Extraction,characterization and biological studies of phytochemicals from Mammea suriga
Mahesha M.Poojary,Kanivebagilu A.Vishnumurthy, Airody Vasudeva Adhikari
Department of Chemistry,National Institute of Technology Karnataka,Surathkal 575025,India
Extraction,characterization and biological studies of phytochemicals from Mammea suriga
Mahesha M.Poojary,Kanivebagilu A.Vishnumurthy, Airody Vasudeva Adhikari✽
Department of Chemistry,National Institute of Technology Karnataka,Surathkal 575025,India
Mammea suriga;
Phytochemical analysis;
Antimicrobial activity;
Antioxidant assay;
FT-IR analysis
The present work involves extraction of phytochemicals from the root bark of a well-known Indian traditional medicinal plant,viz.Mammea suriga,with various solvents and evaluation of their in vitro antimicrobial and antioxidant activities using standard methods.The phytochemical analysis indicates the presence of some interesting secondary metabolites like flavonoids,cardiac glycosides, alkaloids,saponins and tannins in the extracts.Also,the solvent extracts displayed promising antimicrobial activity against Staphylococcus aureus,Bacillus subtilis and Cryptococcus neoformans with inhibition zone in a range of 20-33 mm.Further,results of their antioxidant screening revealed that aqueous extract(with IC50values of 111.51±1.03 and 31.05±0.92 μg/mL in total reducing power assay and DPHH radical scavenging assay,respectively)and ethanolic extract(with IC50values of 128.00±1.01 and 33.25±0.89 μg/mL in total reducing power assay and DPHH radical scavenging assay,respectively)were better antioxidants than standard ascorbic acid.Interestingly,FT-IR analysis of each extract established the presence of various biologically active functional groups in it.
©2015 Xi’an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Nowadays,the use of natural formulations as medicine is gaining more popularity.In fact,several natural formulations which make use of herbal extracts have been found to be safer medicines with minimum side effects when compared to chemical drugs.According to the Bulletin of the World Health Organization(WHO),around 65%of the world’s population relied on medicinal plants as their primary healthcare source[1]. Also,it has been estimated that about 50%of the medicines developed since 1980 have been natural products,their derivatives,or their analogs[2,3].Further,it has been predicted that approximately 25%of the currently used modern medicines are derived from plants[3].Amongst them,analgesics(morphine), cardiotonics(digoxin),anticancer drugs(paclitaxel and the vinca alkaloids)and the antimalarials(quinine and artemisinin) are noteworthy[4].
✽Corresponding author.Tel.:+91 824274000x3203;fax:+91 8242474033.
E-mail addresses:avachem@gmail.com, avchem@nitk.ac.in(A.Vasudeva Adhikari).
Peer review under responsibility of Xi’an Jiaotong University.
2095-1779©2015 Xi’an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BYNC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Mammea suriga is a familiar endemic medicinal plant belonging to the family Clusiaceae,which grows abundantly in Western Ghats of India.The plant is well known for its diverse applications in folk medicine in our region.It is a large tree,growing to a height of 12-18 m and its bud is used as a minor spice.The flower buds possess mild stimulant,carminative and astringent properties and are used in the treatment of dyspepsia and haemorroid[5].Its root-paste is widely used as medicine to cure partial headache[6].The roots of Mammea longifolia(Wight)Planch and Triana are shown to contain interesting molecules,viz.coumarins surangin A,surangin B and taraxerol[7].It was reported that the extract of stem bark of M. longifolia showed one more coumarin named surangin C[8]. Indeed,Mammea coumarins have been investigated to possess a wide array of biological activities.These coumarins are good radical scavengers[9]and are cytotoxic to human tumor cells[9-13]that suppress tumor growth in animal models[14]and also they exhibit anti-HIV[15],antifungal[16]and antibacterial[13,17]activities.
Encouraged by the reported medicinal properties of M.suriga genus and prompted by the usage of root bark extract of M.suriga in many traditional medicinal formulations of our region,it has been contemplated to concentrate our studies on phytochemical studies of this rarely explored species and to investigate its medicinal properties. Accordingly,in the present study,the phytochemicals root bark of M. suriga was extracted with various solvents and these extracts were studied for their in vitro antimicrobial and antioxidant properties. Their antimicrobial activities were determined using disc diffusion assay and the antioxidant activity was evaluated by reducing power assay and DPPH radical scavenging method.In addition,Fourier transform infra-red(FT-IR)spectral analysis of crude extract was carried out in order to predict the presence of various active functional groups which are responsible for their biological activities.
