Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts
Chang-qing Jiang, Jun Hu, Jian-ping Xiang, Jia-kai Zhu, Xiao-lin Liu,, Peng Luo Department of Sports Medicine and Rehabilitation, Peking Universtiy Shenzhen Hospital, Shenzhen, Guangdong Province, China Department of Microscopy, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China The Sixth People’s Hospital of Shenzhen City, Shenzhen, Guangdong Province, China
Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts
Chang-qing Jiang1, Jun Hu2, Jian-ping Xiang2, Jia-kai Zhu2, Xiao-lin Liu2,*, Peng Luo3
1 Department of Sports Medicine and Rehabilitation, Peking Universtiy Shenzhen Hospital, Shenzhen, Guangdong Province, China
2 Department of Microscopy, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China
3 The Sixth People’s Hospital of Shenzhen City, Shenzhen, Guangdong Province, China
How to cite this article:Jiang CQ, Hu J, Xiang JP, Zhu JK, Liu XL, Luo P (2016) Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts. Neural Regen Res 11(11):1845-1850.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This work was supported by grants from the National Natural Science Foundation of China, No. 30170962; the Major Subject of Key Technology of Guangzhou City of China, No. 2002Z1-E0031; science and technology projects of Nanshan district, No. 2014028.
Graphical Abstract

Acellular nerve allografts can help preserve normal nerve structure and extracellular matrix composition. These allografts have low immunogenicity and are more readily available than autologous nerves for the repair of long-segment peripheral nerve defects. In this study, we repaired a 40-mm ulnar nerve defect in rhesus monkeys with tissue-engineered peripheral nerve, and compared the outcome with that of autograft. The graft was prepared using a chemical extract from adult rhesus monkeys and seeded with allogeneic Schwann cells. Pathomorphology, electromyogram and immunohistochemistry findings revealed the absence of palmar erosion or ulcers, and that the morphology and elasticity of the hypothenar eminence were normal 5 months postoperatively. There were no significant differences in the mean peak compound muscle action potential, the mean nerve conduction velocity, or the number of neurofilaments between the experimental and control groups. However, outcome was significantly better in the experimental group than in the blank group. These findings suggest that chemically extracted allogeneic nerve seeded with autologous Schwann cells can repair 40-mm ulnar nerve defects in the rhesus monkey. The outcomes are similar to those obtained with autologous nerve graft.
nerve regeneration; peripheral nerve injury; tissue engineering; rhesus monkey; ulnar nerve; chemical extraction; allogenic nerve; autologous nerve; transplantation; Schwann cells; neural regeneration
Introduction
Traumatic injury to peripheral nerves results in considerable loss of sensory and motor functions, lowering the quality of life. Several research teams have sought to improve regeneration of injured nerves using microsurgery. However, despite the advancement in microsurgical techniques, complete recovery is rarely achieved (Schmidt and Leach, 2003). Currently, nerve autograft is the gold standard for treating peripheral never defects, but obstacles, including limited availability, dysfunction at the donor site, and difficulty in matching diameter and structure, have limited the use of this method.
A successful nerve graft requires the following: (1) the graft should maintain wall thickness and structural integrity to prevent collapse; (2) suitable wall permeability to permit the diffusion, transport and/or controlled release of growth factors through the wall; (3) the tubes should provide an appropriate endoluminal structure so as to prevent neuroma formation at the anastomotic site; (4) the materials should be biodegradable without any cytotoxic reactions and provide a suitable matrix for cell adhesion and migration; and (5) the grafts should be biocompatible and immunologically tolerated by recipients. Acellular nerve allograft derived from natural peripheral nerve retains the natural structure and extracellular matrix components, and elicits a low host immune response. Our previous studies found that acellular nerve graft is a useful way to repair small and large defects in many animal models (Hou et al., 2006; Zhou et al., 2014). However, problems with the use of acellular nerve grafts remain. The use of a bare acellular nerve graft results in a low efficiency of regeneration (Walsh et al., 2009; Yu et al., 2009).
