功率略低于对照组(G=54.53,df=2,P<0.001).线性回归分析显示孵化期与入孵卵重无显著相关性(F1,31=3.25,P=0.081).不同浓度处理下孵出幼体的孵化时间无显著差异(F1,31=0.09,P=0.761,表1).
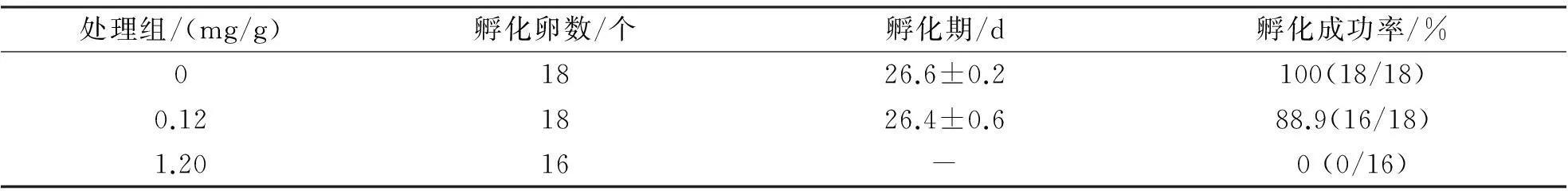
表1 不同毒死蜱浓度对中国石龙子卵孵化期及孵化成功率的影响
毒死蜱显著影响孵出幼体大小(体重:F2,45=47.11,P<0.000 1;体长:F2,45=53.99,P<0.001;尾长:F2,45=120.12,P<0.001).毒死蜱处理使卵内胚胎发育至后期死亡或孵出较小幼体(图1).孵出幼体中,0.12 mg/g处理组幼体的运动表现差于对照组幼体(平均速度:F1,26=15.57,P<0.001;疾跑速:F1,26=38.08,P<0.001).幼体饲养一个月后对照组和0.12 mg/g处理组存活率分别为66.7%(12/18)和56.3%(9/16),但统计上无显著差异(G=0.39,df=1,P=0.533);存活幼体的日生长速率无显著差异(F1,17=0.21,P=0.656,图2).
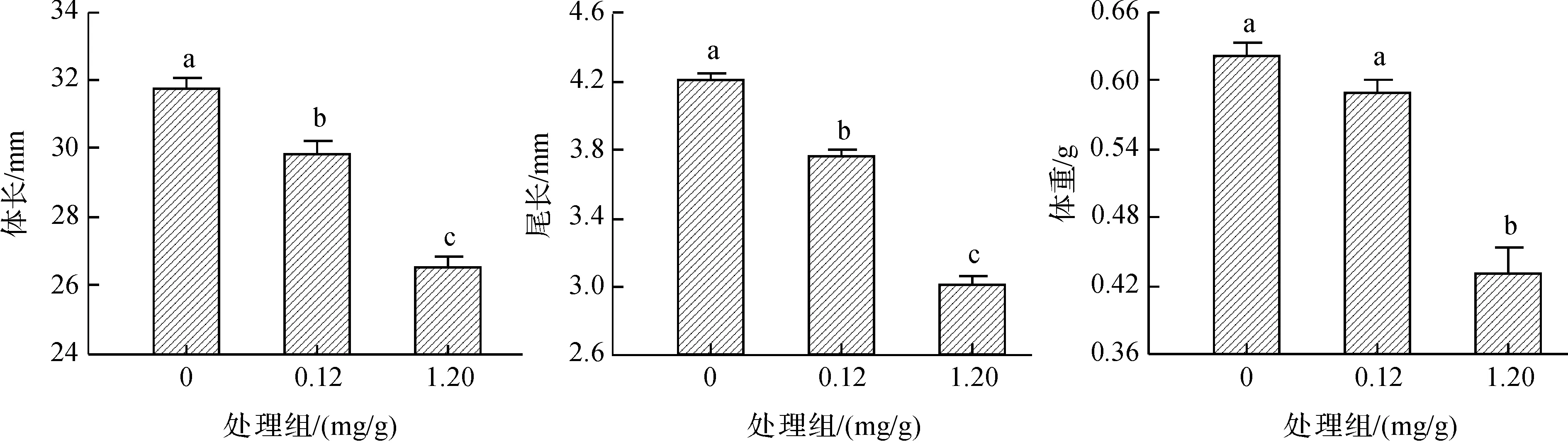
图1 不同毒死蜱浓度对中国石龙子孵出幼体大小的影响Fig. 1 Effects of Chlorpyrifos on body size of Plestiodon chinensis hatchlings
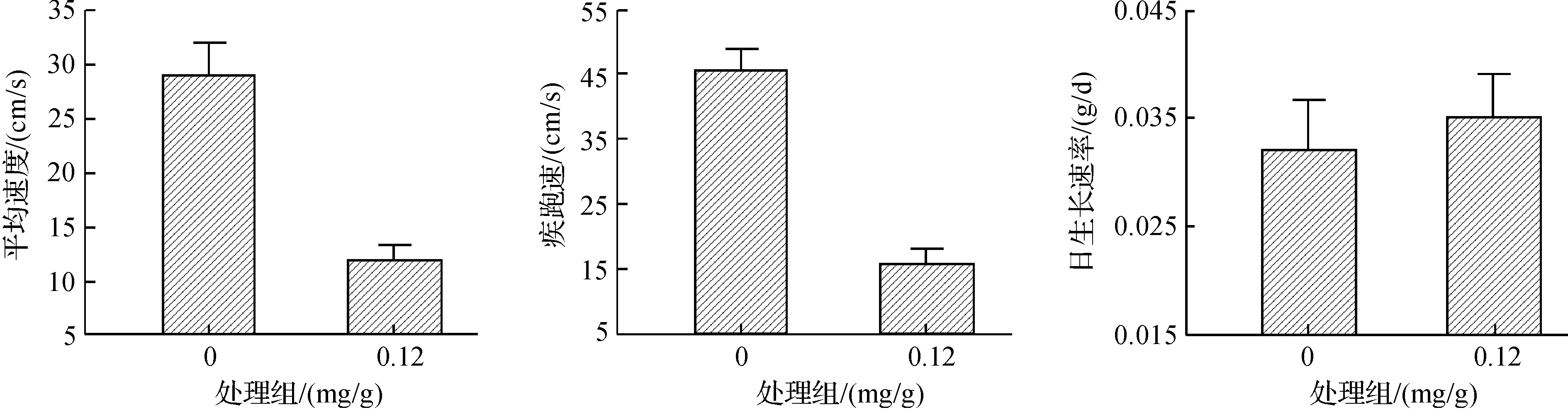
图2 不同毒死蜱浓度对中国石龙子孵出幼体运动表现和生长的影响Fig. 2 Effects of Chlorpyrifos on locomotor performance and early growth rate of Plestiodon chinensis hatchlings
3讨论
