雷公藤红素衍生物合成与活性测定
单伟光,施 航,占扎君
(浙江工业大学 药学院,浙江 杭州 310014)
雷公藤红素衍生物合成与活性测定
单伟光,施航,占扎君
(浙江工业大学 药学院,浙江 杭州 310014)
摘要:雷公藤红素是一种从中药雷公藤中提取的一种具有很强抗癌活性的三萜化合物,为找到具有更好抗癌活性和成药性的雷公藤红素衍生物,在雷公藤红素C-29羧基设计并合成10个新型的衍生物,并通过MMT法,测定了衍生物对A549和HepG2肿瘤细胞的抗癌活性,实验结果显示10个衍生物都具有很好的抗癌活性,化合物C4和C8抗癌活性最强,并且具有良好的水溶性,是较好的体内实验备选化合物.
关键词:雷公藤红素;抗癌;毒性;MTT法
Synthesis and anti-cancer evaluation of novel celastrol analogues
SHAN Weiguang, SHI Hang, ZHAN Zhajun
(College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 3100, China)
Abstract:Celastrol, a natural quinone methide triterpenoid originally isolated from root bark of the traditional Chinese herb “Thunder of God Vine”, showed remarkable anticancer activity. In order to find the celasrol analogues of better anticancer activity and druggability, Ten novel celastrol analogues were designed and synthesized. Their cytotoxicity were evaluated against 2 human cancer cell lines(A549 and HepG2) by MTT method. Ten celastrol analogues all showed better inhibitions than positive drug. Among all of the derivatives, C4 and C6 were the most suitable candidate compounds for in vivo experiment in the future because of their high activity and water solubility.
Key words:celastrol; anticancer; cytotoxicity; MTT method
雷公藤红素(celastrol)是一种五环的木栓烷型三萜化合物,又名南蛇藤碱,是第一个从雷公藤根部提取出来的三萜类化合物[1-2].三萜类化合物雷公藤红素具有独特的化学结构基团,能够和半胱氨酸残基中的巯基发生迈克尔加成,生成共轭加成产物[3],该化合物在体内通过发生加成反应,影响蛋白或酶的活性、调节多种细胞信号途径等,进而产生药理作用.据报道,雷公藤红素具有多种显著的生物活性,例如,抗肿瘤、抗免疫与抗炎、抗病毒以及抗神经衰退性疾病等[4-6].雷公藤红素的抗肿瘤活性在很久之前已经被研究证实,但是很长一段时间机制不明确,直到2006年Yang等[7]首次实验证明雷公藤红素可以诱导癌细胞凋亡,使得肿瘤细胞坏死,从而引发了雷公藤红素抗癌机制的研究热浪;之后相继有研究表明雷公藤红素对多种肿瘤细胞的治疗都有很好的疗效,例如前列腺癌细胞、神经胶质瘤细胞、口腔鳞状癌细胞、黑素瘤、乳腺癌、白血病和肺癌等等[8-10].
国内外开展了不少雷公藤红素修饰物的研究[11-13],主要是针对C-3羟基和C-29羧基成一些酯基和一些酰胺键,本次研究的创新处在于在C-29位的常规修饰基团中加入一些卤元素、苯环、六圆环及羟基,试图改变整个分子的结构以及电子云密度,从而产生不同的抗癌活性.
1合成研究
1.1实验材料与仪器
实验材料:雷公藤红素(课题组自提分离);卤代烷试剂(AR,Aladdin Chemistry Co.Ltd.);碳酸氢钠(CR,天津永大化学试剂有限公司);无水硫酸钠(CR,上海四赫维化工有限公司);硅胶(青岛海洋化工厂);溶剂均为国产分析纯.
