嵌段共聚物刷稳定的纳米银粒子的制备与表征
李爱香, 陈复强, 吕滋建
(山东理工大学 材料科学与工程学院,山东 淄博 255049)
嵌段共聚物刷稳定的纳米银粒子的制备与表征
李爱香, 陈复强, 吕滋建
(山东理工大学 材料科学与工程学院,山东 淄博 255049)
摘要:制备了链中间含三硫代酯基团的苯乙烯-丙烯酸丁酯嵌段共聚物刷保护的纳米银(Ag NPs)粒子.首先采用可逆加成断裂链转移(RAFT)聚合法制备苯乙烯-丙烯酸丁酯三嵌段共聚物PS-b-PBA-b-PS,然后以PS-b-PBA-b-PS为稳定剂,硼氢化钠(NaBH4)为还原剂,原位还原硝酸银得到嵌段共聚物刷保护的纳米银粒子.用红外光谱(IR)、核磁共振氢谱(1H NMR)、GPC等方法对聚合物进行了表征,用紫外可见光谱(UV-Vis)、透射电子显微镜(TEM)等对纳米银复合粒子进行了表征.结果表明,含三硫代酯基团的聚合物不必经过还原和水解成巯基,可直接作为配体制备稳定的纳米银粒子,粒子粒径分布均匀,分散性好.
关键词:三嵌段共聚物;RAFT聚合;原位还原;聚合物刷;纳米银
收稿日期:2015-01-15
基金项目:国家自然科学基金项目(51303096); 山东理工大学青年教师发展支持计划项目
作者简介:李爱香,女,axl@sdut.edu.cn
文章编号:1672-6197(2016)01-0046-05
中图分类号:O631
文献标志码:A
Abstract:Triblock copolymer of styrene and butyl acrylate PS-b-PBA-b-PS containing trithiocarbonate group was synthesized by reversible addition-fragmentation transfer (RAFT) polymerization. And silver nanoparticles (Ag NPs) were prepared by in-situ reduction method using this triblock copolymer as stabilizer, sodium borohydride as reductant. The structure of triblock polymer was characterized by FTIR,1H NMR, and the molecular weight and the distribution of the triblock copolymer and its precursor were determined by gel permeation chromatography (GPC).Ag NPs were characterized by TEM and UV-Vis spectrum. The results showed that the triblock copolymers PS-b-PBA-b-PS could be used to stabilize Ag NPs directly without reductant and hydrolysis. Ag NPs stabilized by tribolck copolymer brushes by in-situ reduction method had good stability and dispersibility.
Preparation and characterization of silver
nanoparticles stabilized by block copolymer brushes
LI Ai-xiang, CHEN Fu-qiang, LYU Zi-jian
(School of Materials Science and Engineering, Shandong University of Technology, Zibo 255049,China)
Key words: triblock copolymer; RAFT polymerization; in-situ reduction; polymer brush; silver nanoparticles
近年来,金属纳米粒子,尤其是金和银,由于在纳米电子、纳米光学、催化、生物和生物医学等领域的潜在应用而吸引了重大的研究兴趣[1-7].含巯基聚合物刷稳定的金属纳米粒子由于组成多样和聚合物优异的加工性能尤其吸引人的关注.
Grafting-from和Grafting-onto方法已经被广泛应用在制备聚合物刷保护的金属纳米粒子上.Grafting from方法首先在纳米粒子的表面引入可以引发聚合的官能团,然后采用适合的聚合方法引发单体聚合,可得到聚合物刷保护的纳米粒子.例如Ohno等[8]首先在含二硫键和溴官能团的化合物存在下,用硼氢化钠还原HAuCl4制备了含多个Br原子的纳米金粒子(Au NPs),然后用此Au NPs为引发剂,引发MMA进行原子转移自由基聚合(ATRP),得到了高密度聚合物刷保护的Au NPs.而Dong等[9]采用相似的方法,制备了高热稳定性聚丙烯酸丁酯保护的Au NPs. Grafting-from法制备聚合物刷保护的金属纳米粒子虽然非常有效,但是仅限于低温聚合,因为Au-S和Ag-S键在高温下不稳定,容易断裂,造成纳米粒子的聚集,因而限制了其应用和发展.