2. Methods
2.1. Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl(DPPH),trichloro acetic acid and ascorbic acid were purchased from Sigma-Aldrich(St.Louis,USA).Meullar Hinton agar media and fluconazole were purchased from HiMedia (Mumbai,India).Clairo mono cefoperazone sublactum was procured from Span Diagnostics Ltd.(Surat,India).Cefoxitin and ticarcilin clavulanic acid were purchased from Oxoid Ltd.(Basingstoke,UK).
2.2. Plant material
The root barks of M.suriga were collected from the Western Ghats of Karnataka,India,during winter season.Plant parts were packed immediately after picking and kept in cold(-20°C)dark storage until processed.The plant specimen was identified with the help of an expert,Prof.P.D.Ramya Rai(Head,Department of Botany,Alva’s College,Mangalore University,India).Voucher specimens(No.T 6789/2011)were prepared and deposited in the herbarium of Alva’s College,Moodbidri,India.
2.3. Extracts preparation
The root bark of M.suriga was washed with water and shade dried at room temperature for 15 days.After drying,the sample was coarsely powdered with a grinder.The dry sample(60 g)was sequentially extracted with petroleum ether,chloroform,ethyl acetate,ethanol and water(200 mL×2,each solvent)for 14 h under constant agitation.The extracts were evaporated to dryness in vacuo and stored in cold until used.The extraction yield was expressed as

2.4. Preliminary phytochemical assay
Freshly prepared extracts were subjected to standard methods of phytochemical analyses[18,19]to detect the presence of phytoconstituents,viz.flavanoids,carbohydrates,glycosides,saponins, tannins,proteins and alkaloids.
2.5. Antibacterial screening
In vitro antibacterial screening of the plant extracts in DMF was carried out against five pathogenic strains,viz.Escherichia coli (ATCC-25922),Staphylococcus aureus(ATCC-25923),Pseudomonas aeruginosa(ATCC-27853),Bacillus subtilis(recultured) and Serratia marcescens(recultured)by the disk-diffusion method[20].To standardize the inoculum density for a susceptibility test,BaSO4turbidity standard,equivalent to a 0.5 McFarland standard,was used.A 0.5 McFarland standard was prepared as described by Andrews et al.[21].The dried surface of a Mueller-Hinton agar plate was inoculated by streaking the swab over the entire sterile agar surface.Sterile filter paper discs of 6 mm diameter were loaded with 20 μL of the plant extract dissolved in DMF(50 mg/mL)to yield a final concentration of 1000 μg/disc.The paper discs were allowed to evaporate and then placed aseptically on the surface of the inoculated agar plates.Standard clairo mono cefoperazone sublactum,cefoxitin and ticarcilin clavulanic acid were used as positive controls while DMF served asa negativecontrol.The experimentwas performed in triplicates under aseptic conditions.Plates were kept at 4°C for 15 min for diffusion and then incubated for 18 h at 37°C.At the end of the incubation period,the antibacterial activity was evaluated by measuring the inhibition zones.The mean value of the diameter of the inhibition zone of the triplicates sets was taken as the final value.
2.6. Antifungal screening
In vitro antifungal testing of the plant extracts in DMF was carried out against two pathogenic fungi Candida albicans(recultured) and Cryptococcus neoformans(recultured)by the disk-diffusion method on a potato dextrose agar plate.The Petri dishes were prepared in triplicates and maintained at 37°C for 2-5 days. Flucanazole was used as the standard.The measurements were carried out as described in the antibacterial assay above.
2.7. Total reducing power assay
Total reducing power of plant extracts was evaluated as described by Barros et al.[22]with minor modifications.Ethyl acetate,ethanolic and aqueous extracts were selected for the analysis.However,petroleum ether and chloroform extracts were not selected because of their insolubility in the experimental solvent system.In the procedure,1.0 mL of plant extract solutions(final concentration 20-1000 μg/mL)was mixed with sodium phosphate buffer(pH 6.6,0.2 M,2.5 mL)and
potassium ferricyanide(1%(w/v),2.5 mL).The mixture was incubated at 50°C for 20 min.Trichloroacetic acid(10%, 2.5 mL)was then added,and the mixture was centrifuged at 1000 rpm for 10 min.The supernatant liquid(2.5 mL)was mixed with deionized water(2.5 mL)and ferric chloride solution(0.1%,0.5 mL),and the absorbance was measured spectrophotometrically at 700 nm.The extract concentration providing 0.5 of absorbance(IC50)was calculated from the graph of absorbance against extract concentration.In the experiments,ascorbic acid was used as the standard.