动物胚胎通常对许多农药反应敏感,包括杀虫剂、除草剂在内的许多农药会显著影响卵生动物的孵化.此类研究主要集中于鱼类及两栖类动物,其胚胎暴露于受农药污染的水体中,会使胚胎致畸致死[13-16].农药对卵生爬行动物胚胎毒害效应的研究仅见于少数几个物种[17].高剂量的草甘膦导致红耳龟(Trachemysscriptaelegans)孵化成功率下降,胚胎畸形率增加[18].与这些研究结果相似,高浓度的毒死蜱导致中国石龙子卵孵化成功率显著降低.事实上,不同农药对卵生爬行动物孵化成功率的影响并不总展现明显的致死效应.在一定剂量范围内,二氯二苯三氯乙烷(DDE)并不影响绿海龟(Cheloniamydas)和红耳龟卵孵化成功率[19-20];百菌清、异丙甲草胺等农药暴露也不导致北美拟鳄龟(Chelydraserpentina)卵孵化率的显著下降[21];毒杀芬不影响美洲短吻鳄(Alligatormississippiensis)卵孵化率[22].除不同农药品种毒性、动物胚胎大小差异导致卵生动物胚胎农药致死效应存在差异外,农药成分从环境转移至卵内累积的效率可能也是一个重要方面.巢址周边基质和农药的理化性质、卵特征(如蛋壳结构与通透性)的差异会导致这种迁移效率上的差别,从而显示出胚胎致死效应的种间差异[23-24].例如,存在于巢址基质中的有机氯农药及重金属容易通过北美牛蛇(Pituophismelanoleucus)、东方强棱蜥(Sceloporusundulatus)等柔性卵而在卵内累积[25-26],但一些污染物仅少量会通过红耳龟等厚壳或硬壳卵.中国石龙子的卵属于壳薄的柔性卵,有机磷农药成分容易透过卵壳而在卵内累积;新生卵内胚胎相对较小而脆弱,对有机磷农药的反应也极为敏感.事实上,本研究设置的毒死蜱浓度远高于土壤残留浓度(7×106~3.5×105mg/g)[27]而接近于0.4~1.3 mg/g的田间实际喷洒浓度,较高浓度的毒死蜱对敏感的石龙子胚胎产生明显的致死效应是可预见的.