Grafting-onto法可事先制备含巯基的聚合物,然后再在聚合物存在下原位还原金属盐制备纳米粒子.例如,Muriel等[10]用grafting-onto的方法,用巯基封端的聚苯乙烯(PS)和聚乙二醇(PEG)作配体,合成了一系列高密度聚合物刷保护的Au NPs.Greiner等[11]首先用阴离子聚合法制备了含巯基的PS,然后用grafting-onto法,以三乙基硼氢化锂为还原剂,在四氢呋喃(THF)中原位还原三氟醋酸银,得到高浓度稳定的银纳米粒子(Ag NPs),此纳米粒子可在PS基体中均匀分散.众所周知,用可逆加成-裂解链转移(RAFT)聚合法可直接制备含二硫代酯或者三硫代碳酸酯基团的聚合物[12-17],经还原和水解可得到含巯基的聚合物,可被用作配体制备金属纳米粒子.例如,Bae等[18]用二苄基三硫碳酸酯(DBTTC)为链转移剂(CTA)通过RAFT聚合苯乙烯制备了含三硫代酯基团的PS,然后经还原和水解产生了巯基封端的PS.用此聚合物为稳定剂,用两相法还原HAuCl4,得到了平均粒径在3~5nm的Au NPs,并用含有Au NPs的PS薄膜制备了有机记忆器件,表现出较好的开关行为.
然而,已经被证明,含二硫代酯基团的聚合物可以不必经过水解成巯基,直接用来作稳定剂制备金属纳米粒子,因为在金属纳米粒子的制备过程中,还原剂可直接将二硫代酯还原为巯基[19-21].而用含三硫代酯基团的聚合物为稳定剂直接制备金属纳米粒子的研究较少被报道.
本文报道了用含三硫代酯基团的苯乙烯-丙烯酸丁酯三嵌段共聚物PS-b-PBA-b-PS为稳定剂,直接原位还原制备银纳米粒子.该方法直接简便,避免了还原和水解等过程,合成的纳米粒子粒径均匀,分散性和稳定性好.
1实验部分
1.1 实验原料
苯乙烯(St)和丙烯酸丁酯(BA)从上海国药集团购买,用之前过碱性三氧化二铝柱子除去阻聚剂,-18℃保存备用;二苄基三硫代碳酸酯(DBTTC)从Sigma-Aldrich公司购买.偶氮二异丁腈(AIBN)用无水乙醇重结晶.其它试剂未做进一步纯化.
1.2 RAFT聚合法合成大分子链转移剂PS-CTA
在一个典型的聚合过程中,将24mL(0.21mol)苯乙烯,0.21g(1.28 mmol)AIBN,0.33 mL (1.16mmol) DBTTC和20 mL甲苯放入100 mL Schlenk瓶中,经过三次冷冻-抽真空-暖化-通高纯氩气,密封反应瓶.将反应瓶浸入80℃油浴中,磁力搅拌反应24 h.冰浴冷却20 min终止反应.将聚合物溶液滴加到100 mL甲醇中沉淀,过滤,将固体聚合物再用THF/甲醇溶解沉淀两次,除去未反应的单体和引发剂.所得固体大分子链转移剂PS-CTA在50℃真空干燥24 h.
1.3 三嵌段共聚物PS-b-PBA-b-PS的合成
取1g PS-CTA,8mg AIBN,2mL BA和6mL甲苯置于100mL Schlenk瓶中,经过三次冷冻-抽真空-暖化-通高纯氩气,密封反应瓶.将反应瓶浸入80℃油浴中,磁力搅拌反应24 h.冰浴冷却20 min终止反应.将聚合物溶液滴加到100mL甲醇中沉淀,过滤,将固体聚合物再用THF/甲醇溶解沉淀两次,除去未反应的单体和引发剂.所得聚合物在50℃真空干燥24 h.
1.4 三嵌段共聚物为稳定制备纳米银粒子
17 mg AgNO3和50 mg的PS-b-PBA-b-PS置于锥形瓶中,加入10 mL DMF,超声溶解,冰浴冷却20 min.取4.2 mg NaBH4溶解于3mL DMF中.剧烈搅拌下,将NaBH4溶液滴加到AgNO3中,继续反应3h.