2.8. DPPH radical scavenging assay
The free radical scavenging capacity of the extracts was determined using DPPH(2,2-diphenyl 1-picryl hydrazyl)radical as described by Barros et al.[22]with minor alterations.Each sample stock solution(1.0 mg/mL)was diluted to final concentrations of 20-1000 μg/mL with 95%methanol.Various concentrations of plant extracts(0.3 mL)were mixed with freshly prepared methanolic solution containing DPPH radicals(0.004% (w/v),2.7 mL).The mixture was shaken vigorously and allowed to stand for 60 min in the dark(until stable absorption values were obtained).The extent of reduction of the DPPH radical was determined by measuring the absorption at 517 nm.Ascorbic acid was used as a reference standard and DPPH solution was used as the control.The radical scavenging activity(RSA)was calculated as a percentage of DPPH discoloration using the following equation:

The scavenging activity was plotted against concentration and IC50(the extract concentration providing 50%of radicals scavenging activity)value was calculated from the graph by linear regression analysis.
2.9. Infra-red spectroscopy
Infra-red spectra of the crude samples were recorded to detect various functional groups responsible for biological activities. Perfectly dried powder/paste of the extracts was placed on the sample chamber of FT-IR spectrophotometer and the spectra were recorded in the range of 3600-600 cm-1on Nicolet Avatar 330 FTIR spectrometer.Important absorption frequencies appeared in functional group region as well as fingerprint region of the spectra were noted down.
2.10. Statistical analysis
All the in vitro experimental results were presented as mean±SEM of three parallel measurements and data were evaluated using student’s t-test.P-values<0.05 were regarded as significant.Results were processed by Microsoft Excel(2007)and BioStat.
3. Results
3.1. Preliminary phytochemical investigation
The yield,color and consistency of different extracts of M.suriga are given in Table 1.It was observed that ethanol extraction produced maximum yield of phytochemicals about 11.5%,whereas petroleum ether extraction yielded only 7.4%.The results of the phytochemical screening of root bark extracts of M.suriga are summarized in Table 2.The results indicated the presence of large amounts of flavonoids,cardiac glycosides and alkaloids in the root bark extracts.Further,active compounds of alkaloids and cardiac glycosides were present in all solvent extracts.Some extracts showed the presence of small amounts of saponins,tannins and proteins.In all the extracts,carbohydrates and reducing sugars were found to be absent.Only aqueous extract showed the presence of proteins to a weaker extent.
3.2. Antibacterial activity
The results of antibacterial screening of root bark extracts are summarized in Table 3.It was observed that E.coli exhibited a moderate inhibition zone 9-10 mm for all the extracts.Petroleum ether,ethyl acetate,chloroform,ethanolic and aqueous extracts of M.suriga showed the inhibition zone between 28 and 33 mm against S.aureus(Fig.1(A)).Among these,petroleum ether extract(32 mm),ethanolic extract(32 mm)and aqueous extract (33 mm)displayed a very good antibacterial activity when compared to other extracts.Presence of active alkaloids and flavonoids in these extract may be the reason for good antibacterial activity.It was observed that only aqueous extract of M.suriga was effective against the bacteria P.aeruginosa and no other extracts displayed any significant activity at the tested concentrations.All the extracts displayed moderate activity ranging from 20 to 25 mm against B.subtilis(Fig.1(B)).Furthermore,against S. marcescens only non-polar solvent extracts,viz.petroleum ether extract and chloroform extract,showed a remarkable activity with inhibition zone 9 mm each and no other extracts exhibited any activity under tested concentrations.The solvent DMF did not show any activity.
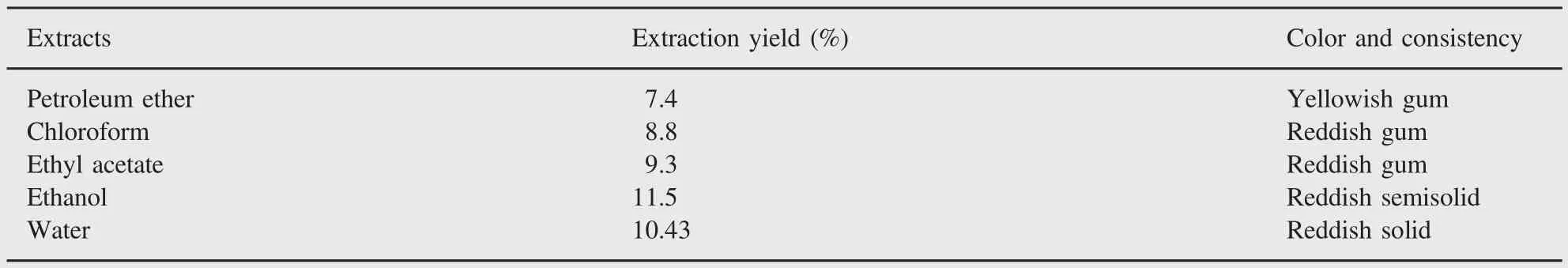
Table 1 Yield,color and consistency of root bark extracts of M.suriga.
