水稻低纤维突变体LCM527-1性状鉴定、遗传分析与基因定位
水稻低纤维突变体LCM527-1性状鉴定、遗传分析与基因定位
童杰鹏, 童川, 王艳, 任三娟, 沈圣泉*
(浙江大学原子核农业科学研究所,杭州310029)
摘要水稻茎秆机械强度是抗倒伏能力强弱的一项重要指标。为了深入研究水稻茎秆机械强度和抗倒伏特性的内在生理、生化、遗传和分子等方面机制,经400 Gy60Co γ射线诱变和筛选,获得了一个特异性低纤维突变体LCM527-1。经考查,该突变体与野生型品种527(WT)相比,除整个生长期间表现出茎秆、叶片、叶鞘、穗颈和枝梗均脆性及极易折断外,还伴随有生长势偏弱、植株变矮、穗数减少、剑叶和穗变短等形态特性。抗倒伏指数、茎秆单位长度的鲜质量和干质量以及倒一节和倒二节抗折断力均明显降低,茎秆中Ca、Si、K等重要组成元素显著减少,茎秆纤维素含量显著下降,半纤维素含量增高。遗传分析表明,该低纤维突变性状受单隐性核基因控制(暂名为lcm527-1),利用SSR分子标记将lcm527-1基因定位于2号染色体的RM3774和RM1092之间,遗传距离分别为10.6 cM和5.1 cM。这些结果将为今后研究低纤维突变及其精细定位提供依据,也为深入研究水稻抗倒伏机制提供分子生物学支持。
关键词水稻; 低纤维; 突变体LCM527-1; 抗倒伏性; 遗传分析; 基因定位
中图分类号Q 343.17; S 511文献标志码A

Character identification, genetic analysis and gene mapping of a low cellulose mutant LCM527-1 in rice。 Journal of ZhejiangUniversity(Agric. & LifeSci.), 2015,41(3):261-268
Tong Jiepeng, Tong Chuan, Wang Yan, Ren Sanjuan, Shen Shengquan*(InstituteofNuclearAgriculturalSciences,ZhejiangUniversity,Hangzhou310029,China)
SummaryLow cellulose mutant in rice is a common type of crop culm mutants as reported in Arabidopsis, maize and barley previously. These mutants have obvious traits of fragility in stem and leaf, low mechanical strength and weaker breaking-resistance. A low cellulose mutant 527-1 (LCM527-1) obtained from the offspring of a rice variety 527 by 400 Gy60Co γ irradiation was applied to study it’s biological phenotype, genetic traits and mutant gene, attempting to find reasons for low cellulose mutation and rice lodging. It exhibited fragility with continuity and integrity during the whole period of rice growth. In the process of experiment, some main investigations were made to identify the variation in biological expression, genetic analysis and gene mapping, concerning phenotype observation, agronomic traits, rice quality, chemical mineral elements, inheritance pattern and location of mutation gene. Four crosses were made to inspect the inheritance pattern and genetic stability between the low cellulose mutant527-1 (female parent) and the wild-type 527, Zhe7954, 9311, Minghui63 (male parents). After then, 505 pairs of SSR molecular markers were chosen to map the mutant gene on chromosome, and three markers: RM3774, RM1092 and RM530 presented polymorphism difference. These mapping populations were derived from a cross between LCM527-1 and Zhe7954.
The mutant LCM527-1 showed dramatically decreased content of cellulose, high lodging index, big changes in some agronomic traits and mineral elements content. Genetic analysis indicated the character of mutant LCM527-1 was controlled by a single recessive gene. And genelcm527-1 was roughly located between SSR marker RM3774 and RM1092 on chromosome 2 with genetic distances of 10.6 cM and 5.1 cM, respectively.
Above all, it was concluded that mutant LCM527-1 was of great importance for research of mechanical strength, especially the ability of lodging resistance. The mutant traits appeared single locus mutation and inheritable stably, suggesting some specific traits of LCM527-1 might be controlled by this mutant genelcm527-1 and resulted in some related changes in agronomic traits and mineral elements content. These results are helpful to make a fine mapping and its gene cloning in the fragile mutant; meanwhile, they could also lay a theoretical basis on molecular biology mechanism of rice lodging resistance.
