肠道微生物对宿主免疫系统的调节及其可能机制
王珊珊 王佳堃刘建新(浙江大学奶业科学研究所,动物分子营养学教育部重点实验室,杭州310058)
肠道微生物对宿主免疫系统的调节及其可能机制
王珊珊 王佳堃∗刘建新
(浙江大学奶业科学研究所,动物分子营养学教育部重点实验室,杭州310058)
摘 要:动物肠道是一个开放的生态系统,栖息着大量的微生物,这些微生物与宿主免疫系统之间协同进化,在维持肠道稳态方面发挥着重要的作用。建立和维持肠道微生物与宿主免疫系统之间有益的相互作用是保障机体健康的关键。为此,本文综述了肠道微生物区系的组成及影响因素、肠道微生物促进宿主免疫系统的发育及调节机体免疫系统的可能机制。
关键词:肠道微生物;宿主;免疫系统发育;免疫平衡
胃肠道是机体与外界接触最为广泛的器官之一。它不仅是消化吸收营养物质的场所,而且是机体阻止肠道细菌移位的一道重要防线。肠道是一个开放的生态系统,其中栖居着大量的微生物。历经长期进化,这些微生物与宿主之间建立了稳定的共生关系,直接影响着宿主的健康。正常生理状态下,肠道微生物可以促进宿主免疫系统的发育,并可通过脂多糖(LPS)、脂蛋白以及代谢产物等特定组分调控宿主的免疫反应,形成一道肠道生物屏障。目前,肠道微生物对宿主免疫系统的影响受到越来越多的关注。为此,本文在介绍肠道微生物区系组成的基础上,就肠道微生物促进宿主免疫系统发育、调节宿主免疫反应进行了探讨。
1 肠道微生物区系的组成及影响因素
动物肠道内微生物区系组成丰富且多样,除细菌外,还包括古细菌、病毒、真菌和原虫。肠道细菌90.0%~99.9%是厌氧菌,它们有的是有益菌,有的是有害菌,也有的是中性菌,构成了肠道的正常菌群。肠道细菌种群多达500种(spe⁃cies),但这些种仅归属于少数几个门[1-2]。其中,人类和小鼠肠道中以厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidetes)为主;鸡肠道中主要以Firmi⁃cutes为主,其次为变形菌门(Proteobacteria)、Bacteroidetes和放线菌门(Actinobacteria)[3];Firm⁃icutes在反刍动物肠道中占主导地位,Bacteroidetes 和Proteobacteria次之[4]。由于消化道不同部位蠕动速度和微环境明显不同,所以其中定植的细菌数量与组成存在着显著差异。从消化道的近端到远端,细菌的数量和多样性逐渐递增[5-6]。
胎儿的肠道是无菌的,出生之后,细菌开始快速定植。在胎儿生长早期,尽管分娩和喂养方式均会影响肠道微生物区系的组成[7-8],但肠杆菌(Enterobacteria)和双歧杆菌(Bifidobacteria)仍然是最早定植的细菌[9];接下来的生长阶段,许多因素都会影响肠道微生物区系的组成。基因型是决定肠道微生物区系组成的首要因素。Ley等[10]研究后发现小鼠后代与母亲的肠道微生物区系组成密切相关。Turnbaugh等[11]研究发现,与非亲缘关系的个体相比,来自同一个家族的个体(双胞胎之间和双胞胎与母亲之间)具有更相似的细菌结构和功能。饲粮和生活环境等也会影响肠道微生物区系组成。Turnbaugh等[12]研究饲喂低脂高糖和高脂高糖饲粮对成年小鼠盲肠内容物中微生物的影响时发现,2种饲粮下其盲肠内容物中细菌多样性不同,饲喂低脂高糖饲粮小鼠的盲肠菌群组成以Firmicutes为主,而饲喂高脂高糖饲粮小鼠的盲肠菌群组成以柔膜菌门(Mollicutes)为主。这一研究结果与Duncan等[13]得出的人类膳食中随碳水化合物含量的减少,肠道细菌中罗氏菌属(Rose⁃buria)所占比例减少的研究结果一致。