Schwann cells (SCs) are the major glial cell type in the peripheral nervous system. A central function of SCs is the myelination of nerves. After peripheral nerve injury, SCs become activated and migrate to the injured area (Oudega and Xu, 2006; Lopez-Leal and Court, 2016). Upon reaching the injured site, SCs proliferate and support axonal regeneration and elongation (Ide, 1996). The remyelination of demyelinated injured nerves is achieved by the differentiation and de-differentiation processes in which the SCs switch between non-myelinating and myelinating stages (Jessen and Mirsky, 2005). The role of SCs also includes synthesizing neurotrophic factors (Acheson et al., 1991), cytokines, and adhesion and growth promoting factors necessary for satisfactory nerve regeneration after injury (Mirsky and Jessen, 1999). SCs are being tested for transplantation therapy for the treatment of spinal cord injury, and SC transplantation has been shown to promote nerve regeneration and improve axon elongation in the peripheral nervous system (Snipes and Suter, 1995; Oudega and Xu, 2006; Flaiz et al., 2009). Wang et al. (2016) used SC transplantation and enhanced the efficacy of acellular nerve allograft in peripheral nerve injury. Lopez-Leal and Court (2016) showed that SCs supply axons with ribosomes, and promote nerve regeneration.
In our study, we generated a long and thick allogenic nerve graft from adult rhesus monkey through chemical extraction. We then repaired a 40-mm rhesus monkey ulnar nerve defect using the tissue-engineered graft seeded with autologous SCs and achieved a good outcome. These allogenic tissue-engineered grafts represent a promising strategy for peripheral nerve repair.
Materials and Methods
Experimental animals
Nineteen adult rhesus monkeys inbred lines were used in this study. Of these, 10 monkeys (5 males and 5 females, 2.5–3.0 years old and weighing 2.1–3.6 kg) were used for preparing acellular basement membrane tubes, and were the source of SCs. Nine monkeys (five males and four females, 2.5–3.0 years old and weighing 2.0–3.7 kg) were subjected to nerve repair. All monkeys were provided by GuangZhou Nine Buddha Primate Research and Development Center of China (license No. SYXK (Yue) 2010-0106). Animal use was approved by the Animal Ethics Committee of Peking University Shenzhen Hospital in China.
Preparation of acellular basement membrane tube from adult rhesus monkey
As performed previously by our laboratory (Wang et al., 2002; Zhu et al., 2004), one 2.5-year-old, 3.5-kg male rhesus monkey was executed after anesthesia with a combination of ketamine (10 mg/kg, intramuscularly) and diazepam (2 mg/kg, intramuscularly). Eighteen segments of nerve, 2.5–3.0 mm in diameter and 45 mm in length, were isolated and transferred to Hank’s solution. Loose connective tissue was removed from the surface of the nerve trunk under a microscope (Olympus BX51; Center Valley, PA, USA) until the smooth epineurium was visible. The nerves were washed five times in Hank’s solution and double extracted with 4% Triton X-100 and 4% sodium deoxycholate (both from Sigma-Aldrich, St. Louis, MO, USA). In total, we obtained 18 acellular allogenic nerve segments, which were kept in phosphate-buffered saline (PBS; Maixin Biotechnology Company, Fuzhou, China) containing 100 U/mL penicillin and 100 μg/mL streptomycin at 0–4°C. The solution was replaced every 2 weeks. Ultrastructure was observed under a scanning electron microscope (Olympus, Tokyo, Japan). A part of these acellular basement membrane tubes in this stage were used as acellular graft. Others were prepared for engineered nerve with SCs.