1.5 分析测试与表征
嵌段共聚物的傅里叶变换红外光谱(FTIR)采用溴化钾压片法,使用岛津8400S型傅立叶红外光谱仪测定.嵌段共聚物的1H NMR谱在Bruck 400 Hz型核磁共振仪上进行测定,CDCl3为溶剂.嵌段共聚物及其前体的分子量及其分布用Waters 1414型凝胶渗透色谱(GPC)仪测试,两根PS色谱柱(styrogel HR 4, 5),折光指数检测器,THF为淋洗剂,流速为1 mL/min,线形PS为标准样品,温度为40℃.紫外可见光谱(UV-Vis)用Scinco S-3150型紫外光谱仪记录,DMF为参比.Ag NPs的尺寸和分散性和用JEOL JEM- 1011型透射电子显微镜(TEM)观察,加速电压100KV.将Ag NPs胶体溶液直接滴在纯碳膜上,在空气中自然晾干观察.
2结果与讨论
首先,用DBTTC为链转移剂用RAFT聚合法聚合苯乙烯制备聚苯乙烯大分子链转移剂(PS-CTA),三硫代酯基团位于高分子链中间;然后以PS-CTA为链转移剂聚合丙烯酸丁酯制备PS-b-PBA-b-PS三嵌段共聚物;最后,利用三硫代酯基团上的S原子和Ag的较强的络合作用,原位还原硝酸银制备聚合物刷保护的Ag NPs.合成路线如图1所示.
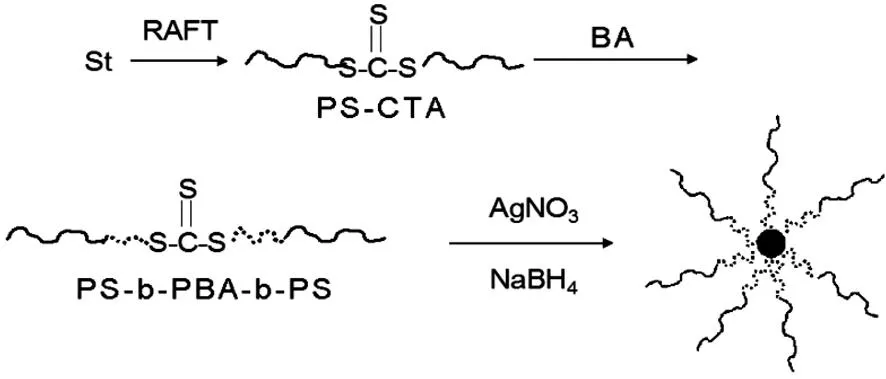
图1 苯乙烯-丙烯酸丁酯三嵌段共聚物PBA-b-PS-b-PBA的合成及聚合物刷保护的Ag NPs的制备
2.1 嵌段共聚物PBA-b-PS-b-PBA的合成
参照文献[14],采用DBTTC为链转移剂,先溶液聚合苯乙烯,制备大分子链转移剂PS-CTA,然后再聚合丙烯酸丁酯合成链中间含有三硫代酯基团的三嵌段共聚物PS-b-PBA-b-PS.图2为PS-b-PBA-b-PS及其前体PS-CTA的GPC谱图.我们可以看到,对应于PS-CTA的峰为一尖锐单峰,分子量分布窄,GPC测得的数均分子量为6300,分子量分布指数为1.27.与PS-CTA的峰相比,嵌段共聚物PS-b-PBA-b-PS的峰明显向高分子量方向移动,峰形仍然为尖锐单峰,表明成功合成了嵌段共聚物.GPC测得的数均分子量为8020,分子量分布指数为1.29.因为GPC测试时使用PS做标准样品,所以测得的嵌段共聚物的分子量是不准确的.