实验仪器:磁力搅拌器(杭州大卫科教仪器);真空干燥箱(上海精宏实验设备有限公司);真空油泵(台州博奥真空设备有限公司);恒温鼓风干燥箱(上海精宏实验设备有限公司);数控超声波清洗器(昆山市禾创超声仪器有限公司);旋转蒸发仪(BUCHI);循环水式多用真空泵(杭州大卫科教仪器);紫外灯(上海顾村电光仪器厂);电子天平(max 220 g,d=0.1 mg,德国塞多利斯);冰箱(青岛海尔股份有限公司).
1.2衍生物的合成
如图1所示,取红素(50 mg, 0.11 mmol)溶于N,N-二甲基甲酰胺(DMF,4 mL)中,加入碳酸氢钠少量,再加入相应的二卤代烷、卤代环烷烃(0.3 mmol),室温下搅拌数小时.TLC法检测反应程度,观察雷公藤红素点消失后,反应终止.加入去离子水(15 mL),乙酸乙酯萃取3 次,合并有机层.有机层再用饱和食盐水洗3次,无水硫酸钠干燥,旋转蒸发仪浓缩得棕色油状物.粗产物经快速柱层析(油醚:丙酮)法分离纯化,产物经减压蒸除溶剂、真空干燥得深棕色固体C1~C10.所得产物经氢谱、碳谱和质谱等方法确定其结构.雷公藤红素衍生物的合成路线为

1.3衍生物的图谱归属
C1:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.12(3H,s,CH3),1.21(3H,s,CH3),1.25(3H,t,J=7.0 Hz,H-2′),1.27(3H,s,CH3),1.45(3H,s,CH3),2.25(3H,s,CH3),3.98(2H,m,H-1′),6.35(1H,d,J=7.0 Hz7.0,H-7),6.55(1H,s,H-1),7.03(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,14.0,18.4,21.6,28.7,29.3,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.2,43.0,44.3,45.1,60.3,117.3,118.2,119.5,127.5,134.3,146.1,164.9,170.4,178.2,178.4.ESI-MS:m/z479.3[M+H]+.
C2:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.45(2H,t,J=6.5 Hz,H-2′),4.10(1H,m,H-1′a),4.27(1H,m,H-1′b),6.31(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.09(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.5,28.4,28.8,29.6,29.8,30.6,30.7,31.5,32.7,33.5,34.6,36.3,38.1,39.4,40.4,42.8,44.2,44.9,63.9,117.0,118.0,119.5,127.3,134.0,146.1,164.6,169.8,177.7,178.2.ESI-MS:m/z559.3[M+H]+.
C3:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.64(2H,t,J=6.5 Hz,H-2′),4.08(1H,m,H-1′a),4.23(1H,m,H-1′b),6.34(1H,d,J=7.0 Hz,H-7),6.53(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.6,21.6,28.6,29.6,29.7,29.8,30.8,31.5,32.7,33.6,34.7,36.3,38.2,39.5,40.5,41.6,42.9,44.3,45.0,64.2,117.1,118.1,119.5,127.4,134.0,146.1,164.7,169.8,177.9,178.4.ESI-MS:m/z513.3[M+H]+.
C4:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.12(3H,s,CH3),1.21(3H,s,CH3),1.26(3H,s,CH3),1.44(3H,s,CH3),2.20(3H,s,CH3),3.78(2H,m,H-2′),4.01(1H,m,H-1′a),4.09(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.53(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.5,21.6,28.7,29.3,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.2,43.0,44.3,45.1,60.9,66.1,117.2,118.2,119.6,127.5,134.1,146.1,164.8,170.0,178.4,178.5.ESI-MS:m/z485.3[M+H]+.
C5:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),0.97(3H,m,H-3′),1.12(3H,s,CH3),1.19(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.24(3H,s,CH3),3.82(1H,m,H-1′a),3.93(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,14.0,18.4,21.6,21.7,28.7,29.3,29.7,30.6,30.7,31.6,32.8,33.5,34.8,36.4,38.2,39.4,40.4,43.0,44.3,45.1,66.3,117.3,118.2,119.5,127.4,134.0,146.0,164.7,170.0,178.3,178.4.ESI-MS:m/z493.1[M+H]+.