Culture of adult rhesus monkey SCs and construction of tissue-engineered nerve grafts
Schwann cells were isolated from adult rhesus monkeys (Yi et al., 2004). The common peroneal nerves from nine monkeys were ligatured in either side and excised 7 days later. Connective tissue was removed under a magnifying lens, and the nerve was cut into pieces. The nerve tissues were digested with 0.2% collagenase (Sigma-Aldrich) for 30 minutes and then with 0.25% trypsin (Gibco) for 20 minutes. Afterwards, serum was added to neutralize trypsin. Samples were filtered with a 400-mesh strainer and centrifuged. Cytarabine (1 × 10–5M; Sigma-Aldrich) was added to inhibit the growth of fibroblasts. SCs were purified and subjectedto S-100 immunocytochemistry. The purity of the SCs was evaluated. Immunocytochemistry was conducted as previously described (Chun et al., 1996).
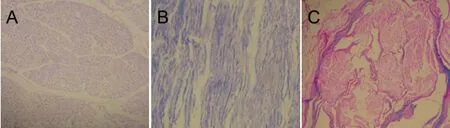
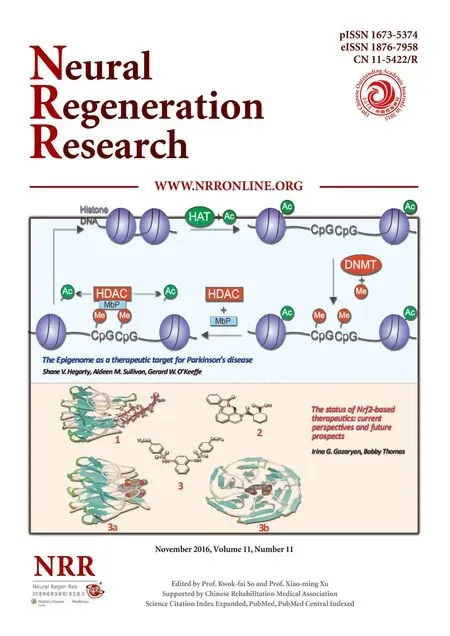
Figure 1 In vitro extraction and culture of Schwann cells and acellular nerve (optical microscope, immunocytochemical staining, × 100).
Figure 2 Empty endoneurial tube of the extracted nerve (scanning electron microscope).

Figure 3 Electrophysiology of the ulnar nerve 5 months after the operation.
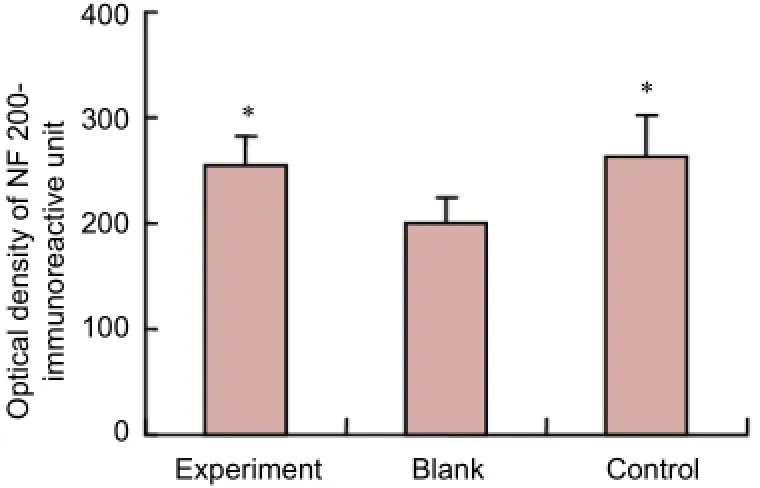
Figure 4 NF 200-immunoreactive units in transverse sections distal to the anastomotic site.
In vivotransplantation of acellular nerve allografts
A total of 18 nerve segments were successfully obtained for transplantation, and randomly divided for use in the following three groups: experimental group, acellular graft and control group. Each group contained six nerve segments. After anesthesia with ketamine (10 mg/kg; Ted Pella, CA, USA), we removed a 40-mm section of ulnar nerve, 1 mm distal to the elbow joint, for reconstruction with the different nerve grafts (bridges).