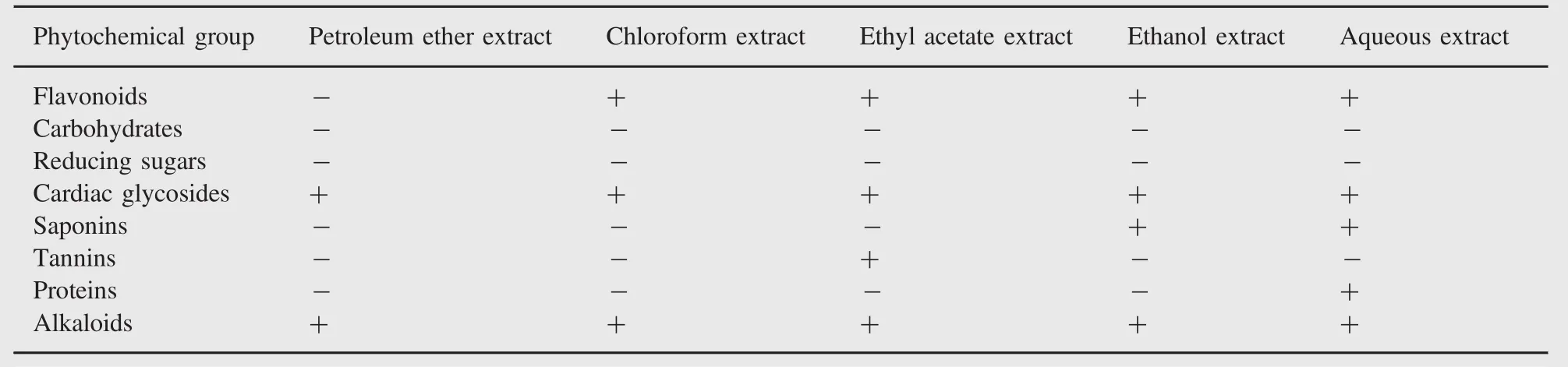
Table 2 Preliminary phytochemical screening.
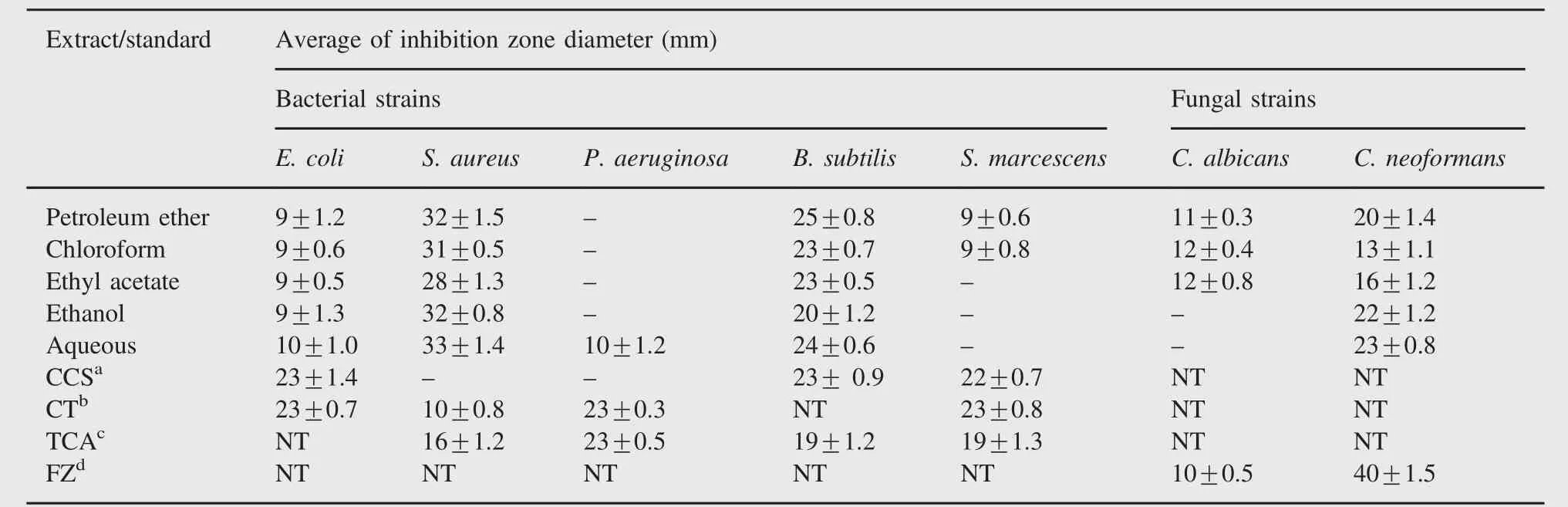
Table 3 Antibacterial and antifungal activities of different extracts of M.suriga.