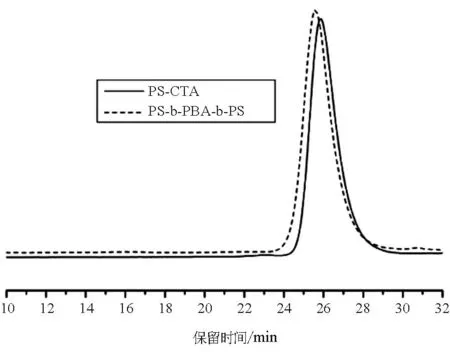
图2 PS-b-PBA-b-PS 及其前体PS-CTA的GPC谱图
图3为PS-b-PBA-b-PS的1H NMR谱图.化学位移δ=4.1×10-6附近的峰归属于PBA链段上与酯基相连的碳上的两个质子峰,而化学位移δ=6.0~7.2×10-6的峰归属于PS链段上苯环上的峰,通过比较这两个峰的峰面积,可计算出嵌段共聚物的分子量为15540.嵌段共聚物的组成为PBA36-b-PS29-b-PBA36.嵌段共聚物及其前体的分子量及其分布表征如表1所示.
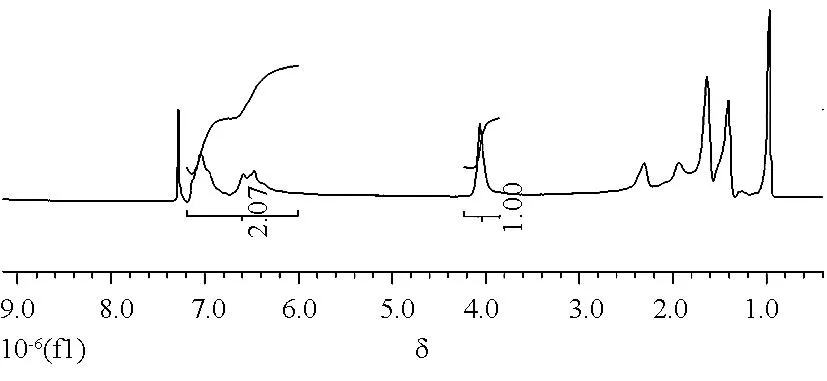
图3 嵌段共聚物PS-b-PBA-b-PS的1H NMR
表1嵌段共聚物PS-b-PBA-b-PS及其前体的分子表征
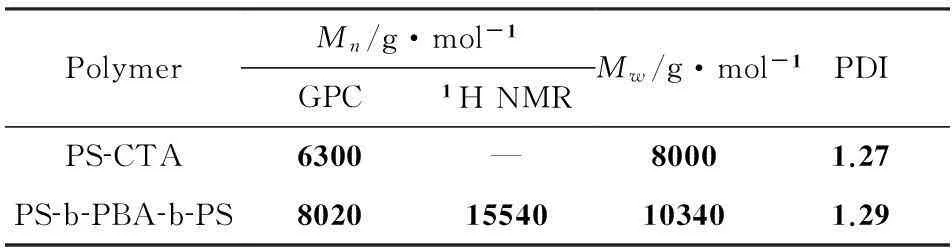
PolymerMn/g·mol-1GPC1HNMR Mw/g·mol-1PDIPS CTA6300—80001.27PS b PBA b PS802015540103401.29
图4为PS-b-PBA-b-PS的FTIR光谱图.1490cm-1处为苯环上的峰,1730cm-1处的峰归属于PBA链段的酯基,也表明成功得到了嵌段共聚物PS-b-PBA-b-PS.
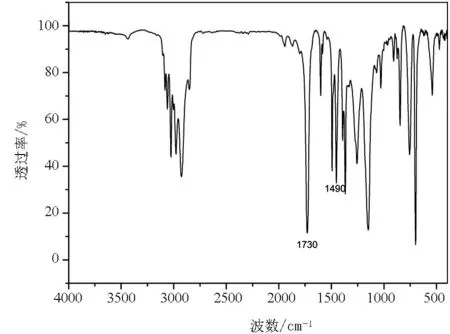
图4 嵌段共聚物PS-b-PBA-b-PS的FTIR谱图
2.2 嵌段聚合物刷PS-b-PBA-b-PS保护的Ag NPs的制备
在PS-b-PBA-b-PS的存在下,用NaBH4作还原剂,在DMF中原位还原硝酸银制备Ag NPs.溶液颜色由无色很快变为浅黄色,至深褐色,表明生成了Ag NPs.溶胶放置数月无任何聚集,表明无需将聚合物还原和水解,三硫代酯基团也对Ag NPs有很好的保护和稳定作用.图5为得到的Ag NPs的UV-Vis谱图.可以看出,Ag NPs在DMF中表现出明显的等离子体共振吸收峰,最大吸收波长在414nm.峰形为单峰,对称性较好,表明Ag NPs为球形,粒径分布窄.