In the experimental group, allogenic cultured SCs were mixed with Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) to obtain a 1 × 106/mL cell suspension. Under magnification, the cell suspension was injected into the nerve bridge, using a 100 μL microinjector (Jackson, PA, USA) at four points. Expansion of the nerve bridge during the injection was observed under a microscope. Four hours after the injection, the nerve segment was placed into a sixwell culture plate with DMEM supplemented with 10% fetal calf serum, and cultured in a 37°C CO2incubator for 24 hours. In the acellular graft, only the acellular nerve allograft was grafted. In the control group, an autologous nerve was grafted. The nerve graft was attached to the cut ends of the ulnar nerve with 8-0 nylon sutures. All nerve bridges were performed by epineurium sutures. Postoperatively, the rhesus monkeys were fed separately in different cages for 1 month, and together for 4 months. Morphology of the palm was observed and compared between preoperative and postoperative time points. Vascularization on the surface of the nerve grafts was observed at the same time.
Electrophysiological assessment
Five months after the operation, all monkeys were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneally). The Keypoint 3.02 Portable system (Nicolet Instrument Corp, Madison, WI, USA) was used to stimulate the proximal anastomotic region of the ulnar nerve. The recording electrode was placed in the hypothenar eminence muscles to record the amplitude of the compound muscle action potential (CMAP). The stimulating electrode was a hook-shaped silver needle electrode, and was placed on the proximal and distal ends of the graft. Normal CMAP of the hypothenar muscles on the contralateral side was also recorded for comparison. A personal computer was used to set the parameters, including the frequency and amplitude of the stimulation signal, and recordings were performed with a NicoletViking Electrodiagnostic System (Nicolet Instrument Corp.). Digital data were stored on the computer. The nerve conduction velocity (NCV) was calculated. Electrophysiological assessments were performed by an expert who was blinded to group assignment.
Immunohistochemical staining
A 10-mm section of nerve tissue between the nerve graft and the distal ulnar nerve was removed from each group and fixed in 4% paraformaldehyde in 0.1 M PBS for 12 hours at room temperature. After being dehydrated through a graded ethanol series, the specimens were cut into 5-mmlong blocks and embedded in paraffin. The sections were pre-incubated in 3% hydrogen peroxide and 10% normal rabbit serum for 10 minutes to block non-specific binding. Afterwards, sections were incubated with monoclonal anti-neurofilament 200 (NF 200) antibody (diluted at 1:400 in phosphate buffer; Sigma) at room temperature overnight. After washing with PBS, the sections were incubated with donkey anti-rabbit IgG (1:300; Jackson ImmunoResearch, West Grove, PA, USA). The sections were then rinsed three times with PBS and mounted on a gelatin-coated slide, and air-dried. Images of the stained sections were captured with a microscope attached to a CCD spot camera and processed with LEICA IM50 software (DFC350FX/DMIRB; Leica, Wetzlar, Germany). Myelinated axons were quantified according to the unbiased counting criteria.
Scanning electron microscopy
The ultrastructure of the nerve was visualized with a scanning electron microscope. The endoneurium and axons were observed.
Image analysis
Ultrathin sections (70 nm) obtained from the fifth slice at the distal part of the anastomotic region were observed using the IBS2.0 image analysis system. Five fields of every slice were evaluated at 100× magnification. The optical density values of NF 200-immunoreactive units (each unit represents the regeneration of nerve fiber, per unit area = 1 μm2) were recorded to compare the effect of different grafts on nerve regeneration.
Statistical analysis
The data, expressed as the mean ± SD, were analyzed with SPSS 13.0 software (SPSS, Chicago, IL, USA). The differences among the experimental, blank and control groups were evaluated with one-way analysis of variance, followed by least significant difference test.P-values less than 0.05 were considered statistically significant.