Key wordsrice; low cellulose mutant; LCM527-1; lodging resistance; genetic analysis; gene mapping
水稻低纤维突变体是作物茎秆突变体中常见的一类,在玉米、拟南芥、大麦、小麦中均有报道[1-4]。这些突变体具有明显的脆硬性状,茎秆易于折断,茎秆成分含量和结构发生变化,机械强度降低,从而影响其抗倒伏能力以及花穗孕育[5]。水稻低纤维相关性状的产生涉及复杂的生理生化和遗传调控过程。它的生长过程表现为连续性和整体性的低纤维脆硬性特点,从幼苗期到成熟期,从茎秆到叶片都有易断易折的特点[6-7]。
关于水稻低纤维或脆性变异性状及其控制基因的研究报道较多,迄今已在水稻中发现近十几个控制该突变性状的基因,如bc1-8、bc10-12、bc14-15等基因编码产物,主要参与纤维素合成、细胞壁组装、胞壁次级修饰等生物学过程[8]。Li等[9]通过γ诱变得到1个水稻突变体基因bc1,经定位和功能分析发现该基因位于3号染色体,编码COBRA磷脂酰锚定蛋白,通过影响纤维素的装配而影响细胞的生长,降低纤维素,增加木质素,从而调控细胞壁的化学组成和茎秆机械强度。Hirano等[10]研究发现,BC3突变体植株的纤维素含量降低28%~36%,厚壁细胞的细胞壁变薄,细胞壁结构异常,功能分析发现bc3编码膜动力蛋白家族成员OsDRP2B,介导质膜与高尔基体反面管网区之间的囊泡转运,可能影响纤维素的转运组装而导致胞壁变薄、茎秆变脆的原因。
由此可见,低纤维突变体的成因和产生途径各有不同,基因突变位点多样,编码蛋白家族丰富,功能作用广泛。为此,开展不同来源的水稻低纤维突变体分子遗传的研究,对于深入理解复杂的水稻机械强度遗传本质和抗倒伏机制意义重大。
本研究以60Co γ射线处理水稻品种527,经诱变后代筛选和鉴定,获得了1份低纤维突变体LCM527-1。该突变体在生长发育过程中,茎、叶纤维素含量较低,有明显脆硬性、机械强度较弱,与以往研究茎脆叶硬或叶脆茎硬的突变体具有明显不同的表型。为此,笔者试图通过对该突变体LCM527-1的生理、生化、遗传等方面进行鉴定和分析,评价该突变体及其相关突变基因的理论意义和应用价值,以便为今后进一步开展分子水平的水稻纤维素合成、植株抗倒伏机制研究提供参考依据。
1材料与方法
1.1材料
于2009年利用400 Gy60Co γ射线辐照含水率为13.5%的水稻品种527干种子,在浙江大学华家池校区实验农场种植M1。此后,在35 000株诱变M2群体中,发现了1株叶色稍淡,茎、鞘、叶、枝梗均表现脆性,成熟期易折断的突变株。经后代M3和M4连续种植、鉴定和筛选,最终获得了1份性状遗传稳定,植株表现脆性,纤维素含量检测较低的脆性、低纤维突变体,暂命名为LCM527-1(low cellulose mutant)。
1.2方法
1.2.1田间试验低纤维突变体LCM527-1与527(WT)特征特性及各种抗倒伏相关性状鉴定试验于2011年夏在浙江大学紫金港校区实验农场进行。5月25日播种,6月25日移栽,种植密度25 cm×25 cm,每小区种植100株,2次重复。田间管理同生产大田。
1.2.2农艺性状考查在成熟期,每小区分别取5株样品,室内进行水稻形态性状和穗部特征的指标考查,包括株高、每株穗数、剑叶长、穗长、每穗总粒数、每穗实粒数、结实率、千粒重、着粒密度等。
1.2.3稻米品质性状鉴定收取成熟期稻谷,按中华人民共和国农业部标准(NY147-88)进行稻米品质性状的鉴定,包括米粒长、米粒宽、长宽比、垩白粒率、垩白度、透明度、碱消值(糊化温度)、胶稠度、直链淀粉含量等。
1.2.4抗倒伏指数、茎秆单位长度的鲜质量和干质量以及抗折断力测定参照Ookawa等[11]方法并稍加修改。具体做法:抗倒伏指数测定是水稻乳熟期在田间选取10株,用成串的回形针挂于穗颈节处,让其自然垂至地面,计算出每单位长度茎秆自然弯曲下所承受的回形针质量(g/cm),该值即为抗倒伏指数。另取10株的主茎剥去叶鞘,测得茎高(根部与穗颈节之间距离),称得茎秆的鲜质量和干质量(60 ℃烘干至恒质量),计算得到茎秆单位长度的鲜质量和干质量,g/cm。再取10株的主茎,剥去叶鞘,剪下倒一节(即穗颈下第一节)和倒二节,置于测定器,节间中点与测定器中点对应(支点间距5 cm),在节间中点处挂一盘子,加砝码至茎秆断裂,此时砝码及盘子的质量即为节间的抗折力。
1.2.5茎秆矿质元素含量测定参照郝虎林等[12]的方法,取乳熟期生长正常植株的茎秆,先将待测样品105 ℃杀青30 min,70 ℃烘干粉碎,200 ℃电热板上完全碳化,马弗炉中550 ℃左右灰化6 h,冷却后用5 mL体积1∶1优级纯盐酸溶解灰分,用纯水定量到50 g左右,最后用ICP-MS(Agilent 7500a)测定Ca、Cu、Fe、K、Mg、Mn、Mo、Na、P、S、Si和Zn等12种矿质元素含量。