3.3. Antifungal activity
The antifungal activity of different extracts from root bark of M.suriga is tabulated in Table 3.Only petroleum ether,ethyl acetate and chloroform extracts of M.suriga showed a moderate activity against the pathogenic fungus C.albicans with the inhibition zone 11-12 mm.However,ethanolic and aqueous extracts did not show any activity.On the other hand,all the extracts showed a significant activity against the fungus C. neoformans.The maximum zone of inhibition 23±0.8 mm was observed for aqueous extract,while minimum activity was found for chloroform extract(13±1.1 mm).From these results it can be noted that extracts of M.suriga contain certain phytochemicals which inhibit fungal metabolism.
3.4. Total reducing power
Reducing power of various extracts and their IC50values are represented in Figs.2 and 3,respectively.In general,the lower the IC50value,the higher the antioxidant activity or the free radical scavenging activity would be.From the results of antioxidant testing of ethyl acetate,ethanolic and aqueous extracts of M. suriga,it was observed that aqueous extract as well as ethanolic extract exhibited a very good reducing capacity with low IC50values of 111.51±1.03 and 128.00±1.01 μg/mL,respectively, which were better than that of standard ascorbic acid that showed an IC50value 136.80±0.46 μg/mL.However,ethyl acetate extract displayed a moderate activity with an IC50value 174.77±0.32 μg/ mL.The observed higher reducing power of aqueous extract as well as ethanolic extract is attributed to presence of various chemical constituents including alkaloids and flavonoids.
3.5. DPPH radical scavenging
The radical scavenging activity and IC50values of different extracts of M.suriga are summarized in Figs.4 and 5,respectively.Normally,the higher%RSA and lower IC50values indicate a higher antioxidant activity.Among the eight different concentrations used in the study(20-1000 μg/mL),concentration of 1000 μg/mL showed the highest scavenging activity 99.10%and 98.10%in aqueous and ethanolic extracts,respectively,whereas ascorbic acid at the same concentration showed 97.3%,which were very close to each other(Fig.4).Similarly,aqueous and ethanolic extracts exhibited an excellent radical scavenging activity with IC50values 31.05±0.92 and 33.25±0.89 μg/mL, respectively,showing even better antioxidant behavior when compared to standard ascorbic acid(IC50=75.30±0.34 μg/mL). On the other hand,ethyl acetate extract displayed very weak
antioxidant behavior with an IC50value 236.2±0.49 μg/mL.The variation in the antioxidant activity among the extracts was mainly attributed to presence of different extent of flavonoids and alkaloids.
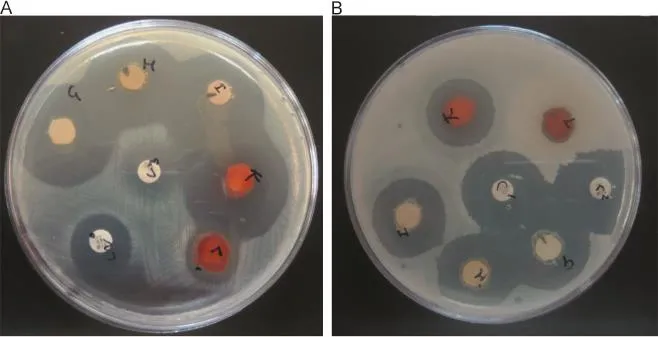
Fig.1 Petri dishes showing an antimicrobial activity with inhibition zones.(A)Staphylococcus aureus and(B)Bacillus subtilis.

Fig.2 Total reducing power assay of extracts.
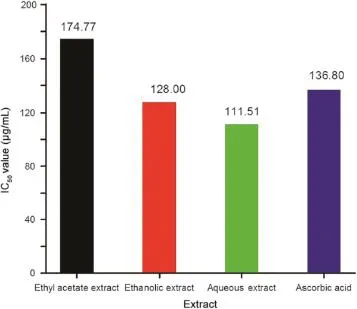
Fig.3 IC50values for different extracts in reducing power assay.

Fig.4 DPPH scavenging assay of extracts.
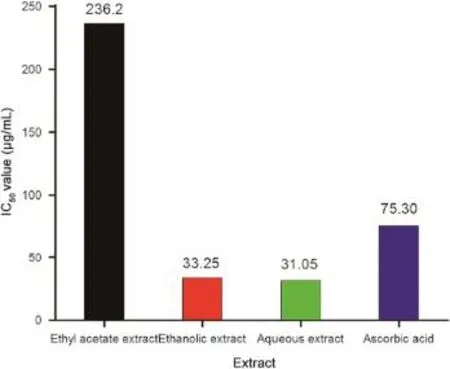
Fig.5 IC50values for different extracts in DPPH radical scavenging assay.