C6:1H-NMR(CDCl3,500 MHz):0.54(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.44(2H,t,J=6.5 Hz H-3′),3.98(1H,m,H-1′a),4.10(1H,m,H-1′b),6.33(1H,d,J=7.0 Hz,H-7),6.52(1H,s,H-1),7.00(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.6,21.6,28.6,29.2,29.3,29.6,29.8,30.6,30.7,31.5,32.7,33.5,34.6,36.3,38.1,39.4,40.4,42.8,44.3,44.9,62.0,117.0,118.1,119.5,127.4,134.0,146.0,164.6,169.7,178.3,178.2.ESI-MS:m/z573.3[M+H]+.
C7:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.56(2H,t,J=6.5 Hz H-3′),3.95(1H,m,H-1′a),4.09(1H,m,H-1′b),6.31(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.1,18.6,21.5,28.5,29.6,29.7,29.8,30.4,30.7,31.5,32.6,33.4,34.7,36.3,38.1,39.3,40.5,41.0,42.8,44.2,44.9,61.0,117.1,118.1,119.5,127.3,133.9,146.o,164.6,169.8,177.9,178.2.ESI-MS:m/z527.3[M+H]+.
C8:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.10(3H,s,CH3),1.18(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.21(3H,s,CH3),3.68(2H,m,H-3′),4.01(1H,m,H-1′a),4.13(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.5,21.6,28.7,29.3,29.7,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.5,43.0,44.3,45.1,59.4,61.5,117.2,118.2,119.6,127.5,134.1,146.1,164.8,170.0,178.4,178.5.ESI-MS:m/z499.3[M+H]+.
C9:1H-NMR(CDCl3,500 MHz):0.50(3H,s,CH3),1.09(3H,s,CH3),1.20(3H,s,CH3),1.26(3H,s,CH3),1.43(3H,s,CH3),2.21(3H,s,CH3),4.94(1H,d,J=7.5 Hz,H-1′a),5.02(1H,d,J=7.5 Hz,H-1′b),6.33(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6),7.27-7.35(5H,m,H-Ar).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.6,28.6,29.3,29.8,30.6,30.8,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.4,42.9,44.2,45.0,66.3,117.3,118.1,119.6,127.4,127.5,128.2,128.3,128,6 134,1 134.1,135,7,146.0,164.7,170.1,177.9,178.3.ESI-MS:m/z541.3[M+H]+.
C10:1H-NMR(CDCl3,500 MHz):0.53(3H,s,CH3),1.13(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.46(3H,s,CH3),2.22(3H,s,CH3),3.64(H,m,H-1′a),3.80(H,m,H-1′b),6.36(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.03(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.6,25.7,25.8,28.7,29.7,29.7,29.8,29.9,30.6,30.8,31.6,32.8,33.5,34.9,36.5,38.2,39.5,40.5,43.0,44.4,45.1,69.7,117.0,118.1,119.5,127.5,134.0,146.1,164.7,169.9,178.3,178.4.ESI-MS:m/z547.3[M+H]+.
2衍生物抗肿瘤活性研究
2.1实验材料
实验细胞(人肺癌细胞株A549,人肝癌细胞株HepG2[14];MEM培养基(南京凯基生物);DMEM培养基(南京凯基生物);RPMI-1640培养基(南京凯基生物);胎牛血清(Hyclone);胰酶(吉诺生物医药);细胞冻存液(南京凯基生物);PBS(吉诺生物医药);MTT(Sigma);DMSO(CR,上海凌峰化学有限公司).
2.2实验仪器
超净台(SW-CJ-1F,上海博迅实业医疗设备厂);CO2培养箱(Thermo scientific);倒置相差显微镜(Olympus, CKX41);酶标仪(TECAN Sunrise);高压蒸汽灭菌锅(上海博迅实业医疗设备厂);96孔培养板(Corning Incorporated);高速离心机(LG10-2.4A).