Results
Cell culture and nerve grafts
SCs were purified and subjected to S-100 immunocytochemistry, and their purity was evaluated (Figure 1). The purity of the SCs was approximately 92%. Axons were not visible. Only ten empty endoneurial tubes were seen after two extraction procedures (Figure 2). Both primary and first passage cells were viable and suitable for transplantation.
Morphology of muscles and nerve grafts
Five months after surgery, no palmar erosion or ulcers were found in the experimental, blank or control groups, and the hypothenar eminence showed no significant differences in morphology or elasticity postoperatively. There were various degrees of vascularization on the surface of nerve grafts, which had a smooth structure, and no neuroma was observed in the groups that received nerve grafts.
Electrophysiological changes after tissue-engineered nerve grafting for ulnar nerve defect in rhesus monkeys
Five months after surgery, electromyograms of the rhesus monkeys in the experimental, blank and control groups demonstrated no significant differences compared with the preoperative state, and the electromyogram waveform at 1 week after operation was flat without any spike potentials.
We were able to record action potentials at the hypothenar eminence in response to every stimulus on the proximal side of the proximal anastomotic site. There was no significant difference in the maximum amplitude of the latent period between the experimental and control groups (P> 0.05). Compared with the experimental and control groups, the blank group displayed a prolonged latent period and decreased maximum amplitude (P< 0.05;Figure 3).
Electrophysiology of tissue-engineered peripheral nerve allografts
When electrical stimulation was performed on the proximal side of the anastomosis, CMAP was recorded on the hypothenar muscles in the experimental, blank and control groups. There was no significant difference in the CMAP maximum amplitude between the experimental and control groups (P> 0.05), but there were significant differences in the CMAP maximum amplitude between the blank and experimental groups, as well as between the blank and control groups (P< 0.05). There was no significant difference in NCV among the experimental, blank and control groups.
Morphology of tissue-engineered nerve grafts in rhesus monkeys after transplantation
Nerve grafts seeded with cells and autologous nerve grafts had a dark blue appearance (because of the dye), which on transverse sections had a regular circular appearance. All specimens contained light-yellow neurofilaments by immunohistochemical staining. Nerve grafts without cell seeding had a faint light-yellow staining which was distributed irregularly on transverse sections. Nerve grafts with cell seeding and autologous nerve grafts showed an intense light-yellow staining, which was uniform and circular on transverse sections. Nerve fibers were visible beyond the anastomosis on longitudinal sections of the distal anastomotic site in the experimental, blank and control groups. Nerve fibers in thecontrol and experimental groups were distributed in a regular and tightly-arranged manner, in direct contrast to the blank group.
Effects of allogeneic tissue-engineered peripheral nerve grafts on the regeneration of anastomotic nerve fibers in macaque monkeys
No significant difference was found in NF 200 immunoreactivity between the experimental and control groups in transverse sections 0.5 cm distal to the distal anastomotic site (P> 0.05). NF 200 immunoreactivity was significantly less in the blank group than in the experimental and control groups (P< 0.05;Figure 4).
Discussion
Acellular nerve scaffold and tissue-engineered peripheral nerve
Peripheral nerves with a longer than 2 cm defect must be repaired by nerve bridging, and autologous transplantation is the gold standard for nerve grafting (Wiberg and Terenghi, 2003, Pettersson et al., 2011; Tzou et al., 2011). However, autologous transplantation is limited by the lack of donor sites, as well as by dysfunction of the donor nerve (Al-Zer and Kalbouneh, 2015; Li et al.. 2015), which is often a cutaneous nerve with a thin endoneurial tube that affects the regeneration of thick axons. Consequently, tissue-engineered peripheral nerve grafting is currently a hot topic in the field of neural regeneration.