1.2.6茎秆纤维素、半纤维素、木质素和灰分含量测定按van Soest等[13]方法并稍加改动。取乳熟期植株的茎秆,85 ℃烘干,研磨成粉末;准确称取1.000 g样品,放入250-mL三角瓶中,加入100 mL酸性洗涤剂或中性洗涤剂(含2~3 g正辛醇),三角瓶套上冷凝装置于电炉上加热,在5~10 min内煮沸,从开始沸腾计时,回流60 min,煮沸完毕后,取下三角烧瓶,将内容物转入已知质量的坩埚式抽滤器进行减压抽滤,用90~100 ℃的沸水冲洗3~4次,直至酸性洗涤剂洗净或者滤液呈中性为止。用40 mL丙酮冲洗滤器2~3次,抽滤。将坩埚置于100 ℃鼓风式干燥箱中干燥3 h,在干燥器中冷却30 min,称质量,直至恒质量。按van Soest方法测定和计算茎秆纤维素、半纤维素、木质素和灰分含量。
1.2.7遗传分析以突变体LCM527-1为母本,水稻品种(系)527、9311、浙7954和明恢63为父本,配制LCM527-1×527、LCM527-1×9311、LCM527-1×浙7954、LCM527-1×明恢63等4个杂交组合。种植并观察记录F1的植株及其茎、叶部的特征;次年,种植F2并考查植株和茎、叶的性状分离情况,分析突变基因的遗传特性,计算分离比,进行2测验。
1.2.8突变基因定位选取LCM527-1×浙7954组合,以F2的485个单株作为突变基因的定位群体,提取总DNA。同时,选择F2分离群体中的正常型株和低纤维突变型株各10株提取DNA,分别混合,构成正常株和低纤维突变体的近等基因池。
抽穗期按单株取叶片,DNA提取参考Porebski等[14]和韩美丽等[15]的方法并加以改正。根据Temnykh等[16-17]公布的相关SSR分子标记信息,选取水稻12条染色体中的505个SSR标记用于连锁标记基因的初定位。连锁标记得到后,在http://www.gramene.org搜索距离较近区段的SSR分子标记,寻找连锁SSR分子标记。SSR引物由上海Sangon公司合成。PCR反应体系:10×PCR缓冲液2 μL,25 mmol/L dNTPs 1.5 μL,SSR引物2 μL,模板DNA 50~100 ng,5 U/μLTagDNA聚合酶0.2 μL,用ddH2O补足体积至20 μL。反应程序设计为94 ℃预变性5 min;94 ℃变性失活45 s,55 ℃退火复性45 s,72 ℃ 延伸1 min,共35个循环,最后72 ℃延伸7 min。扩增产物用8%聚丙烯酰胺凝胶电泳,银染检测。
根据SSR分析结果,分别对低纤维突变体池和正常池的个体赋值,将表现为突变体带型单株记为1,正常亲本带型单株记为2,同时具有2种带型单株记为3,利用MAPMAKER 3.0[18]软件对分离群体的表型和分子标记分离的数据进行连锁分析,并用Kosambi函数将重组率转化成遗传图距,构建目标基因区域的分子标记连锁图谱。
2结果与分析
2.1突变体LCM527-1的植株性状和田间表现
LCM527-1生长较为正常,与527(WT)相比,生长势稍弱、植株变矮、穗数减少、剑叶和稻穗变短,茎秆、叶片、叶鞘及至穗部枝梗均易折易断,表现出明显的脆性,不同于正常对照水稻折断时的韧性和机械强度,凭手感即可分辨(图1)。

图1 突变体LCM527-1和527(WT)的表型特征 Fig.1 Phenotype characteristics of mutant LCM527-1 and 527 (WT)
对LCM527-1主要农艺性状考查发现,株高、每株穗数、穗长、剑叶长、每穗总粒数、每穗实粒数、结实率等7个性状均比527(WT)显著降低,千粒重和着粒密度两者则未见较大差异(表1),推测该基因突变可能对水稻的整体生长有着或大或小的影响。
2.2突变体LCM527-1稻米品质性状表现
对LCM527-1和527(WT)进行主要稻米品质性状鉴定,结果表明,LCM527-1与527(WT)相比,粒型相似,均属细长粒,长宽比3.05。垩白粒率,垩白度、透明度、糊化温度(碱消值)、胶稠度和直链淀粉含量均无显著差异(表2),表明该突变基因对稻米品质影响不大。
2.3突变体LCM527-1茎秆抗倒伏指数和抗折断力
取LCM527-1 和527(WT)乳熟期植株,田间测定抗倒伏指数,并将其带回室内进行抗倒伏相关机械强度性状检测,包括茎秆高度、单位长度的鲜质量和干质量以及抗折断力性状。结果表明,LCM527-1与527(WT)相比,抗倒伏指数显著较小,茎秆单位长度的鲜质量和干质量均明显降低,倒一节和倒二节抗折断力也显著减弱(表3)。可见,低纤维突变基因对植株抗倒伏机械强度方面相关性状有显著影响。
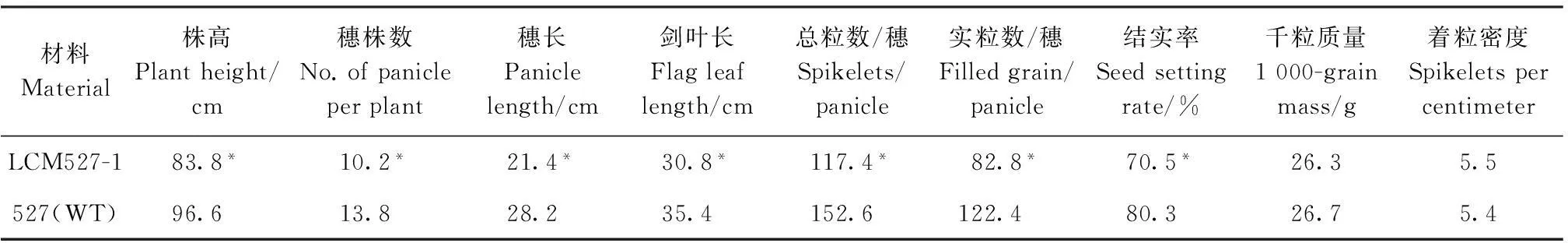
表1 突变体LCM527-1和527(WT)主要农艺性状比较
*表示在P<0.05水平差异有统计学意义。
* indicate statistically significant difference at the 0.05 probability level.