图6a和图b为Ag NPs的TEM图和粒径分布柱形图.可以看出,Ag NPs为球形,无任何聚集,粒子平均粒径为5.6 nm.
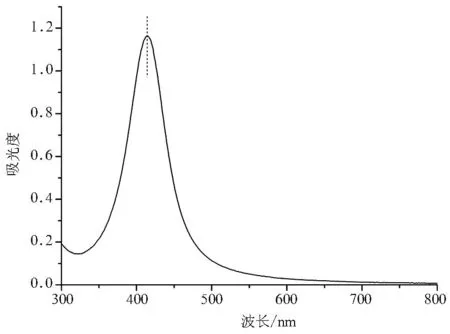
图5 Ag NPs的UV-Vis谱图
3结论

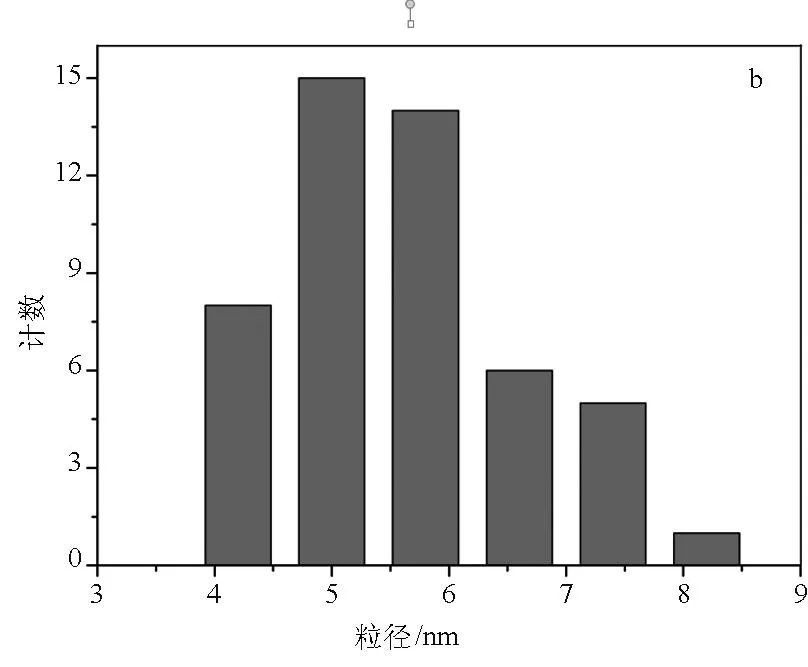
图6 Ag NPs的TEM图(a)和粒径分布柱形图(b)
用RAFT聚合法先聚合苯乙烯,再聚合丙烯酸丁酯,制备了含三硫代酯基团的苯乙烯-丙烯酸丁酯三嵌段共聚物,并以此共聚物为稳定剂,制备了嵌段共聚物刷保护的纳米银粒子,粒子粒径分布均匀,稳定性好.该方法简单易行,具有普适性.若将第二单体换成可交联的单体,如乙烯基苄基氯,4-乙烯基吡啶等,得到嵌段聚合物稳定的纳米银粒子以后可进一步交联,得到高热稳定性交联壳和聚合物刷保护的复合粒子.
参考文献
[1]Brust M, Bethell D, Kiely C J,etal. Self-assembled gold nanoparticle thin films with nonmetallic optical and electronic properties [J]. Langmuir, 1998, 14: 5425-5429.
[2] Liu S Q, Tang Z Y. Nanoparticle assemblies for biological and chemical sensing [J]. J Mater Chem, 2010, 20: 24-35.
[3]Brett D M, Jake F, ZhengW,etal. Generation of fluorescent silver nanoscale particles in reverse micelles using gamma irradiation [J]. Chem Commun, 2012, 48: 10657-10659.