2.3实验操作
采用MTT法[15-16],在37 ℃,体积比为5%的CO2培养箱内,取对数生长期的各细胞(3×104个/mL),接种于96孔培养板(180 μL/孔)中贴壁生长.24 h后,加入不同浓度药物及阳性对照物,以含细胞培养基为空白对照孔,每个浓度设5个平行孔.置于37 ℃,体积比为5% CO2培养箱中培养.第48 h时加入5 mg/mL MTT溶液(20 μL/孔),混匀后,继续孵育4 h.第52 h时,停止培养,小心倒去96孔板内培养基,加入DMSO(150 uL/孔),振荡溶解,10 min内酶标仪于570 nm波长处测定OD值.最后根据IC50计算公式和软件计算出各化合物IC50值.
2.4IC50实验结果和讨论
选取雷公藤红素以及10个衍生物,以卡铂作为阳性药,测出IC50值,如表1所示.合成所得的化合物C1~C10活性都要高于阳性对照药卡铂.C9和C10这两个衍生物,芳烃衍生物C9活性要好于环烷烃衍生物C10.在直链烃衍生物中,羟基衍生物C4和C8活性最强.
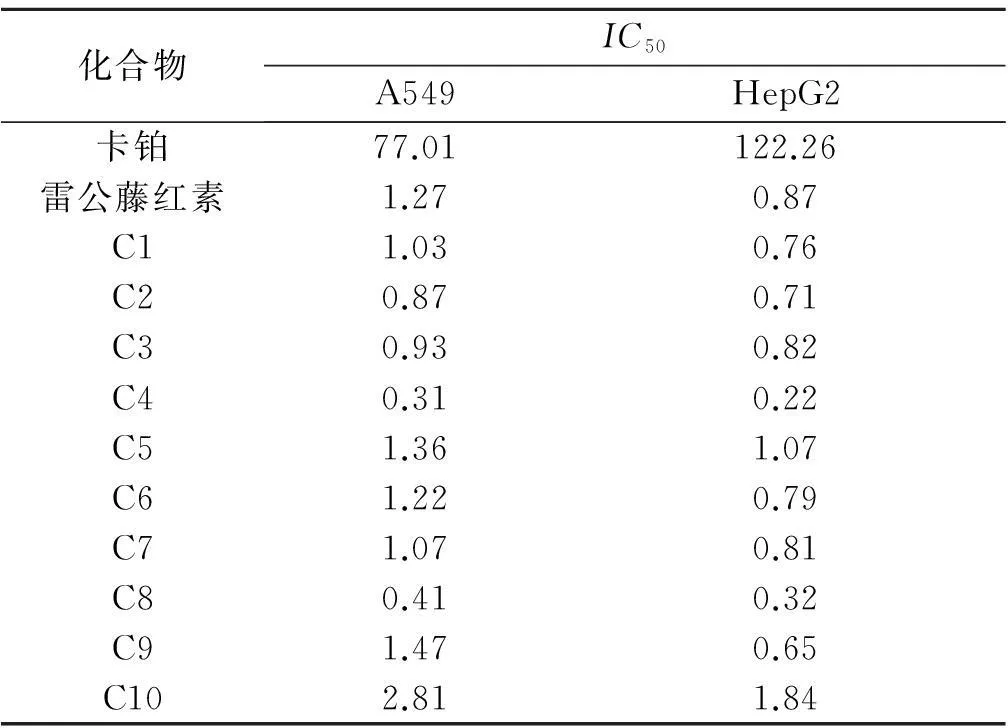
表1 雷公藤红素衍生物的IC50
3结论
本实验对具有较强抗癌活性的木栓烷型三萜化合物雷公藤红素C-29-羧基进行修饰,得到了10个新型的衍生物,并对衍生物进行抗癌活性测定,采用人肺癌细胞株A549和人肝癌细胞株HepG2,运用MTT法算出了IC50.衍生物C1~C4以及C6~C8都表现出了比雷公藤红素强的抗癌活性,引入了卤素、苯环、羟基都明显提升了化合物抗癌活性,而引入六圆环化合物活性有明显下降.衍生物中化合物C4和C8抗癌活性最强,且C4和C8是衍生物中唯一两个极性大于红素的衍生物,可以作为未来体内实验的候选药物.