虽然低剂量的农药并不一定直接造成卵生动物的胚胎死亡,但可能会对其孵出幼体具有深远影响.0.12 mg/g处理组的石龙子卵孵化成功率并不明显低于对照组,但其孵出幼体明显较小、运动表现和早期生长情况较差.不同农药显著影响卵生动物幼体表型的结果已被频繁地报道[28-30].卵生爬行动物胚胎农药毒性试验的结果通常是一致的.例如,毒杀芬和硫丹暴露分别使美洲短吻鳄和宽吻凯门鳄(Caimanlatirostris)孵出幼体减小[22,31];高浓度的甲萘威会显著降低游蛇的游泳能力[32].幼体大小、运动表现和生长速率与其适合度相关联,因此可以推测环境中的农药残留可能会通过影响孵出幼体表型而影响其生存.
大多数有机农药属于内分泌干扰物,在动物胚胎或体内积累引起机体内分泌失衡而造成活动异常.值得注意的是内分泌干扰物以模拟类固醇化合物来干扰类固醇的合成与活性而阻断正常的性别决定途径,使胚胎发育过程中性器官形成发生逆转[22,32].一些内分泌干扰物会导致红耳龟、佛罗里达红腹龟(Chrysemysnelsoni)、美洲短吻鳄等雄性胚胎雌性化[33-36].毒死蜱在一些动物中表现出类似雌激素作用已有报道[37],但是否会影响爬行动物的性别决定有待于进一步确定.
参考文献:
[1] XING H J, ZHANG Z W, YAO H D, et al. Effects of atrazine and chlorpyrifos on cytochrome P450 in common carp liver[J]. Chemosphere,2014,104:244-250.
[2] LONG G G, SCHEIDT A B, EVERSON R J, et al. Age related susceptibility of newborn pigs to the cutaneous application of chlorpyrifos[J]. Veterinary and Human Toxicology,1986,28(4):297-299.
[3] FOXENBERG R J, MCGARRIGLE B P, KNAAK J B, et al. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos[J]. Drug Metabolism and Disposition: the Biological Fate of Chemicals,2007,35(2):189-193.
[4] AKHTAR N, SRIVASTAVA M K, RAIZADA R B. Assessment of chlorpyrifos toxicity on certain organs in rat, rattus norvegicus[J]. Journal of Environmental Biology,2009,30(6):1047-1053.
[5] SLANINOVA A, SMUTNA M, MODRA H, et al. A review: oxidative stress in fish induced by pesticides[J]. Neuro Endocrinology Letters,2009,30(S1):2-12.
[6] VALAVANIDIS A, VLAHOGIANNI T, DASSENAKIS M, et al. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants[J]. Ecotoxicology and Environmental Safety,2006,64(2):178-189.
[7] GALLOWAY T, HANDY R. Immunotoxicity of organophosphorous pesticides[J]. Ecotoxicology,2003,12(1):345-363.