[4] Arcadi A. Alternative synthetic methods through new developments in catalysis by gold [J]. Chem Rev, 2008, 108: 3266-325.
[5] Liu S, Han M. Synthesis, functionalization, and bioconjugation of monodisrse, silica-coated gold nanoparticles: robust bioprobes [J]. Adv Funct Mater, 2005, 15: 961-967.
[6] Nam J, Won N, Jin H,etal. pH-Induced aggregation of gold nanoparticles for photothermal cancer therapy [J]. J Am Chem Soc, 2009, 131: 13639-13645.
[7] Rycenga M, Cobley C M, Zeng J,etal. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications [J]. Chem Rev, 2011, 111: 3669-3712.
[8] Ohno K, Koh K, Tsujii Y,etal. Synthesis of gold nanoparticles coated with well-defined, high-density polymer brushes by surface-initiated living radical polymerization [J]. Macromolecules, 2002, 35: 8989-8993.
[9] Dong H, Zhu M, Yoon J A,etal. One-pot synthesis of robust core/shell gold nanoparticles [J]. J Am Chem Soc, 2008, 130: 12852-12853.
[10] Corbierre M K, Cameron N S, Lennox R B. Polymer-stabilized gold nanoparticles with high grafting densities [J]. Langmuir, 2004, 20: 2867-2873.
[11] Bokern S, Getze J, Agarwal S,etal. Polymer grafted silver and copper nanoparticles with exceptional stability against aggregation by a high yield one-pot synthesis [J]. Polymer, 2011, 52: 912-920.
[12] Moad G, Rizzardo E, Thang S H. Radical addition-fragmentation chemistry in polymer synthesis [J]. Polymer, 2007, 49: 1079-1131.
[13] Zhang J, Dong A, Cao T,etal. Carbazyl RAFT agents synthesized by an improved aqueous phase method and their applications in RAFT polymerizatiol [J]. Eur Polym J, 2008, 44: 1071-1080.
[14] Chernikova E V, Terpugova P S, Garina E S,etal. Controlled radical polymerization of styrene and n-butyl acrylate mediated by trithiocarbonates [J]. Polym Sci, Ser A, 2007, 49: 108-119.
[15] Freal-Saison S, Save M, Bui C,etal. Emulsifier-free controlled free-radical emulsion polymerization of styrene via RAFT using dibenzyltrithiocarbonate as a chain transfer agent and acrylic acid as an ionogenic comonomer: batch and spontaneous phase inversion processes [J]. Macromolecules, 2006, 39: 8632-8638.
[16] Zhou D, Zhu X, Zhu J,etal. Preparation and characterization of poly(styrene)/metal composites via reversible addition-fragmentation chain transfer (RAFT) polymerization [J]. React Funct Polym, 2009, 69: 55-61.
[17] Liu X, Chen J, Sun P,etal. Grafting modification of ramie fibers with poly(2,2,2-trifluoroethyl methacrylate) via reversible addition-fragmentation chain transfer (RAFT) polymerization in supercritical carbon dioxide [J]. React Funct Polym, 2010, 70: 972-979.
[18] Bae S K, Lee S Y, Hong S C. Thiol-terminated polystyrene through the reversible addition-fragmentation chain transfer technique for the preparation of gold nanoparticles and their application in organic memory devices [J]. React Funct Polym, 2011, 71: 187-194.
[19] Deng Y, Sun Y Y, Wang P. Nonlinear optical properties of silver colloidal solution by in situ synthesis technique [J]. Current Applied Physics, 2008, 8: 13-17.
[20] Shan J, Nuopponen M, Jiang Ha,etal. Amphiphilic gold nanoparticles grafted with poly(N-isopropylacrylamide) and polystyrene [J]. Macromolecules, 2005, 38: 2918-2926.
[21] Lowe A B, Sumerlin B S, Donovan M S,etal. Facile preparation of transition metal nanoparticles stabilized by well-defined (co)polymers synthesized via aqueous reversible addition-fragmentation chain transfer polymerization [J]. J Am Chem Soc, 2002, 124: 11562-11563.
(编辑:姚佳良)