生活在不同环境下的近交系小鼠,其ASF(altered Schaedler flora)丰度存在显著差异[14]。除了人类和小鼠,以上因素也会对反刍动物和单胃动物的肠道微生物区系组成产生影响。对于分别饲喂代乳料、代乳料加开食料的断奶前犊牛,其肠道细菌的种系组成明显不同[15]。Lunedo等[16]利用荧光定量PCR技术对饲喂不同饲粮的肉鸡肠道微生物进行了分析,发现从7~42日龄,饲喂玉米饲粮的肉鸡回肠黏膜中肠球菌(Enterococcus)数量逐渐减少,而饲喂高粱饲粮的肉鸡却没有发生任何变化。此外,断奶应激会改变仔猪肠道的菌群结构[17]。因此,在动物个体发育的过程中,基因型、饲粮和环境等因素相互作用使得肠道菌群具有多样性和宿主特异性。
2 肠道微生物促进宿主免疫系统的发育
2.1 刺激杯状细胞分泌黏蛋白,保障黏液层完整
肠道黏液层由杯状细胞分泌的黏蛋白组成,覆盖于上皮细胞之上。黏蛋白-2(MUC2)是小鼠和人类肠道主要的糖基化黏蛋白[18]。黏液层不仅具有润滑剂功效,有利于肠道蠕动,而且其网状结构可有效阻止细菌穿过肠黏膜上皮,经淋巴管到达肠系膜淋巴结,再进入其他脏器和血液循环,为宿主提供保护屏障。胃和结肠的黏液层由内、外2层构成,外层为疏松的黏液层,内层为紧质的黏液层[19]。正常生理情况下,仅可在外黏液层检测到细菌[20],而MUC2缺失或黏蛋白糖基化异常时,肠屏障功能受损、通透性增加,可观察到细菌在黏液层过度增殖,其结果可触发全身性反应和多系统器官功能衰竭[21-23]。
黏液层的存在可有效防止细菌移位(bacterial translocation,BT),而肠道微生物的存在则可激发杯状细胞分泌黏蛋白,保障黏液层结构的完整,从而发挥屏障作用。很多无菌动物的肠黏膜均会变薄,例如,在无菌鸡中,肠道黏蛋白的产生和分泌下降导致小肠黏膜发育不成熟[24];无菌小鼠的结肠杯状细胞减少,黏液层变薄[25],而受细菌LPS或肽聚糖(PGN)刺激后,杯状细胞分泌黏蛋白,使黏液层恢复到普通小鼠厚度[26]。
2.2 诱导淋巴组织发育
集合淋巴小结[又称派伊尔淋巴结(Peyer’s patches,PP)]、孤立淋巴滤泡(isolated lymphoid follicles,ILF)和肠系膜淋巴结(mesenteric lymph nodes,MLN)共同组成肠道黏膜相关淋巴组织(gut⁃associated lymphoid tissues,GALT)[27]。PP 和MLN的发育起始于出生前,由形成于胎儿肝脏的淋巴组织诱导细胞(lymphoid tissue inducer,LTi)诱导[28]。而出生后两者的发育则要依赖于肠道微生物的定植[29-30]。肠道菌群是一种重要的抗原,可刺激GALT的发育成熟,而GALT产生的免疫反应又受控于肠道菌群组成及其代谢活性。
细菌刺激GALT发育主要是通过树突状细胞(dendritic cells,DC)识别细菌及其代谢产物而实现[31]。DC对细菌的识别主要有3条途径:1)通过PP肠腔面的M细胞(microfold cell)吞饮,传递给PP内的DC;2)弥散分布于整个肠道上皮黏膜固有层(lamina propria,LP)的DC从肠腔上皮细胞间伸出突触与细菌接触;3)细菌代谢产物直接经细胞旁途径进入肠黏膜固有层,进而与DC反应。这一识别过程中,DC表面的Toll样受体(Toll⁃like receptors,TLR)是微生物成分引发DC活化的桥梁。TLR4可以识别革兰氏阴性菌LPS;TLR2的配体较TLR4的广泛,包括脂蛋白、脂多肽、脂壁酸(LTA)、阿拉伯甘聚糖(LAM)及酵母多糖等;TLR3特异识别病毒复制的中间产物双链RNA(ds⁃RNA);TLR9识别细菌的胞嘧啶-磷酸-鸟嘌呤二核苷酸(CpG⁃DNA)。