参考文献:
[1]刘为萍,刘素香,唐慧珠,等.雷公藤研究新进展[J].中草药,2010,41(7):1215-1218.
[2]韦登明,黄光照.雷公藤及其单体的药理和毒理病理学研究进展[J].中药材,2003,26(12):894-897.
[3]SREERAMULU S, GANDE S L, GOBEL M, et al. Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37 a kinome chaperone-cochaper one by triterpene celastrol[J]. Angewandte Chemie International Edition,2009,48:5853-5855.
[4]胡凯,葛卫红.雷公藤红素药理活性研究进展[J].亚太传统医药,2012,8(11):179-181.
[5]CHAPELSKY S, BATTY S, FROST M, et al. Inhibition of anthrax lethal toxin-induced cytolysis of RAW264.7 cells by celastrol[J]. Plos One,2008(1):1-6.
[6]钱利武,周国勤,陈师农.雷公藤红素抗精神衰退性疾病研究概况[J].中国现代应用药学,2011,7(28):629-633.
[7]YANG H J, CHEN D, CUI C, et al. Celastrol a triterpene extracted from the chinese “Thunder of God Vine” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice[J]. Cancer Research,2006,66(9):4758-4765.
[8]WESTERHEIDE S D. Celastrols as inducers, of the heat shock response and cytoprotection[J]. Journal of Biological Chemistry,2004,279(53):56053-56060.
[9]GE P, JI X, DING Y, et al. Celastrol causes apoptosis and cell cycle arrest in rat glioma cells[J]. Neurological Research,2010,32(1):94-100.
[10]PENG B, XU L, CAO F, et al. HSP90 inhibitor celastrol arrests, human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way[J]. Molecular Cancer,2010,9(1):79.
[11]SUN H, XU L, YU P, et al. Synthesis and preliminary evaluation of neuroprotection of celastrol analogues in PC12 cells[J]. Bioorganic & Medicinal Chemistry Letters,2010,20(13):3844-3847.
[12]王家强,刘军峰,刘珂.雷公藤红素衍生物的合成与评价[J].中草药,2009,40:201.
[13]ABBAS S, BHOUMIK A, DAHL R, et al.Preclinical studies of celastrol and acetyl isogambogic acid in melanoma[J]. Clinical Cancer Research,2007,13(22):6769-6778.
[14]宋必卫,骆钱芬.BAPTA-AM对OGD-再灌致HepG-2细胞损伤的保护作用研究[J].浙江工业大学学报,2013,41(2):133-137.
[15]竺佳,朱泱平,徐佳,等.七叶皂苷钠对P388小鼠白血病细胞的体内外增殖抑制作用[J].浙江工业大学学报,2009,37(2):123-125.
[16]唐岚,赵亚,单海峰,等.甜瓜蒂中葫芦素类成分分离及体外抗癌活性研究[J].浙江工业大学学报,2012,40(4):388-391.
(责任编辑:陈石平)
文章编号:1006-4303(2015)06-0607-04
中图分类号:O626.4
文献标志码:A
作者简介:单伟光(1961—),男,浙江宁波人,教授,博士生导师,主要从事天然产物化学以及药品质量控制标准的研究,E-mail:swg@zjut.edu.cn.
基金项目:国家自然科学基金资助项目(20702049)
收稿日期:2015-04-15