[8] NAKAMURA R, KIMURA Y, MATSUOKA H, et al. Effects of transplacental and trans-breast milk exposure to the organophosphate compound chlorpyrifos on the developing immune system of mice[J]. Bulletin of National Institute of Health Sciences,2011(129):105-110.
[9] SHIN H S, SEO J H, JEONG S H, et al. Exposure of pregnant mice to chlorpyrifos-methyl alters embryonic h19 gene methylation patterns[J]. Environmental Toxicology,2014,29(8):926-935.
[10] LIU L L, XU Y X, XU L L, et al. Analysis of differentially expressed proteins in zebrafish (Daniorerio) embryos exposed to chlorpyrifos[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2015,167:183-189.
[11] RIVADENEIRA P R, AGRELO M, OTERO S, et al. Different effects of subchronic exposure to low concentrations of the organophosphate insecticide chlorpyrifos in a freshwater gastropod[J]. Ecotoxicology and Environmental Safety,2013,90:82-88.
[12] SOTOMAYOR V, CHIRIOTTO T S, PECHEN A M, et al. Biochemical biomarkers of sublethal effects inRhinellaarenarumlate gastrula exposed to the organophosphate chlorpyrifos[J]. Pesticide Biochemistry and Physiology,2015,119:48-53.
[13] GREULICH K, PFLUGMACHER S. Differences in susceptibility of various life stages of amphibians to pesticide exposure[J]. Aquatic Toxicology,2003,65(3):329-336.
[14] KÖPRÜCÜ K, AYDIN R. The toxic effects of pyrethroid deltamethrin on the common carp (CyprinuscarpioL.) embryos and larvae[J]. Pesticide Biochemistry and Physiology,2004,80(1):47-53.
[15] 侯立静,杨晓梅,马跃,等.丙线磷对中华大蟾蜍早期胚胎发育的影响[J].农业环境科学学报,2005,24(4):682-685.
[16] BISHOP C A, NG P, PETTIT K E, et al. Environmental contamination and developmental abnormalities in eggs and hatchlings of the common snapping turtle (Chelydraserpentinaserpentina) from the Great Lakes-St Lawrence River basin (1989-1991)[J]. Environmental Pollution,1998,101(1):143-156.
[17] HOPKINS W A. Reptile toxicology: challenges and opportunities on the last frontier in vertebrate ecotoxicology[J]. Environmental Toxicology and Chemistry,2000,19(10):2391-2393.
[18] SPARLING D W, MATSON C, BICKHAM J, et al. Toxicity of glyphosate as Glypro®and LI700 to red-eared slider (Trachemysscriptaelegans) embryos and early hatchlings[J]. Environmental Toxicology and Chemistry,2006,25(10):2768-2774.
[19] PODREKA S, GEORGES A, MAHER B, et al. The environmental contaminant DDE fails to influence the outcome of sexual differentiation in the marine turtle Chelonia mydas[J]. Environmental Health Perspectives,1998,106(4):185-188.
[20] WILLINGHAM E. Embryonic exposure to low-dose pesticides: effects on growth rate in the hatchling red-eared slider turtle[J]. Journal of Toxicology and Environmental Health Part A:Current Issues,2001,64(3):257-272.
[21] DE SOLLA S R, PALONEN K E, MARTIN P A. Toxicity of pesticides associated with potato production, including soil fumigants, to snapping turtle eggs (Chelydraserpentina)[J]. Environmental Toxicology and Chemistry,2014,33(1):102-106.
[22] MILNESA M R, ALLEN D, BRYAN T A, et al. Developmental effects of embryonic exposure to toxaphene in the American alligator (Alligatormississippiensis)[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology,2004,138(1):81-87.
[23] MCDIARMID R W, MITCHELL J C. Diversity and distribution of amphibians and reptiles[M]//SPARLING D W, LINDER G, BISHOP C A. Ecotoxicology of Amphibians and Reptiles. Pensacola:FL,2000:15-69.
[24] SMITH P N, COBB G P, GODARD-CODDING C, et al. Contaminant exposure in terrestrial vertebrates[J]. Environmental Pollution,2007,150(1):41-64.