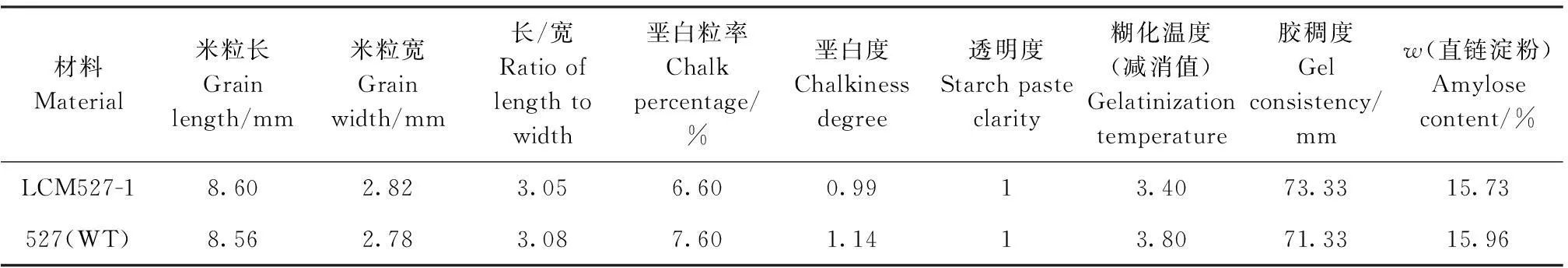
表2 突变体LCM527-1和527(WT)主要稻米品质性状

表3 突变体LCM527-1和527(WT)茎秆机械强度性状
*表示在P<0.05水平差异有统计学意义。
* indicate statistically significant difference at the 0.05 probability level.
2.4突变体LCM527-1茎秆矿质元素含量
乳熟期,取植株茎秆进行主要矿质元素检测。结果发现,LCM527-1和527(WT)相比,Ca、K、Mn、Na、Si、Zn等6种元素含量均有明显降低,Fe含量有所增高,Cu、Mo、P、S含量无显著差异(表4)。由于Ca元素是细胞壁重要组成,Si元素对加强植株硬度有显著作用,说明低纤维突变基因表达使植株对Ca和Si的吸收和积累减少,可能是导致茎秆脆性和易倒的主要原因之一。
2.5突变体LCM527-1纤维素、半纤维素和木质素含量
取LCM527-1和527(WT)乳熟期植株,测定茎秆中纤维素、半纤维素和木质素含量。结果表明,LCM527-1茎秆中纤维素含量显著低于527(WT),表现出明显低纤维、脆硬性状;半纤维素含量增高,其差异达显著水平;木质素含量有升高趋势,但差异不显著(表5)。因而,推测该突变体可以通过植物自身“补救”途径维持茎秆生长。
2.6突变体LCM527-1遗传分析

表4 突变体LCM527-1和527(WT)茎秆矿质元素含量
*表示在P<0.05水平差异有统计学意义。
* indicate statistically significant difference at the 0.05 probability level.
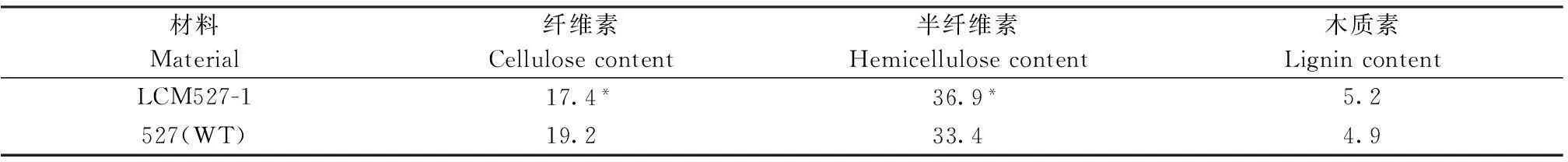
表5 突变体LCM527-1和527(WT)茎秆纤维素、半纤维素和木质素含量
*表示在P<0.05水平差异有统计学意义。
* indicate statistically significant difference at the 0.05 probability level.
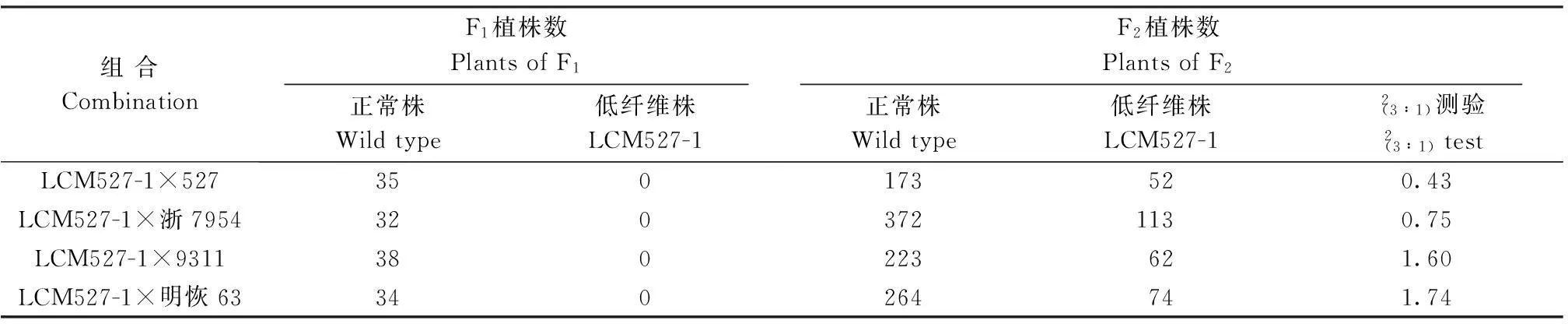
表6 突变体LCM527-1制配的4个杂交组合遗传性状分离比
2.7突变体LCM527-1基因定位
选择覆盖整个水稻基因组,均匀分布于12条染色体的505对SSR标记,逐条进行2个池之间SSR标记多态性分析,筛选出80对具有多态性差异的SSR标记。利用这80对分子标记对F2的群体进行验证和分析,以明确这种低纤维变异性状是否与这些SSR之间存在连锁关系。结果显示,在第2号染色体上存在有3对微卫星引物RM3774,RM1092和RM530,在低纤维突变池与正常株混合池间表现出多态性,可能与低纤维突变性状连锁。利用这些多态性的微卫星标记进一步检测,确认低纤维突变基因与RM3774、RM1092和RM530这3个分子标记存在不同程度的连锁(图2、图3)。
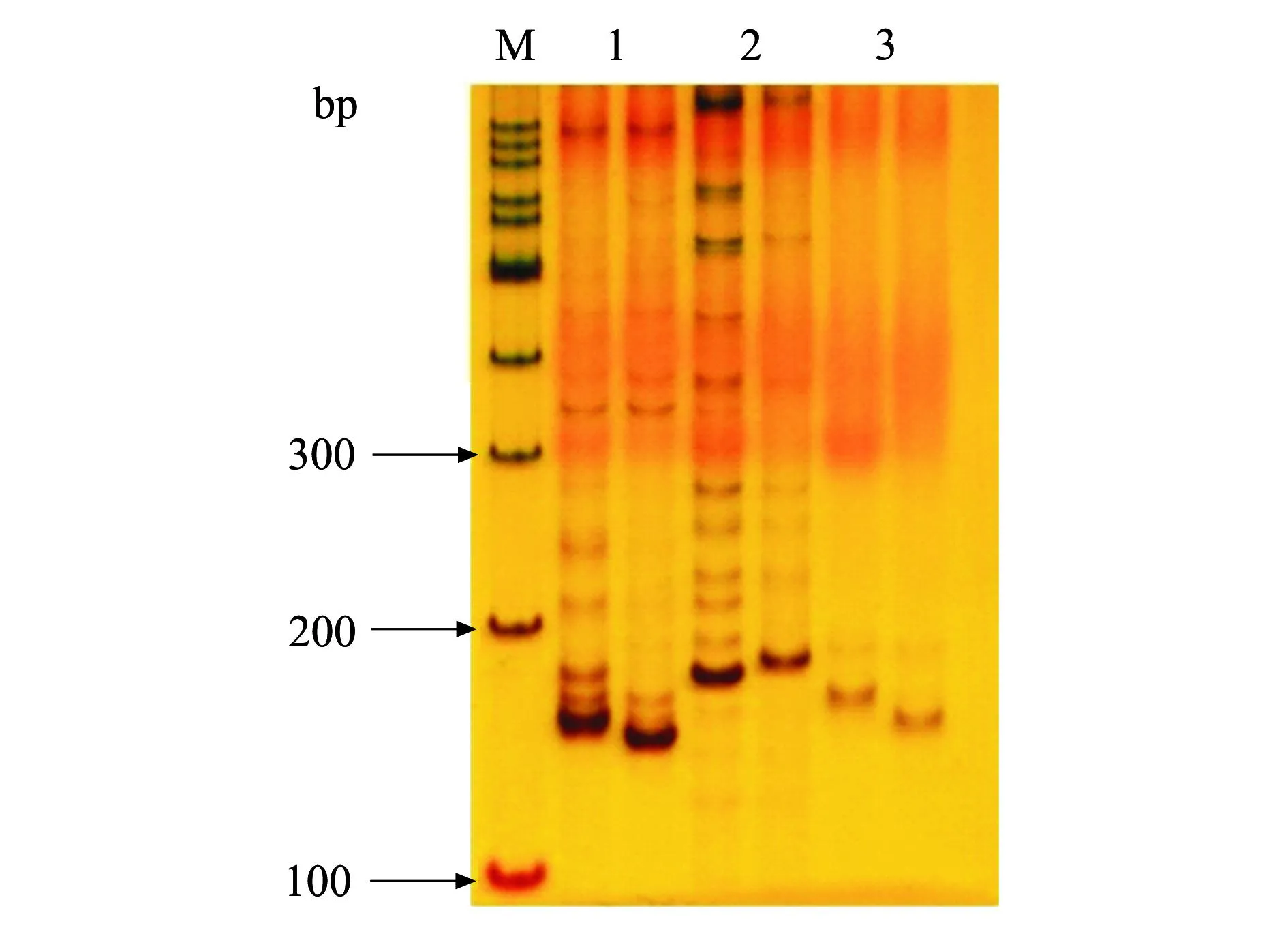
1: RM3774; 2: RM1092; 3: RM530. 图2 3个标记在亲本间的多态性 Fig.2 Polymorphisms of three markers between parents
