Determination of Heavy Metals in Tobacco by ICP—MS and Analysis of Uncertainty
, , , *
1. Yunnan Institute of Metrology and Testing Technology, Kunming 650228, China; 2. College of Tobacco Science, Yunnan Agricultural University, Kunming 650201, China
1 Introduction
Due to the universality of environmental factors, the soil pollution is likely to cause excessive heavy metals in tobacco[1]. In the early 1990s, Cr, As, Hg, Cd and Pb were included in Hoffmann list of harmful gas substances[2-3]. Currently, the commonly used methods to detect trace elements and heavy metals in tobacco include atomic absorption spectrometry, atomic fluorescence spectrometry, inductively coupled plasma atomic emission spectrometry (ICP-AES) and ultraviolet-visible spectrophotometry[4-8]. Inductively coupled plasma mass spectrometry (ICP—MS) is a trace analysis technique rapidly developed in recent years, and it has been applied to the determination of heavy metals and trace elements in various matrices[9-10]. Uncertainty measurement is an indicator to assess the measurement level, and a basis to judge the reliability of measurement results. At present, uncertainty has been used in many laboratories and measurement institutions. During the measurement process by ICP—MS method, there are many factors affecting the accuracy of the results[11-13]. By the evaluation of uncertainty measurement, we can quantitatively evaluate the quality of measurement results. Based on CNAS-GL06: 2006EvaluationGuideforChemicalAnalysisUncertainty[14]and JJF 1059 evaluation of uncertainty measurement[15], this paper uses ICP—MS to determine the content of some heavy metals in tobacco, and evaluates the uncertainty about determination of heavy metals in tobacco, in order to provide a more rational and scientific basis for the correct evaluation and use of test data.
2 Materials and methods
2.1MaterialsandreagentsThe materials are selected from the middle leaves of Yunyan 99 in Yunnan Province. Concentrated nitric acid (65%, mass fraction) and hydrogen peroxide (30%, mass fraction), guarantee reagent; deionized water; Cr, Cu, Cd, As, Hg, Pb single element standard solution (100 μg/mL, National Research Center for Certified Reference Materials, relative uncertainty of Cr at 2.2%, relative uncertainty of other elements at 0.8%); mixed element standard solution (1 μg/mL, 1 mL of single element standard solution is suctioned and diluted with 2% nitric acid to 100 mL); 1 μg/mL Bi, Sc, Ge, In internal standard solution (diluted 100 times by 100 μg/mL mixed solution).
2.2InstrumentsCP224S electronic balance (accuracy of 0.000 1 g, Sartorius, Germany); Agilent 7 700X inductively coupled plasma mass spectrometer (ICP—MS) (Agilent, USA); EXCEL2010 microwave digestion system (Shanghai Yiyao); Millipore Mill-Q ultrapure water machine (Millipore, USA).
2.3InstrumentoperatingconditionsThe tuned liquid is used to tune the inductively coupled plasma mass spectrometer to the optimum operating conditions, and the optimized ICP-MS operating parameters: RF power (1.55 kW); sampling depth (10 mm); plasma gas flow rate (15.0 L·min-1); carrier gas flow rate (1.02 L·min-1); spray chamber temperature (2℃); He flow rate (4.5 mL·min-1); number of repetitions (3).
2.4MethodsAfter the main vein of the flue-cured tobacco samples is removed, they are dried at 60℃ for 2 h, and sifted through a 100-mesh sieve. About 0.4 g of samples are taken, accurately weighed and placed into the microwave digestion tank. 5 mL of nitric acid and 2 mL of H2O2are added for digestion. Temperature program is used, and it is heated to 120℃ at 20℃/s, holding 5 min; it is heated to 160℃ at 120℃/s, holding 20 min; it is heated to 190℃ at 160℃/s, holding 15 min. After completion of digestion, the digestion tank is taken, and when the digestion solution is cooled to room temperature, the digestion solution is transferred into 100 mL volumetric flask. A small amount of water is used for washing 3 to 4 times, and the washing liquid is put into a sample tube, and it is diluted with water to the mark and shaken. Meanwhile, the reagent blank solution is prepared.