Both natural and artificial materials have been studied for application in tissue-engineered peripheral nerve grafts (Lin et al., 2013; Jiang et al., 2014), and most have been found to have limitations for clinical application. We and others have found that,in vivo, axons can regenerate in the presence of bioactive seed cells, such as SCs (Chun et al., 1996; Gainer et al., 2016; Tuffaha et al., 2016), which function as an endoneurial tube, allowing axons to traverse the nerve bridge (Cunningham et al., 1983; Hirano et al., 1988; Yang et al., 1994). An effective bridging agent must have the following features: (1) allow easy vascularization; (2) can be invaded by large numbers of functional axons, with no fibrosis; (3) can be myelinated like normal nerve fibers; (4) be compatible with the host, with no rejection reaction; (5) easily operated. Allogenic nerves obtained through chemical extraction and used to prepare tissue-engineered peripheral nerve grafts fulfill each of these requirements. It has an endoneurial tube, similar to the allogenic nerve, through which nerve fibers can easily pass through. Its loose structure promotes vascularization. Additionally, it can be made biocompatible with the host by eliminating antigenicity by chemical methods. The extracted endoneurial tube retains the basement membrane, which functions as an extracellular matrix that promotes seed cell adhesion and nerve fiber invasion. Furthermore, these nerve grafts are elastic, flexible and easily operated. Owing to these advantages, we chose this material as the nerve graft scaffold (bridge) in our experiment. We conclude that the nerve graft seeded with SCs provides an outcome similar to that of autologous nerve transplantation. The outcome of the nerve graft without cell implantation was significantly inferior. However, we do not know whether this tissue-engineered nerve graft degrades over timein vivo.
In the present study, we repaired a peripheral nerve defect with the graft, and the SCs culturedin vitrowere similar to the host SCs at the cut ends of the nerve. The graft with cell seeding and the allogenic nerve graft provided similar outcomes, suggesting thatin vitro-cultured SCs indeed promote nerve regeneration.
In previous studies, grafts were neural or non-neural tissues, biological or non-biological, with or without cell implantation, and used to bridge the sciatic nerve with 6–8 mm defects in mice, providing good outcomes 3–6 months postoperatively. The reasons for the high efficacy are unclear. However, small mammals, such as mice, might have a high capacity for regeneration. Additionally, the short gap length might contribute to the good outcome. Therefore, we chose the rhesus monkey for its evolutionary and genetic similarity to humans. We evaluated cell migration and regeneration of the ulnar nerve with a 40-mm defect repaired by grafts, and our results demonstrated the superiority of the tissue-engineered graft.
Prospects for tissue-engineered nerve grafts
For over a century, researchers have studied strategies for repairing the peripheral nerve (Ozkan et al., 2016; Wu et al., 2016), and have made substantial progress (Wang et al., 2015; Flores and Socolovsky, 2016; Oprych et al., 2016). However, nerve regeneration is far from ideal. Currently, implantation of SCs (Aghayan et al., 2012; Cao et al., 2013; Zhou et al., 2015) as the seed cells into an appropriate bridging agent to generate a tissue-engineered peripheral nerve is a favored approach for peripheral nerve repair.
Chemically extracted allogeneic nerve has no antigenicity, and the basement membrane is adhesive to SCs and possesses a three-dimensional structure similar to normal nerve (Arai et al., 2000; Zhong et al., 2003; Luo et al., 2015). Moreover, it is considered the ideal material for nerve defect repair. There are various approaches and agents for chemical extraction. We successfully extracted rhesus monkey peripheral nerve for transplantation. Future studies should evaluate methods for extracting and using the comparatively longer and thicker human nerve.
Author contributions:CQJ and XLL designed this study. CQJ, JH, JPX, JKZ and XLL performed experiments. PL was responsible for experimental data acquisition and data compilation. CQJ, JH, JPX and JKZ analyzed data. CQJ and JH wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA (1991) Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron 7:265-275.