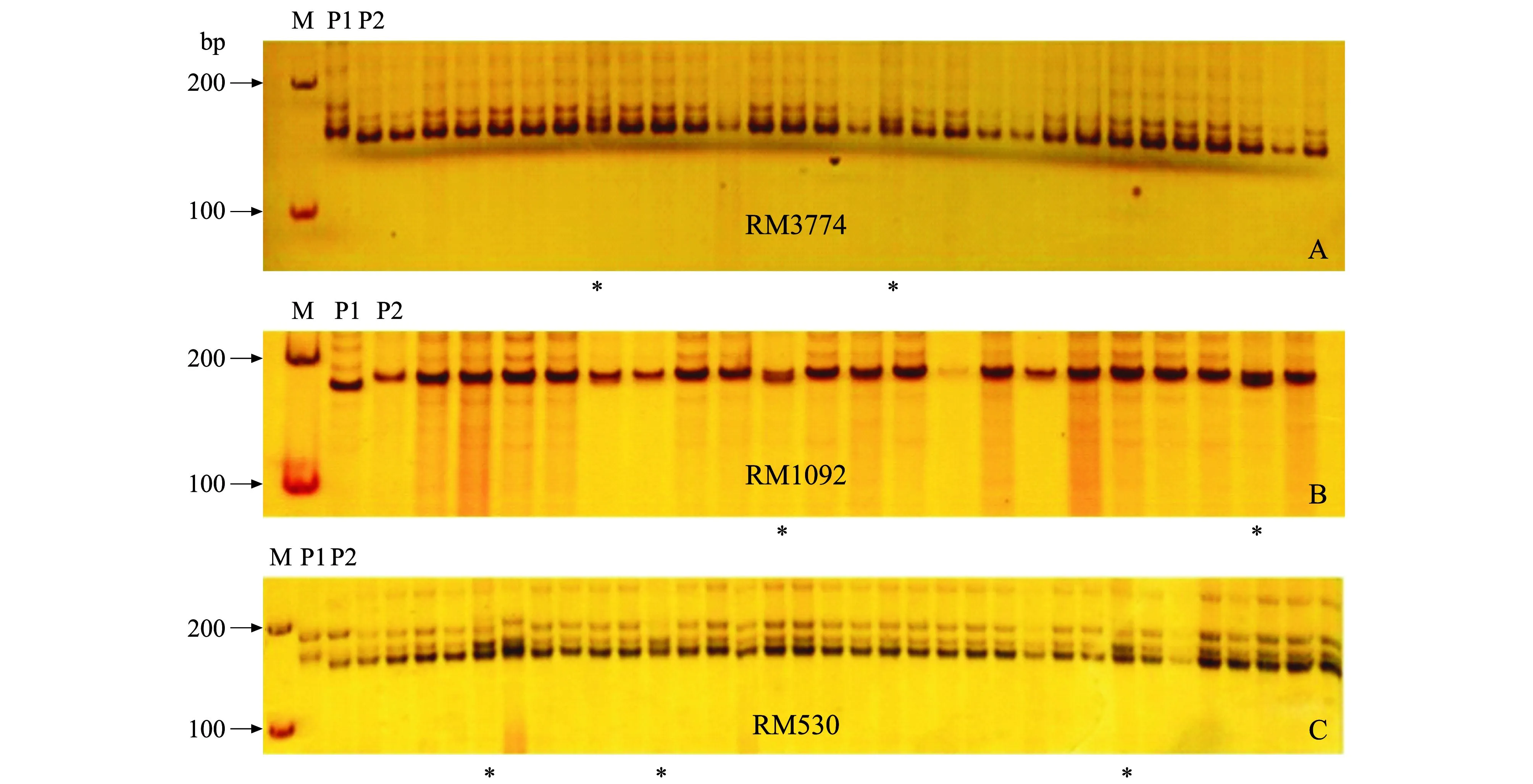
M:DNA标志物; P1:野生亲本;P2:突变亲本;*:基因型交换植株. M: 100 bp DNA ladder; P1: Wild parent; P2: Mutant parent; *: Genotypes of the plant exchanged. 图3 SSR标记RM3774,RM1092和RM530在F 2突变株中的差异分离 Fig.3 Separation of F 2 mutants by RM3774, RM1092 and RM530
根据 F2群体的表型和SSR分子标记分离的数据进行连锁分析,构建了局部连锁图,低纤维突变基因lcm527-1位于第2染色体微卫星标记RM3774和RM1092之间,与RM3774和RM1092的遗传距离分别为10.6 cM和5.1 cM(图4)。
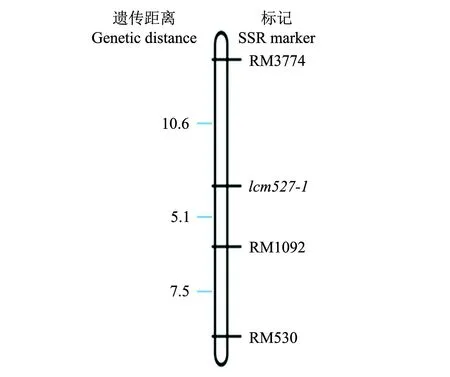
图4 水稻低纤维突变基因lcm527-1在2号染色体上的部分连锁图谱 Fig.4 Linkage map of a low cellulose mutant lcm527-1 on chr2
3讨论
水稻低纤维、脆性突变体的产生途径多样,如化学诱变、电离辐射、基因插入等,涉及的生理、生化、遗传和分子机制也不尽相同[19]。目前有关水稻低纤维脆秆相关基因主要有bc1、bc2、bc3、bc4、bc7、bc8、bc12、bc15等,分别位于水稻第3、5、2、6、1、7、9、4等染色体上[20-26]。其中bc1编码1种COBRA样蛋白,通过作用于纤维素的组装而影响细胞壁的厚度和机械强度;bc3通过影响纤维素次生细胞壁合成相关基因CesA4的表达丰度而导致纤维素合成减少,脆性增加;bc12主要参与组织细胞分裂和次生细胞壁加厚过程,其编码蛋白结合赤霉素合成关键基因,导致细胞生长和分裂受到影响;诸如上述不同调控水稻细胞壁成分的基因还有很多,它们以不同的方式作用,最终都导致水稻茎秆易折变脆。
本文的低纤维突变体LCM527-1生物学性状不仅表现出茎、叶、鞘、穗部枝梗均脆,抗折断力变弱,抗倒伏指数减小,Ca、Si等主要矿质成分下降,纤维素含量明显降低,还伴随着株高变矮、穗数减少、结实率下降等农艺性状的改变。农艺性状和纤维素等理化测定结果与刘斌美等[27]和王川丽等[28]的研究结果相近,而抗折断力和矿质元素等测定更加全面考察了突变体脆性相关因素。从实验结果可以推测突变基因lcm527-1的表达具有多效性,除了影响植株机械强度外,对植株营养生长和生殖生长也存在一定的负效应。本试验遗传分析和基因定位发现,lcm527-1位于第2染色体SSR标记RM3774和RM1092之间。鉴于与目前国内外已发表的相关或类似突变基因的比对,尤其是水稻2号染色体上有关控制低纤维突变体的基因至今发现较少的事实,作者认为该突变基因lcm527-1很可能是新的。推测本试验低纤维变异性状的产生可能受lcm527-1控制,通过表达产物影响水稻茎叶细胞壁化学成分的含量改变,或影响细胞壁成分的组装,从而导致细胞壁组装材料不足,壁变薄,结构形态有别于正常生长型水稻,最终表现出机械强度减小,抗倒伏能力减弱的低纤维特性。
由于本试验采用籼/籼交构建遗传群体的多态性较少,难以对该突变基因lcm527-1进行精细定位。因此,作者正构建相关新的籼/粳交遗传群体,并借助于上述基因初定位和多个性状的鉴定结果,旨在对该基因进行精细定位、测序和克隆等,进而深入了解突变基因的遗传表达机制,为阐明水稻植株纤维素合成、代谢及抗倒性分子机制提供理论依据。