2.5MathematicalmodelThe element is calculated as follows:
whereX(Cr, Cu, As, Cd, Hg, Pb) is the concentration of each element in the sample, mg/kg;cis the concentration of elements in the sample solution,μg/mL;Vis the total volume of sample, mL;mis the sample weight, g.
3 Results and analysis
3.1Standardcurveanddetectionlimit1 μg/mL standard stock solution is mixed with 0, 0.1, 1, 10 mL of element standard solution, respectively, and it is diluted with 2% nitric acid to a constant volume of 100 mL, shaken and prepared into different concentration standard solution. It is analyzed according to the aforesaid instrument working conditions. Linear regression is performed with ratio and concentration. The standard curve linear relationship among six elements is good, and the correlation coefficient is 1.0000 (Table 1).
Table1Linearrelationshipanddetectionlimit
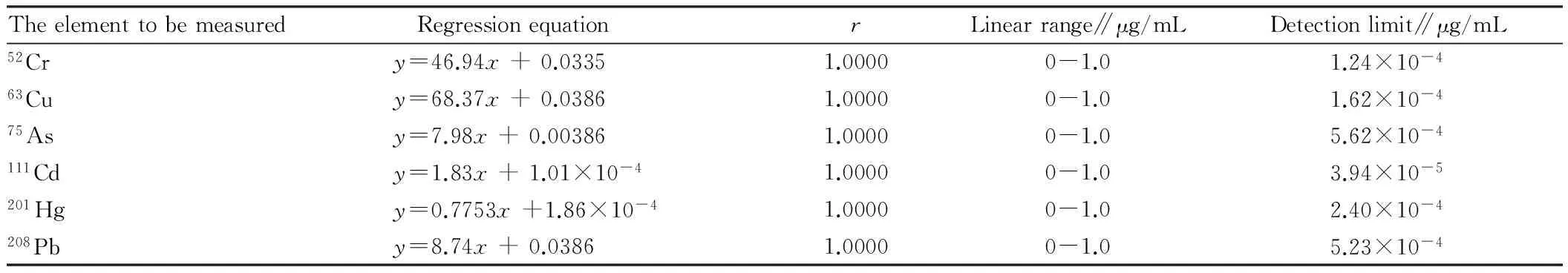
Theelementtobemeasured RegressionequationrLinearrange∥μg/mLDetectionlimit∥μg/mL52Cry=46.94x+0.03351.00000-1.01.24×10-463Cuy=68.37x+0.03861.00000-1.01.62×10-475Asy=7.98x+0.003861.00000-1.05.62×10-4111Cdy=1.83x+1.01×10-41.00000-1.03.94×10-5201Hgy=0.7753x+1.86×10-41.00000-1.02.40×10-4208Pby=8.74x+0.03861.00000-1.05.23×10-4
3.2Short-termstabilityoftheinstrumentThe solution standard material (indium, beryllium, bismuth mixed standard solution) calibrated by ICP—MS instrument is taken, and the continuous sample injection is conducted 10 times.RSDis calculated based on the ion count of each element.RSDof each element is less than 3%, indicating that the short-term stability of the instrument is good.
3.3Actualsampletestandprecision8 groups of 0.4 g tobacco samples are taken for operation based on the preparation method of sample solution, and determined according to the conditions in "2.2". The content of various elements is calculated as follows:52Cr 2.918 mg/kg;63Cu 3.635 mg/kg;75As 0.467 mg/kg;111Cd 3.118 mg/kg;208Pb 4.621 mg/kg;201Hg 0.035 mg/kg. The corresponding precision is 7.5%, 4.3%, 3.3 %, 1.5%, 5.7% and 11.0%, respectively. From the experimental data, the ICP—MS method plays a good role in determining six kinds of metal elements in tobacco.