Aghayan HR, Arjmand B, Norouzi-Javidan A, Saberi H, Soleimani M, Tavakoli SA, Khodadadi A, Tirgar N, Mohammadi-Jahani F (2012) Clinical grade cultivation of human Schwann cell, by the using of human autologous serum instead of fetal bovine serum and without growth factors. Cell Tissue Bank 13:281-285.
Al-Zer H, Kalbouneh H (2015) Dental pulp stem cells-derived schwann cells for peripheral nerve injury regeneration. Neural Regen Res 10:1945-1946.
Arai T, Kanje M, Lundborg G, Sondell M, Liu XL, Dahlin LB (2000) Axonal outgrowth in muscle grafts made acellular by chemical extraction. Restor Neurol Neurosci 17:165-174.
Cao J, Cheng X, Zhou Z, Sun H, Zhou F, Zhao J, Liu Y, Cui G (2013) Changes in the Foxj1 expression of Schwann cells after sciatic nerve crush. J Mol Histol 44:391-399.
Chun JT, Gioio AE, Crispino M, Giuditta A, Kaplan BB (1996) Differential compartmentalization of mRNAs in squid giant axon. J Neurochem 67:1806-1812.
Cunningham BA, Hoffman S, Rutishauser U, Hemperly JJ, Edelman GM (1983) Molecular topography of the neural cell adhesion molecule N-CAM: surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci U S A 80:3116-3120.
Flaiz C, Ammoun S, Biebl A, Hanemann CO (2009) Altered adhesive structures and their relation to RhoGTPase activation in merlin-deficient Schwannoma. Brain Pathol 19:27-38.
Flores LP, Socolovsky M (2016) Phrenic nerve transfer for reconstruction of elbow extension in severe brachial plexus injuries. J Reconstr Microsurg.
Gainer H, House S, Kim DS, Chin H, Pant HC (2016) Squid giant axon contains neurofilament protein mRNA but does not synthesize neurofilament proteins. Cell Mol Neurobiol doi:10.1007/a10571-016-0382-z.
Hirano T, Iwasaki K, Suzuki R, Taguchi A, Ide S (1988) Scintigraphical observation to predict fracture healing in intracapsular fracture of the femoral neck. Nihon Seikeigeka Gakkai Zasshi 62:595-600.
Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK (2006) Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience 140:101-110.
Ide C (1996) Peripheral nerve regeneration. Neurosci Res 25:101-121.
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671-682.
Jiang G, Di Bernardo J, Maiden MM, Villa-Diaz LG, Mabrouk OS, Krebsbach PH, O’Shea KS, Kunisaki SM (2014) Human transgene-free amniotic-fluid-derived induced pluripotent stem cells for autologous cell therapy. Stem Cells Dev 23:2613-2625.
Li HF, Wang YR, Huo HP, Wang YX, Tang J (2015) Neuroprotective effects of ultrasound-guided nerve growth factor injections after sciatic nerve injury. Neural Regen Res 10:1846-1855.
Lin MY, Manzano G, Gupta R (2013) Nerve allografts and conduits in peripheral nerve repair. Hand Clin 29:331-348.
Lopez-Leal R, Court FA (2016) Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol 36:429-436.
Luo H, Zhu B, Zhang Y, Jin Y (2015) Tissue-engineered nerve constructs under a microgravity system for peripheral nerve regeneration. Tissue Eng Part A 21:267-276.
Mirsky R, Jessen KR (1999) The neurobiology of Schwann cells. Brain Pathol 9:293-311.
Oprych KM, Whitby RL, Mikhalovsky SV, Tomlins P, Adu J (2016) Repairing peripheral nerves: is there a role for carbon nanotubes? Adv Healthc Mater 5:1253-1271.
Oudega M, Xu XM (2006) Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma 23:453-467.