参考文献(References):
[1]Sindhu A, Langewisch T, Olek A,etal. Maize Brittle stalk2 encodes a COBRA-like protein expressed in early organ development but required for tissue flexibility at maturity.PlantPhysiology, 2007,145(4):1444-1459.
[2]Taylor N G, Scheible W R, Cutler S,etal. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis.ThePlantCellOnline, 1999,11(5):769-779.
[3]Burton R A, Ma G, Baumann U,etal. A customized gene expression microarray reveals that the brittle stem phenotype fs2 of barley is attributable to a retroelement in the HvCesA4 cellulose synthase gene.PlantPhysiology, 2010,153(4):1716-1728.
[4]Nalam V J, Vales M I, Watson C J W,etal. Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (TriticumturgidumL.).TheoreticalandAppliedGenetics, 2006,112(2):373-381.
[5]Zhang B, Zhou Y. Rice brittleness mutants: A way to open the ‘Black Box’ of monocot cell wall biosynthesis free access.JournalofIntegrativePlantBiology, 2011,53(2):136-142.
[6]张水金,郑轶,朱永生,等.水稻脆性突变体研究进展.福建农业学报,2011,26(5):895-898.
Zhang S J, Zheng T, Zhu Y S,etal. Advance in research on brittleness mutant of rice.FujianJournalofAgriculturalSciences, 2011,26(5):895-898. (in Chinese with English abstract)
[7]吴超,朱丽,林凤.水稻脆茎突变体的研究进展.安徽农业科学,2007,35(17):5085-5086.
Wu C, Zhu L, Lin F. Research advance in brittle culm mutant of rice.JournalofAnhuiAgriculturalSciences, 2007,35(17):5085-5086. (in Chinese with English abstract)