TLR识别细菌及其代谢产物后,进一步激活髓样分化蛋白88(MYD88)接头蛋白样蛋白(MAL)-MYD88和含Toll/白细胞介素-1受体(TIR)结构域能诱导β型干扰素(IFN⁃β)的接头分子(TRIF)相关接头分子(TRAM)-TRIF信号通路(图1),使DC活化。活化后的DC诱导PP生发中心的T细胞增殖,促使B细胞分泌免疫球蛋白(Ig)A;通过传入淋巴血管迁移至MLN,诱导效应T细胞增殖,使MLN发育成熟;刺激B细胞和募集T细胞,导致隐窝小结发育成为成熟的ILF。Hamada等[32]报道无菌小鼠中的ILF发育不完全,而革兰氏阴性菌的PGN可以诱导无菌小鼠ILF的形成[33]。与此相似,淋巴组织(如PP)以及分泌IgA的浆细胞在无菌仔猪中的发育也不成熟[34],但ASF定植无菌仔猪肠道后,导致大部分ASF菌株形成,从中筛选出Bristol微生物,它们可以提高血清IgA和IgM水平,为进一步研究微生物定植对宿主肠道黏膜和免疫系统的发育提供了一种生物模型[35]。
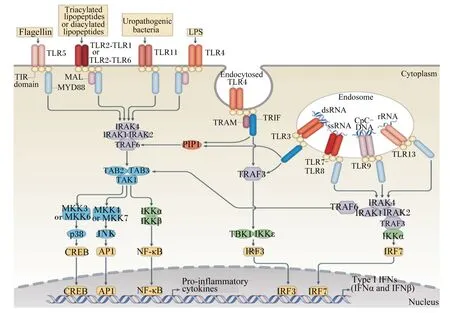
图1 TLR信号通路Fig.1 TLR signaling pathway[36]
3 肠道微生物调节机体免疫系统的可能机制
T细胞是维持肠道免疫稳态的重要执行者。DC通过TLR识别肠道细菌的过程中,不同类型的细菌被不同家族的TLR识别,激活MAL⁃MYD88和TRAM⁃TRIF信号通路,产生不同的细胞因子,进而调节T细胞向不同亚群分化,实现细菌耐受与免疫的平衡。T细胞对肠黏膜免疫屏障的调节主要表现在辅助性T(helper T,Th)细胞和调节性T(regulatory T,Treg)细胞的调节。Th细胞前体细胞在白细胞介素-12(IL⁃12)作用下定向分化为Th1细胞;白细胞介素-4(IL⁃4)作用下定向分化为Th2细胞;白细胞介素-6(IL⁃6)、白细胞介素-23(IL⁃23)和β型转化生长因子(TGF⁃β)作用下定向分化为Th17细胞。Th1和Th2细胞合成淋巴因子的种类不同,两者分别介导迟发型和速发型超敏反应。白细胞介素-17(IL⁃17)是Th17细胞的主要效应因子,能有效介导机体的炎症反应和自身免疫性疾病,如实验性自身免疫性脑炎(EAE)、哮喘、类风湿性关节炎等。研究发现,Th1和Th2细胞分别产生的γ型干扰素(IFN⁃γ)和IL⁃4可共同下调Th17细胞反应[37]。白细胞介素-27(IL⁃27)是一种新的IL⁃12家族细胞因子,可以通过下调IL⁃6和TGF⁃β的表达对Th17细胞的分化起到抑制作用,但Th1细胞释放的TNF可增强Th17细胞的发育(图2)。Th细胞前体细胞在TGF⁃β单独诱导下分化为Treg细胞,分泌TGF⁃β并表达转录因子叉头/翼状螺旋转录因子3(Foxp3),抑制Th17和Th1细胞的促炎反应[38]。
分节丝状菌(segmented filamentous bacteria,SFB)已被证实是Th17细胞强有力的诱导者[39]。SFB定植可以上调SAA的表达,刺激CD11c+DC产生IL⁃6和IL⁃23,促进Th17细胞分化[40]。而有害菌鼠伤寒沙门氏菌(Salmonella typhimunum)能诱导T细胞分化为产生IFN⁃γ的Th1细胞。脆弱型拟杆菌(Bacteroides fragilis)产生的多糖A(pol⁃ysaccharide A,PSA)能刺激Treg细胞分化,分泌白细胞介素-10(IL⁃10),对抗Th17和Th1细胞反应(图3)。此外,梭状芽孢杆菌(Clostridium)Ⅳ簇和ⅪⅤa簇能刺激结肠上皮细胞产生TGF⁃β,诱导T细胞分化为Treg细胞[41],与Bacteroides fragilis具有相似的调控效应。因此,正常情况下,肠道菌群的存在并不诱发机体出现炎症反应。