Ozkan HS, Karatas Silistreli O, Ergur B, Irkoren S (2016) Repairing peripheral nerve defects by vein grafts filled with adipose tissue derived stromal vascular fraction: an experimental study in rats. Ulus Travma Acil Cerrahi Derg 22:7-11.
Pettersson J, McGrath A, Kalbermatten DF, Novikova LN, Wiberg M, Kingham PJ, Novikov LN (2011) Muscle recovery after repair of short and long peripheral nerve gaps using fibrin conduits. Neurosci Lett 500:41-46.
Schmidt CE, Leach JB (2003) Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng 5:293-347.
Snipes GJ, Suter U (1995) Molecular basis of common hereditary motor and sensory neuropathies in humans and in mouse models. Brain Pathol 5:233-247.
Tuffaha SH, Singh P, Budihardjo JD, Means KR, Higgins JP, Shores JT, Salvatori R, Hoke A, Lee WP, Brandacher G (2016) Therapeutic augmentation of the growth hormone axis to improve outcomes following peripheral nerve injury. Exp Opin Ther Targets 1-7.
Tzou CH, Aszmann OC, Frey M (2011) Bridging peripheral nerve defects using a single-fascicle nerve graft. Plast Reconstr Surg 128:861-869.
Walsh S, Biernaskie J, Kemp SW, Midha R (2009) Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience 164:1097-1107.
Wang EW, Zhang J, Huang JH (2015) Repairing peripheral nerve injury using tissue engineering techniques. Neural Regen Res 10:1393-1394.
Wang H, Wu J, Zhang X, Ding L, Zeng Q (2016) Study of synergistic role of allogenic skin-derived precursor differentiated Schwann cells and heregulin-1beta in nerve regeneration with an acellular nerve allograft. Neurochem Int 97:146-153.
Wiberg M, Terenghi G (2003) Will it be possible to produce peripheral nerves? Surg Technol Int 11:303-310.
Wu R, Wang L, Chen F, Huang Y, Shi J, Zhu X, Ding Y, Zhang X (2016) Evaluation of artificial nerve conduit and autografts in peripheral nerve repair in the rat model of sciatic nerve injury. Neurol Res 38:461-466.
Yang P, Major D, Rutishauser U (1994) Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem 269:23039-23044.
Yu H, Peng J, Guo Q, Zhang L, Li Z, Zhao B, Sui X, Wang Y, Xu W, Lu S (2009) Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery 29:330-336.
Zhong HB, Lu SB, Hou SX, Zhao Q (2003) Acellular nerve allograft by chemical extraction in humans. Zhonghua Wai Ke Za Zhi 41:60-63.
Zhou LN, Zhang JW, Liu XL, Zhou LH (2015) Co-graft of bone marrow stromal cells and schwann cells into acellular nerve scaffold for sciatic nerve regeneration in rats. J Oral Maxillofac Surg 73:1651-1660.
Zhou X, He B, Zhu Z, He X, Zheng C, Xu J, Jiang L, Gu L, Zhu J, Zhu Q, Liu X (2014) Etifoxine provides benefits in nerve repair with acellular nerve grafts. Muscle Nerve 50:235-243.
Copyedited by Patel B, Wysong S, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Xiao-lin Liu, M.D., Ph.D., jcq-006@163.com.
orcid: 0000-0002-5231-2749 (Chang-qing Jiang)
10.4103/1673-5374.194757
Accepted: 2016-10-23
- 中国神经再生研究(英文版)的其它文章
- Cortical spreading depression-induced preconditioning in the brain
- Nerve growth factor protects against palmitic acidinduced injury in retinal ganglion cells
- HLA class II alleles and risk for peripheral neuropathy in type 2 diabetes patients
- Rab27a/Slp2-a complex is involved in Schwann cell myelination
- Key genes expressed in different stages of spinal cord ischemia/reperfusion injury
- Methylprednisolone promotes recovery of neurological function after spinal cord injury: association with Wnt/β-catenin signaling pathway activation