[8]童川,童杰鹏,孙出,等.水稻脆性基因的功能研究进展.分子植物育种,2013,11(2):286-292.
Tong C, Tong J P, Sun C,etal. Research progress on functions of brittle culm gene in rice.MolecularPlantBreeding, 2013,11(2):286-292. (in Chinese with English abstract)
[9]Li Y, Qian Q, Zhou Y,etal.BRITTLECULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants.ThePlantCellOnline, 2003,15(9):2020-2031.
[10]Hirano K, Kotake T, Kamihara K,etal. RiceBRITTLECULM3 (BC3) encodes a classical dynamin OsDRP2B essential for proper secondary cell wall synthesis.Planta, 2010,232(1):95-108.
[11]Ookawa T, Ishihara K. Varietal difference of physical characteristics of the culm related to lodging resistance in paddy rice.JapaneseJournalofCropScience, 1992,61:419-425.
[12]郝虎林,魏幼璋,杨肖娥,等.供氮水平对稻株铁,锰,铜,锌含量和稻米品质的影响.中国水稻科学,2007,21(4):411-416.
Hao H L, Wei Y Z, Yang X E,etal. Effects of different nitrogen fertilizer levels on concentrations of Fe, Mn, Cu and Zn and grain quality in rice (Oryzasativa).ChineseJournalofRiceScience, 2007, 21(4):411-416. (in Chinese with English abstract)
[13]van Soest P J, Robertson J B, Lewis B A. Methods for dietary fibre, neutral detergent fibre and non starch polysaccharides in relation to animal nutrition,JournalofDairyScience, 1991,74:3583- 3597.
[14]Porebski S, Bailey L G, Baum B R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components.PlantMolecularBiologyReporter, 1997,15(1):8-15.
[15]韩美丽,陆荣生,霍秀娟,等.水稻DNA快速提取及RAPD分析体系的优化.江西农业学报,2009,21(5):1-3.