图2 Th细胞分化与调节Fig.2 Cell differentiation and regulation of helper T cell[37]
除了细菌的直接调节外,肠道细菌产生的代谢产物,如短链脂肪酸(SCFA)等,也可从肠腔转位到肠黏膜固有层中,影响宿主免疫相关基因的表达(表1)。
4 小 结
虽然肠道微生物在宿主免疫系统的成熟过程中扮演着重要角色,并且可通过LPS、脂蛋白以及代谢产物等特定组分调节宿主的免疫反应,影响肠道内环境的稳定,但肠道微生物调控宿主免疫反应的确切机制仍有待进一步研究。其次,肠道内病毒、噬菌体等与宿主免疫系统之间的相互作用也越来越成为研究的热点,也值得深入探究。
正常情况下,动物体内的肠道微生物区系结构相对恒定,而当外环境发生变化时,肠道微生物区系的组成也会随之改变。肠道菌群一旦失调,就会引发宿主免疫系统的功能紊乱,导致炎症性肠道疾病的发生,如急性结肠炎、克罗恩病(Crohn’s disease)等。因此,研究肠道微生物的免疫调控机制,为治疗免疫相关疾病提供了新的思路,对维护动物健康具有重要的意义。

图3 共生细菌驱动的免疫稳态Fig.3 The commensal microbiota drives immune homeostasis[40]

表1 常见的肠道细菌代谢产物及其免疫调节作用Table 1 Common gut bacteria metabolites and their immunoregulatory functions

续表1
参考文献:
[1] The Human Microbiome Project Consortium.Struc⁃ture,function and diversity of the healthyhuman mi⁃crobiome[J].Nature,2012,486(7402):207-214.
[2] QIN J J,LI R Q,RAES J,et al.A human gut microbi⁃al gene catalogue established by metagenomic sequen⁃cing[J].Nature,2010,464(7285):59-65.
[3] CHOI J H,KIM G B,CHA C J.Spatial heterogeneity and stability of bacterial community in the gastrointes⁃tinal tracts of broiler chickens[J].Poultry Science,2014,93(8):1942-1950.
[4] DURSO L M,HARHAY G P,SMITH P L,et al.Ani⁃mal⁃to⁃animal variation in fecal microbial diversity a⁃mong beef cattle[J].Applied and Environmental Mi⁃crobiology,2010,76(14):4858-4862.
[5] DAVE M,HIGGINS P D,MIDDHA S,et al.The hu⁃man gut microbiome:current knowledge,challenges,and future directions[J].Translational Research,2012,160(4):246-257.