3.4Evaluationofmeasurementuncertainty
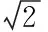
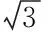
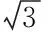
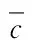
Table2Uncertaintyofelementsinstandardsolution
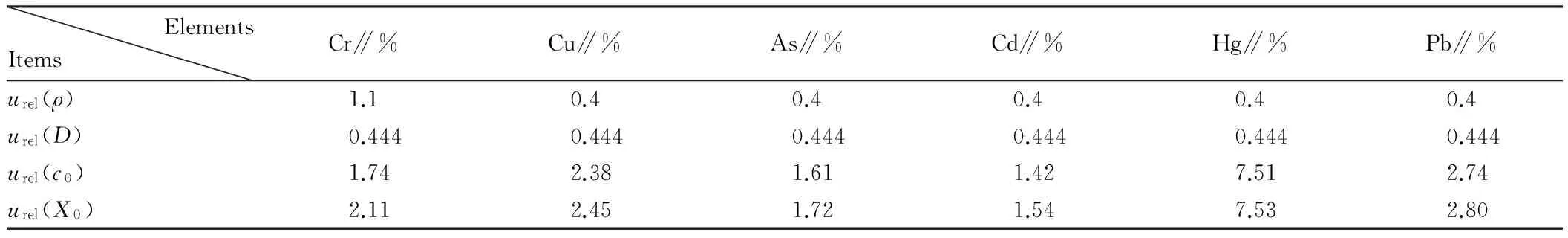
ElementsItemsCr∥%Cu∥%As∥%Cd∥%Hg∥%Pb∥%urel(ρ)1.10.40.40.40.40.4urel(D)0.4440.4440.4440.4440.4440.444urel(c0)1.742.381.611.427.512.74urel(X0)2.112.451.721.547.532.80
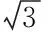
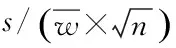
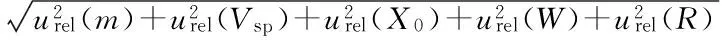
3.4.7Uncertainty of determination results. Using the ICP-MS method, the content of Cu, As, Cd, Hg, Pb in tobacco is determined. When the sample weight is 0.4105 g, the confidence probability is 95%,k=2, and the determination results are as follows: Cr (2.92±0.24) mg/kg, Cu (3.64±0.24) mg/kg, As (0.467±0.025) mg/kg, Cd (3.12±0.15) mg/kg, Hg (0.035±0.006) mg/kg, Pb (4.62±0.36) mg/kg.
Table3Calculationofexpandeduncertainty

ElementsItemsSymbolCrCuAsCdHgPbWeight∥10-5kgurel(m)1.9981.9981.9981.9981.9981.998Constantvolume∥%urel(Vsp)0.0840.0840.0840.0840.0840.084Standardsolution∥%urel(X0)2.112.451.721.547.532.80Instrumentstability∥%urel(W)1.7321.7321.7321.7321.7321.732Repeatability∥%urel(R)2.751.521.180.543.912.01Relativecombineduncertainty∥%urel(C)3.883.362.712.388.663.86Theaveragecontentofelement∥mg/kgC2.9183.6350.4673.1180.0354.621Standarduncertainty∥mg/kgu(C)0.120.120.0130.0740.0030.18Expandeduncertainty∥mg/kgU0.240.240.0250.150.0060.36
4 Conclusions
The results show that the content of test elements in standard solution and sample repeatability are the main sources of uncertainty in the determination of heavy metals in tobacco leaves by ICP-MS. The uncertainty caused by sampling amount and constant volume of test sample solution can be ignored. The standard solution dilution and linear fitting, stability of the instrument’s response to the signal, uniformity of the sample, preparation of the sample, operating skills of the experimenters, can cause great uncertainty. By calculation, it can be found that when the content of element in the sample is extremely low (especially the mercury element), the uncertainty components brought by standard curve fitting make the most important contribution to the total uncertainty. Therefore, by selecting feasible test scheme and increasing the concentration of test elements in standard solution and samples, we can effectively reduce uncertainty and ensure the accuracy and reliability of the measurement results.
[1] SHI HZ, LIU GS, CHANG SM,etal. Research progress on heavy metal in tobacco and agricultural measures of harm reduction[J].Acta Tabacaria Sinica,2011,17(3) : 89-94.(in Chinese).