Han M L, Lu R S, Huo X J,etal. Rapid extraction of DNA and establishment of RAPD system in rice.ActaAgricultureJiangxi, 2009,21(5):1-3. (in Chinese with English abstract)
[16]Temnykh S, DeClerck G, Lukashova A,etal. Computational and experimental analysis of microsatellites in rice (OryzasativaL.): Frequency, length variation, transposon associations, and genetic marker potential.GenomeResearch, 2001,11(8):1441-1452.
[17]Temnykh S, Park W D, Ayres N,etal. Mapping and genome organization of microsatellite sequences in rice (OryzasativaL.).TheoreticalandAppliedGenetics, 2000,100(5):697-712.
[18]Lander E S, Green P, Abrahamson J,etal. MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations.Genomics, 1987,1(2):174-181.
[19]韦存虚,谢佩松,周卫东,等.水稻脆性突变体叶的解剖结构和化学特性.作物学报,2008,34(8):1417-1423.
Wei C X, Xie P S, Zhou W D,etal. Anatomical structure and chemical features of leaf in brittle mutant of rice.ActaAgronomicaSinica, 2008,34(8):1417-1423. (in Chinese with English abstract)
[20]Li X, Yang Y, Yao J,etal.FLEXIBLECULM1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice.PlantMolecularBiology, 2009,69(6):685-697.
[21]李文丽,吴先军.一个水稻脆性突变体的遗传分析与基因定位.核农学报,2006,20(6):500-502.
Li W L, Wu X J. Genetic analysis and gene mapping of a fragile rice mutant.JournalofNuclearAgriculturalSciences, 2006,20(6):500-502. (in Chinese with English abstract)
[22]Kotake T, Aohara T, Hirano K,etal. Rice brittle culm 6 encodes a dominant-negative form of CesA protein that perturbs cellulose synthesis in secondary cell walls.JournalofExperimentalBotany, 2011,62(6):2053-2062.
[23]Wu B, Zhang B, Dai Y,etal. Brittle culm 15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice.PlantPhysiology, 2012,159(4):1440-1452.
[24] Zhang M, Zhang B, Qian Q,etal. Brittle culm 12, a dual-targeting kinesin-4 protein, controls cell-cycle progression and wall properties in rice.ThePlantJournal, 2010,63(2):312-328.
[25]Yan C, Yan S, Zeng X,etal. Fine Mapping and Isolation of Bc7(t), allelic toOsCesA4.JournalofGeneticsandGenomics, 2007,34(11):1019-1027.
[26]Brown D M, Zeef L A H, Ellis J,etal. Identification of novel genes inArabidopsisinvolved in secondary cell wall formation using expression profiling and reverse genetics.ThePlantCellOnline, 2005,17(8):2281-2295.
[27]刘斌美,叶亚峰,章忠贵,等.一个籼稻脆性突变体的生物学特性及基因定位研究.植物遗传资源学报,2011,12(5):259-264.
Liu B M, Ye Y F, Zhang Z G,etal. Characterizations and gene mapping of a brittle culm and leaf mutant inindicarice.JournalofPlantGeneticResources, 2011,12(5):259-264. (in Chinese with English abstract)
[28]王川丽,王令强,牟同敏.水稻脆性突变体nbc(t)的主要特性和脆性基因的初步定位.华中农业大学学报,2012,31(2):159-164.
Wang C L, Wang L Q, Mou T M. Main characteristics and prime gene mapping of brittle culm mutantnbc(t) of rice.JournalofHuazhongAgriculturalUniversity, 2012,31(2):159-164. (in Chinese with English abstract)
Foundation item: Project supported by National Natural Science Foundation of China (No. 31260179).
*Corresponding author: Yang Bin, E-mail: yangbin48053@163.com
Biography: Wang Dawei, E-mail: wdwchem@163.com
Received: 2014-11-11; Accepted: 2014-12-12; Published online: 2015-05-19
URL:http://www.cnki.net/kcms/detail/33.1247.s.20150519.1252.004.html
Interruption effects of green leaf volatiles to a forest pest pine shoot beetle,Tomicusyunnanensis(Coleoptera, Scolytidae)
Wang Dawei1, Zhao Ning1, Ze Sangzi2, Zhu Jiaying3, Yang Bin1*(1.KeyLaboratoryofForestDisasterWarningandControlinYunnanProvince,SouthwestForestryUniversity,Kunming650224,China; 2.YunnanForestryTechnologicalCollege,Kunming650224,China; 3.CollegeofForestry,SouthwestForestryUniversity,Kunming650224,China)
SummaryThe pine shoot beetle,Tomicusyunnanensis, is one of the most damaging pests of Yunnan pine (Pinusyunnanensis) in southwestern China. In this study, the laboratory behavioral bioassays and field investigations were employed to examine the effects of three most abundant green leaf volatiles (GLVs) [(E)-2-hexenal, (E)-2-hexen-1-ol, (Z)-3-hexen-1-ol] from non-host trees on host location behavior of this forest pest. Y-tube olfactometer bioassays showed that adultT.yunnanensiswere significantly repelled by single GLVs compound or their blends. Field experiments showed that, the increase rates of infested twigs (fed byT.yunnanensis) in all the Yunnan pine groups treated with GLVs compound were lower than control group, and had significant differences from control group. The GLVs and their blends played a negative role in the feeding behavior ofT.yunnanensisin field, and decreased the damage to Yunnan pine. The above results show that, as the interrupting factors, GLVs from non-host plants have the potential to be used in field integrated managements ofT.yunnanensisin the future.
Key wordspine shoot beetle;Tomicusyunnanensis; green leaf volatiles; non-host plant; olfactory bioassay
CLC numberQ 968Document codeA