[6] SEKIROV I,RUSSELL S L,ANTUNES L C,et al.Gut microbiota in health and disease[J].Physiological Reviews,2010,90(3):859-904.
[7] DOMINGUEZ⁃BELLO M G,COSTELLO E K,CON⁃TRERAS M,et al.Delivery mode shapes the acquisi⁃tion and structure of the initial microbiota across mul⁃tiple body habitats in newborns[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(26):11971-11975.
[8] MOUNTZOURIS K C,MCCARTNEY A L,GIBSON G R.Intestinal microflora of human infants and current trends for its nutritional modulation[J].British Journal of Nutrition,2002,87(5):405-420.
[9] XU J,GORDON J I.Honor thy symbionts[J].Pro⁃ceedings of the National Academy of Sciences of the United States of America,2003,100(18):10452-10459.
[10] LEY R E,BÄCKHED F,TURNBAUGH P,et al.Obe⁃ sity alters gut microbial ecology[J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(31):11070-11075.
[11] TURNBAUGH P J,HAMADY M,YATSUNENKO T,et al.A core gut microbiome in obese and lean twins[J].Nature,2009,457(7228):480-484.
[12] TURNBAUGH P J,BÄCKHED F,FULTON L,et al.Diet⁃induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome[J].Cell Host&Microbe,2008,3(4):213-223.
[13] DUNCAN S H,BELENGUER A,HOLTROP G,et al.Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate⁃producing bacteria in feces[J].Applied and Environmental Microbiology,2007,73(4):1073-1078.
[14] ORCUTT R P,GIANNI F J,JUDGE R J.Develop⁃ment of an“altered Schaedler flora”for NCI gnotobi⁃otic rodents[J].Microecology Therapeutics,1987,17:5-9.
[15] NILUSHA M,MEIJU L,LAKSIRI A G,et al.Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune respon⁃ses and tight junctions in dairy calves during weaning transition[J].Journal of Dairy Science,2013,96(5):3189-3200.
[16] LUNEDO R,FERNANDEZ⁃ALARCON M F,CAR⁃VALLHO F M,et al.Analysis of the intestinal bacteri⁃al microbiota in maize⁃or sorghum⁃fed broiler chick⁃ens using real⁃time PCR[J].British Poultry Science,2014,55(6):795-803.
[17] 朱伟云,姚文,毛胜勇.变性梯度凝胶电泳法研究断奶仔猪粪样细菌区系变化[J].微生物学报,2003,43(4):503-508.
[18] JOHANSSON M E V,LARSSON J M,HANSSON G C.The two mucus layers of colon are organized by the MUC2 mucin,whereas the outer layer is a legislator of host⁃microbial interactions[J].Proceedings of the Na⁃tional Academy of Sciences of the United States of A⁃merica,2011,108(Suppl.1):4659-4665.
[19] ATUMA C,STRUGALA V,ALLEN A,et al.The ad⁃herent gastrointestinal mucus gel layer:thickness and physical state in vivo[J].American Journal of Physiol⁃ogy Gastrointest and Liver Physiology,2001,280:G922-G929.
[20] JOHANSSON M E,PHILLIPSON M,PETERSSON J,et al.The inner of the two Muc2 mucin⁃dependent mucus layers in colon is devoid of bacteria[J].Pro⁃ceedings of the National Academy of Sciences of the United States of America,2008,105(39):15064-15069.
[21] AN G Y,WEI B Y,XIA B,et al.Increased suscepti⁃bility to colitis and colorectal tumors in mice lacking core 3⁃derived O⁃glycans[J].Journal of Experimental Medicine,2007,204(6):1417-1429.
[22] FU J X,WEI B,WEN T,et al.Loss of intestinal core 1⁃derived O⁃glycans causes spontaneous colitis in mice [J].Journal of Clinical Investigation,2011,121(4):1657-1666.