[2] ZHANG YL, ZHOU HP. Summary on study of heavy metal elements in tobacco[J].Tobacco Science & Technology,2004(12) : 20-23,27.(in Chinese).
[3] ZHU SX, JIANG JL, SI W,etal. Study on regionalism and correlation of seven heavy metals in tobacco leaf[J].Tobacco Science & Technology,2015,48(3) : 47-52.(in Chinese).
[4] HUANG X, XU ZG. Determination of the heavy metal elements in tobacco by ICP-MS with microwave digestion[J].Journal of Zhejiang University(Sciences Edition),2007,34(6) : 658-660.(in Chinese).
[5] SHI J, LI L, HU QY,etal. Advance in determination of trace elements and heavy metals in tobacco[J].Tobacco Science & Technology,2006(2) : 40-45.(in Chinese).
[6] ZHANG XJ, ZHU FP, HU QY,etal. Simultaneous determination of Cr, Ni, Cu, As, Tl and Pb in soil by inductively coupled plasma mass spectrometry[J].Acta Tabacaria Sinica,2009,15(6) : 18-22.(in Chinese).
[7] HE B, CHEN ZY, WANG L,etal. Study on the determination of arsenic and mercury in tobacco by atomic fluorescence spectrometry[J]. Journal of Hebei Agricultural Sciences,2010,14(4) : 154-155.(in Chinese).
[8] SHI J, LI L, HU QY,etal. Simultaneous determination of Cr, Ni, As, Se, Cd, Hg and Pb in tobacco with ICP-MS[J]. Tobacco Science & Technology,2006(12) : 29-34,37.(in Chinese).
[9] DONG ZH, LU LX, LIU ZG,etal. Determination of heavy metals in ceramic food packaging containers by ICP-MS[J].Spectroscopy and Spectral Analysis,2012,32(11) : 3139-3141.(in Chinese).
[10] LI X, PANG YQ, ZHU FP,etal. Determination of six heavy metal elements in sidestream cigarette smoke by inductively coupled plasma mass spectrometry[J].Tobacco Science & Technology,2015,48(3) : 28-32.(in Chinese).
[11] CHEN LY. Evaluation of the uncertainty for determining the content of heavy metal in the soil by ICP-AES[J].Chemical Analysis And Meterage,2004,13(3) : 6-8.(in Chinese).
[12] ZHAO T, HAO L, WANG L,etal. Determination of trace elements in mussel by ICP-MS and evaluation of uncertainty[J].Chinese Journal of Spectroscopy Laboratory,2008,25(5) : 874-877.(in Chinese).
[13] CHEN J, QIAO F, JIN HY,etal. Evaluation of the uncertainty for the determination of heavy metals in strychni semen by ICP- MS[J].Chinese Journal of Pharmaceutical Analysis,2013,33(12) : 2176-2182.(in Chinese).
[14] China National Accreditation Service for Conformity Assessment. The evaluation guidelines of uncertainty measurement for chemical analysis: CNAS-GL06: 2006[S]. Beijing: Chinese Metrology Press,2006.(in Chinese).
[15] The State Bureau of Quality and Technical Supervision.Measurement uncertainty evaluation and expression: JJF 1059. 1-2012[S]. Beijing: Chinese Metrology Press,2012.(in Chinese).
 Asian Agricultural Research2016年10期
Asian Agricultural Research2016年10期
- Asian Agricultural Research的其它文章
- Static and Dynamic Analysis on the Environmental Efficiency of 267 Cities in China during 2004-2012
- Analysis of Patterns and Benefits of Cultivated Land Transfer in Rural Areas in the Loess Plateau
——A Case Study of Yuanzhou District of Ningxia - Genetic Variation Analysis of Watermelon Genomes with Different Ploidy
- A Study on the Adaptability of Yunnan Tea Cultivars in Southern Fujian
- An Empirical Study on Rural Economic Growth in Hubei Province Based on New C-D Production Function
- Evaluation and Comparative Study of Industrial Competitiveness in the Beijing-Tianjin-Hebei Area