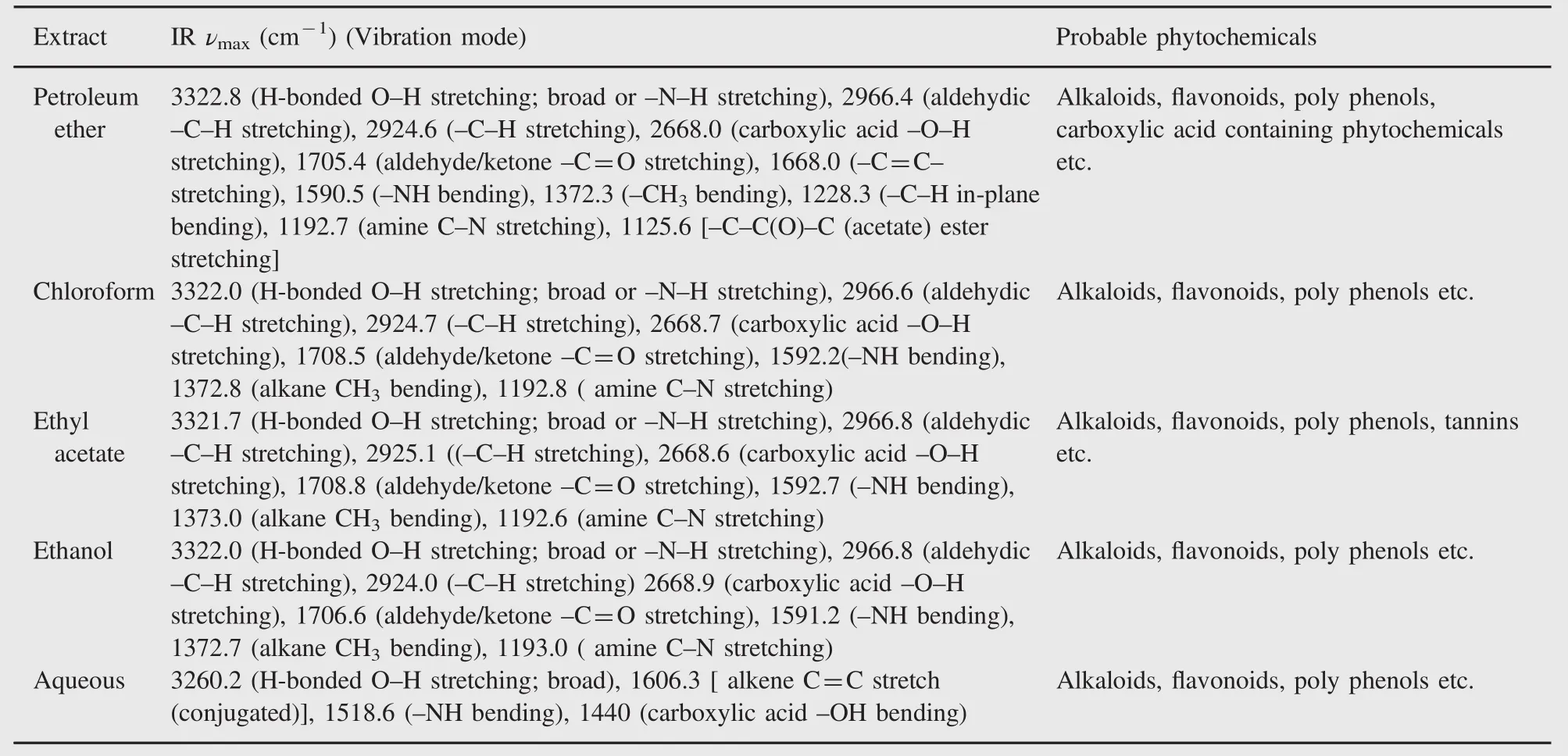
Table 4 Major bands observed in the FT-IR spectra of various extracts.
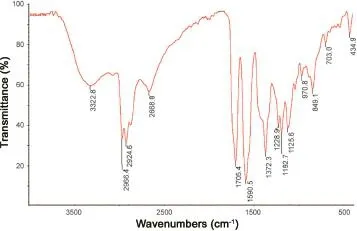
Fig.6 FT-IR spectrum of petroleum ether extract.