[23] HEAZLEWOOD C K,COOK M C,ERI R,et al.Ab⁃errant mucin assembly in mice causes endoplasmic re⁃ticulum stress and spontaneous inflammation resemb⁃ling ulcerative colitis[J].PLoS Medicine,2008,5 (3):e54.
[24] CHELED⁃SHOVAL S L,GAMAGE N S,AMIT⁃RO⁃MACH E,et al.Differences in intestinal mucin dynam⁃ics between germ⁃free and conventionally reared chickens after mannan⁃oligosaccharide supplementa⁃tion[J].Poultry Science,2014,93(3):636-644.
[25] SHARMA R,SCHUMACHER U,RONAASEN V,et al.Rat intestinal mucosal responses to a microbial flora and different diets[J].Gut,1995,36(2):209-214.
[26] PETERSSON J,SCHREIBER O,HANSSON G C,et al.Importance and regulation of the colonic mucus bar⁃rier in a mouse model of colitis[J].American Journal of Physiology:Gastrointestinal and Liver Physiology,2011,300(2):G327-G333.
[27] KOBOZIEV I,KARLSSON F,GRISHAM M B.Gut⁃associated lymphoid tissue,T cell trafficking,and chronic intestinal inflammation[J].Annals of the New York Academy of Sciences,2010,1207(Suppl.1):e86-e93.
[28] MEBIUS R E.Organogenesis of lymphoid tissues[J].Nature Reviews Immunology,2003,3(4):292-303.
[29] ROUND J L,MAZMANIAN S K.The gut microbiota shapes intestinal immune responses during health and disease[J].Nature Reviews Immunology,2009,9 (5):313-323.
[30] RENZ H,BRANDTZAEG P,HORNEF M.The im⁃pact of perinatal immune development on mucosal ho⁃meostasis and chronic inflammation[J].Nature Re⁃views Immunology,2012,12(1):9-23.
[31] MAYNARD C L,ELSON C O,HATTON R D,et al.Reciprocal interactions of the intestinal microbiota and immune system[J].Nature,2012,489(7415):231-241.
[32] HAMADA H,HIROI T,NISHIYAMA Y,et al.Identi⁃fication of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine[J].The Journal of Immunology,2002,168(1):57-64.
[33] BOUSKRA D,BRÉZILLON C,BÉRARD M,et al.Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis[J].Nature,2008,456:507-510.
[34] BUTLER J E,LAGER K M,SPLICHAL I,et al.The piglet as a model for B cell and immune system devel⁃opment[J].Veterinary Immunology and Immunopa⁃thology,2009,128(1/2/3):147-170.
[35] LAYCOCK G,SAIT L,INMAN C,et al.A defined intestinal colonization microbiota for gnotobiotic pigs [J].Veterinary Immunology and Immunopathology,2012,149(3/4):216-224.
[36] O’Neill L A J,GOLENBOCK D,BOWIE A G.The history of Toll⁃like receptors⁃redefining innate immu⁃nity[J].Nature Reviews Immunology,2013,13(6):453-460.
[37] STEINMAN L.A brief history of TH17,the first major revision in the TH1/TH2 hypothesis of T cell⁃mediated tissue damage[J].Nature Medicine,2007,13(2):139-145.
[38] JOLLER N,LOZANO E,BURKETT P R,et al.Treg cells expressing the coinhibitory molecule TIGIT se⁃lectively inhibit proinflammatory Th1 and Th17 cell responses[J].Immunity,2014,40(4):569-581.
[39] GABORIAU⁃ROUTHIAU V,RAKOTOBE S,LÈCUYER E,et al.The key role of segmented fila⁃mentous bacteria in the coordinated maturation of gut helper T cell responses[J].Immunity,2009,31(4):677-689.
[40] IVANOV I I,ATARASHI K,MANEL N,et al.Induc⁃tion of intestinal Th17 cells by segmented filamentous bacteria[J].Cell,2009,139(3):485-498.
[41] ATARASHI K,TANOUE T,SHIMA T,et al.Induc⁃tion of colonic regulatory T cells by indigenous Clos⁃tridium species[J].Science,2011,331(6015):337-341.