[26] BRASFIELD S M, BRADHAM K, WELLS J B, et al. Development of a terrestrial vertebrate model for assessing bioavailability of cadmium in the fence lizard (Sceloporusundulatus) and in ovo effects on hatchling size and thyroid function[J]. Chemosphere,2004,54(11):1643-1651.
[27] 张斌,尧水红.环境中的农药 中国典型集约化农区土壤、水体和大气农药残留状况调查[R/OL].(2013-06-25)[2015-04-20].http://files.instrument.com.cn/bbs/upfile/files/20130625/2013625143215.pdf.
[28] SCHIRLING M, BOHLEN A, TRIEBSKORN R, et al. An invertebrate embryo test with the apple snail Marisa cornuarietis to assess effects of potential developmental and endocrine disruptors[J]. Chemosphere,2006,64(10):1730-1738.
[29] DE SOLLA S R, MARTIN P A, MIKODA P. Toxicity of pesticide and fertilizer mixtures simulating corn production to eggs of snapping turtles (Chelydraserpentina)[J]. The Science of the Total Environment,2011,409(20):4306-4311.
[30] SANTOS D, MATOS M, COIMBRA A M. Developmental toxicity of endocrine disruptors in early life stages of zebrafish, a genetic and embryogenesis study[J]. Neurotoxicology and Teratology,2014,46:18-25.
[31] BELDOMENICO P M, REY F, PRADO W S, et al.Inovumexposure to pesticides increases the egg weight loss and decreases hatchlings weight ofCaimanlatirostris(Crocodylia: Alligatoridae)[J]. Ecotoxicology and Environmental Safety,2007,68(2):246-251.
[32] HOPKINS W A, WINNE C T. Influence of body size on swimming performance of four species of neonatal natricine snakes acutely exposed to a cholinesterase-inhibiting pesticide[J]. Environmental Toxicology and Chemistry,2006,25(5):1208-1213.
[33] WILLINGHAM E, CREWS D. Sex reversal effects of environmentally relevant pesticide concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination[J]. General and Comparative Endocrinology,1999,113(3):429-435.
[34] BERGERON J M, CREWS D, MCLACHLAN J A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination[J]. Environmental Health Perspectives,1994,102(9):780-781.
[35] GUILLETTE L J Jr, GROSS T S, MASSON G R, et al. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida[J]. Environmental Health Perspectives,1994,102(8):680-688.
[36] GUILLETTE L J Jr, PICKFORD D B, CRAIN D A, et al. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment[J]. General and Comparative Endocrinology,1996,101(1):32-42.
[37] ANDERSEN H R, VINGGAARD A M, RASMUSSEN T H, et al. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro[J]. Toxicology and Applied Pharmacology,2002,179(1):1-12.
Effects of Chlorpyrifos on the Embryonic Development and Hatchling
Phenotype of Chinese skink,Plestiodonchinensis
XU Chunxia, XU Wei, LU Hongliang, DANG Wei
(College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou 310036, China)
Abstract:Taking Chinese skink (Plestiodonchinensis)as the animal model, the effects of organophosphorus pesticide chlorpyrifos on embryonic development and hatchling phenotypes are studied. The results indicate that 1.2 mg/g high concentration of chlorpyrifos seriously inhibits embryonic development, and egg hatching success of 0.12 mg/g treated group is significantly lower than that of the control group. The incubation period of 0.12 mg/g treated group is not different from the control group. However, the hatchlings from 0.12 mg/g treated group are significantly smaller and performed worse than those from the control group. In the first month, hatchling survival rate and growth rate have no significant difference.
Key words:egg incubation; larval morphology; locomotor performance; larval development
文章编号:1674-232X(2016)01-0040-06
中图分类号:Q955
文献标志码:A
doi:10.3969/j.issn.1674-232X.2016.01.008
通信作者:党伟(1984—),女,助理研究员,博士,主要从事爬行动物生态学研究.E-mail:dang.wei@hotmail.com
基金项目:浙江省自然科学基金项目(LQ12C03003).
收稿日期:2015-05-13