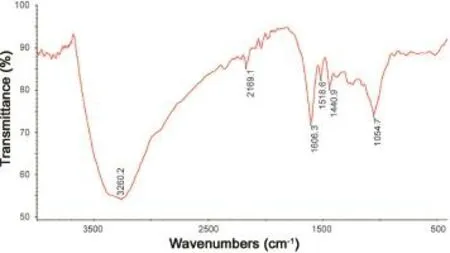
Fig.7 FT-IR spectrum of aqueous extract.
3.6. FT-IR spectral analysis
FT-IR spectral analysis data of various extracts revealed the occurrence of multiple functional groups in them.Spectral data of most of the extracts confirmed the presence of bioactive functional groups such as-OH,>NH,-CHO,-COOH and -COOR.All extracts exhibited the presence of a broad peak for hydrogen bonded-OH stretching in functional group region. Important IR absorption frequencies are tabulated in Table 4 and FT-IR spectra of a representative extract are shown in Figs.6 (petroleum ether extract)and 7(aqueous extract).
4. Discussion
4.1. Phytochemical screening
It is interesting to note that the extracts of M.suriga showed the presence of flavonoids and alkaloids in abundant quantity.Several flavonoid derivatives were reported to be effective antimicrobial substances against different microorganisms.Their mode of activity may be due to their ability to complex with extracellular and soluble proteins as well as to complex with bacterial cell wall.The flavonoids being more lipophilic may also disrupt microbial membranes.In addition to being effective against bacteria,these compounds exhibit inhibitory effects against viruses and parasites[23].It has been well established that flavonoids in nature are the potential antioxidants. Quercetinm,a flavonoid that exists in numerous plants,possesses a very good antioxidant activity and hence it is currently being used in health food stores[24].Another class of natural products,viz.alkaloids, are complex heterocyclic nitrogenous compounds commonly found to possess antimicrobial properties.They are quite useful against viral and protozoan infections.In case of highly aromatic planar quaternary alkaloids,their mechanism of action is due to their ability to intercalate with DNA[23].Saponins,which are amphipathic glycosides,may be mono-or polydesmodic,depending on the number of attached sugar moieties.These bio-active ingredients are commonly present in licorice root(Glycyrrhizaglabra),and possess expectorant,bacteriostatic and antiviral activities[25].The ginsenosides are a class of natural saponins
from Panax ginseng,and are believed to be responsible for immunostimulant and antinociceptive(pain-relieving)properties[26].Similarly, tannins are well-known for their antimicrobial and antioxidant activities [27].According to some reports,certain tannins are considered to be potential cytotoxic and antineoplastic agents[28].Against this background,our work on M.suriga proves to be quite interesting due to the presence of all the above-mentioned important classes of bioactive phytochemicals in the plant.Further,it provides scientific validation for usage of the plant extracts in folk medicine in our region.The present work on preliminary phytochemical screening of root bark of M.suriga certainly encourages future advanced research activities on chromatographic isolation of these compounds in their pure state.
4.2. Antimicrobial assay
At present,there is a lot of scope and importance for development of new antimicrobials in treatment of microbial infections.The latest trend shows that the plant-based antimicrobial agents have an enormous therapeutic potential since they do not show any major side effects on human beings[29].In our present work,the root bark extracts of M.suriga showed a good antimicrobial activity against two pathogenic bacteria,viz.S.aureus and B. subtilis,and a fungus C.neoformans.Hence,it can be expected that the plant possesses unique phytochemicals which are responsible for inhibition of both bacterial and fungal metabolism.
4.3. Antioxidant assay
According to Halliwell et al.[30],an antioxidant is considered to be "any substance that,when present at low concentrations compared to that of an oxidizable substrate,significantly delays or inhibits oxidation of that substrate".Generally,antioxidants present in the body normally scavenge the free radicals produced and prevent the damage caused by them.They can greatly reduce the harm due to oxidants by the free radicals before they can attack the cells and prevent damage to lipids,proteins,enzymes,carbohydrates and DNA.In effect,an imbalance between antioxidants and reactive oxygen species results in oxidative stress,leading to cellular damage [31].Such oxidative stress has been linked to aging and certain neurodegenerative diseases like Parkinson’s disease,Alzheimer’s disease,multiple sclerosis and amyolotrophic lateral sclerosis[32]. Hence,there is an urgent need to develop potent new antioxidants which can effectively reduce the above-mentioned problems.Rathee et al.[33]reported that methanol extract as well as water-ethanol extract of M.longifolia bud showed an impressive antioxidant activity via their ability to scavenge various biologically relevant reactive oxygen species(ROS)and inhibit lipid peroxidation.In the present work,aqueous and ethanolic extracts of root bark of M. suriga showed a very good antioxidant activity,even better than that of the standard reference ascorbic acid.It has been predicted that the bioactivity of these extracts is due to their respective phenolics, flavonoids and alkaloids contents.The observed lower IC50values of these extracts support the significance of M.suriga root bark extracts as promising natural source of antioxidants and hence they can be used in nutritional or pharmaceutical areas for the prevention of free-radical-mediated diseases.