[42] NICHOLSON J K,HOLMES E,KINROSS J,et al.Host⁃gut microbiota metabolic interactions[J].Sci⁃ence,2012,336(6086):1262-1267.
[43] ARPAIA N,CAMPBELL C,FAN X Y,et al.Metabo⁃lites produced by commensal bacteria promote periph⁃eral regulatory T⁃cell generation[J].Nature,2013,504 (7480):451-455.
[44] FURUSAWA Y,OBATA Y,FUKUDA S,et al.Com⁃mensal microbe⁃derived butyrate induces the differen⁃tiation of colonic regulatory T cells[J].Nature,2013,504(7480):446-450.
[45] MASLOWSKI K M,VIEIRA A T,NG A,et al.Regu⁃lation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43[J].Nature,2009,461(7268):1282-1286.
[46] INAN M S,RASOULPOUR R J,YIN L,et al.The lu⁃minal short⁃chain fatty acid butyrate modulates NF⁃κB activity in a human colonic epithelial cell line[J].Gastroenterology,2000,118(4):724-734.
[47] VAVASSORI P,MENCARELLI A,RENGA B,et al.The bile acid receptor FXR is a modulator of intestinal innate immunity[J].The Journal of Immunology,2009,183(10):6251-6261.
[48] MYSZKA M,KLINGER M.The immunomodulatory role of vitamin D[J].Postepy higieny imedycyny doswiadczalnej(Online),2014,68:865-878.
[49] REYES⁃BECERRIL M,ASCENCIO⁃VALLE F,TO⁃VAR⁃RAMÍREZ D,et al.Effects of polyamines on cellular innate immune response and the expression of immune⁃relevant genes in gilthead seabream leuco⁃cytes[J].Fish Shellfish Immunology,2011,30(1):248-254.
(责任编辑 菅景颖)
[50] FENG T,WANG L F,SCHOEB T R,et al.Microbiota innate stimulation is a prerequisite for T cell spontane⁃ous proliferation and induction of experimental colitis [J].The Journal of Experimental Medicine,2010,207 (6):1321-1332.
[51] DRAPER E,DECOURCEY J,HIGGINS S C,et al.Conjugated linoleic acid suppresses dendritic cell acti⁃vation and subsequent Th17 responses[J].The Journal of Nutritional Biochemistry,2014,25(7):741-749.
Regulation of Host Immune System by Gut Microbiota and Its Possible Mechanisms
WANG Shanshan WANG Jiakun∗LIU Jianxin
(Key Laboratory of Molecular Animal Nutrition of Ministry of Education,Institute of Dairy Sciences,Zhejiang University,Hangzhou 310058,China)
∗Corresponding author,associate professor,E⁃mail:jiakunwang@zju.edu.cn
Abstract:An animal gut is an open ecological system that is colonized by large numbers and variety of micro⁃organisms.The microbiota has coevolved relationship with the host immune system and plays an important role in maintaining intestinal homeostasis.Establishing and maintaining beneficial interactions between gut microbio⁃ta and host immunity are key requirements for host health.Therefore,this article reviewed the composition of gut microbiota,the development of the host immune system under regulation of gut microbes and the possible mechanisms of gut microbiota regulating immune system.[Chinese Journal of Animal Nutrition,2015,27 (2):375⁃382]
Key words:gut microbiota;host;immune system development;immune homeostasis
通信作者:∗王佳堃,副教授,博士生导师,E⁃mail:jiakunwang@zju.edu.cn
作者简介:王珊珊(1990—),女,山东济南人,硕士研究生,从事肠道屏障功能发育研究。E⁃mail:shanlan1128@163.com
基金项目:中央高校基本科研业务费专项资金(2014QNA6027)
收稿日期:2014-09-22
doi:10.3969/j.issn.1006⁃267x.2015.02.007
文章编号:1006⁃267X(2015)02⁃0375⁃08
文献标识码:A
中图分类号:S811.2