4.4. Functional group analysis
In any bio-organic molecule,its functional groups influence its biological activity considerably as they contribute significantly to their inherent acid-base properties,solubility,partition coefficient, crystal structure,stereochemistry,and so on.All these properties are supposed to influence the absorption,distribution,metabolic extraction,and toxicity of bioactive molecules[34].Hence, functional group analysis plays a vital role in understanding the overall physicochemical properties of the extract.Also,identification of the functional group helps to evaluate their structureactivity relationships.In the present work,FT-IR spectral analysis of the root bark extracts of M.suriga showed the presence of phytochemicals carrying hydrogen bonded-OH functional group. It is well established that hydroxyl functionality is an integral part of most of the phenolic phytochemicals such as flavonoids and tannins.Recent studies show that several plant products,including polyphenolic substances(e.g.,flavonoids and tannins)and various herbal extracts,show antioxidant[35-38]and anti-inflammatory activities[38].Our results indicate that the root bark extracts of M.suriga contain various biologically active functional groups, viz.alcoholic,ester,aldehydic etc.,and hence we can confirm that the plant possesses bioactive phytochemicals.
5. Conclusions
The present study revealed that the root bark extract of M.suriga displayed strong antimicrobial and antioxidant activities.Therefore,it suggests that root bark extracts of M.suriga are a potential source of natural antimicrobial and antioxidant agents.It is hoped that the present study would direct to the establishment of some compounds that could be used to investigate new and more potent antimicrobials and antioxidants of the plant origin.Further research is needed to isolate and identify active molecules from the crude extract and also to evaluate in detail in vivo biological activities of such isolated compounds.
Acknowledgments
The authors are grateful to Dr.Harish Poojari(Department of Chemical Engineering,National Institute of Technology Karnataka, Surathkal,India)for his assistance during the antimicrobial analysis and thankful to Mrs.P.D.Ramya Rai(Department of Botany, Alva’s College,Moodbidri,India)for helping in identification of plant species.
[1]D.S.Fabricant,N.R.Farnsworth,The value of plants used in traditional medicine for drug discovery,Environ.Health Perspect. 109(Suppl.1)(2001)S69-S75.
[2]D.J.Newman,G.M.Cragg,Natural products as sources of new drugs over the last 25 years,J.Nat.Prod.70(2007)461-477.
[3]R.Verpoorte,Overview and introduction,in:L.Mander,H.-W.Lui (Eds.),Comprehensive Natural Products-II-Chemistry and Biology, vol.3,Elsevier Science,Oxford,UK,2010,pp.1-4.
[4]K.G.Ramawa,S.Das,M.Mathur,The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants, in:K.G.Ramawa(Ed.),Herbal Drugs:Ethnomedicine to Modern Medicine,Springer,Berlin Heidelberg,Germany,2009,pp.7-32.
[5]S.S.Kubal,V.S.Shirke,K.K.Shirke,et al.,Triple-seeds in Mammea suriga(Buch.-Ham.Ex Roxb.)an avenue tree,Res.Rev.J.Bot.Sci. 2(2013)1-2.
[6]A.B.Prusti,K.K.Behera,Ethno-medico botanical study of Sundargarh District,Orissa,India,Ethnobot.Leafl.11(2007)148-163.
[7]B.S.Joshi,V.N.Kamat,T.R.Govindachari,et al.,Isolation andstructure of surangin A and surangin B,two new coumarins from Mammea longifolia (Wight)Planch and Triana,Tetrahedron 25 (1969) 1453-1458.
[8]M.Mahandu,V.Ravindran,Surangin C,a coumarin from Mammea longifolia,Phytochemistry 25(1986)555-556.
[9]H.Yang,P.Protiva,R.R.Gil,et al.,Antioxidant and cytotoxic isoprenylated coumarins from Mammea americana,Planta Med.71 (2005)852-860.
[10]K.-H.Lee,H.-B.Chai,P.A.Tamez,et al.,Biologically active alkylated coumarins from Kayea assamica,Phytochemistry 64(2003)535-541.
[11]R.Reyes-Chilpa,E.Estrada-Muñiz,T.R.Apan,et al.,Cytotoxic effects of mammea type coumarins from Calophyllum brasiliense, Life Sci.75(2004)1635-1647.
[12]J.L.Lopez-Perez,D.A.Olmedo,E.del Olmo,et al.,Cytotoxic 4-phenylcoumarins from the leaves of Marila pluricostata,J.Nat. Prod.68(2005)369-373.
[13]B.M.W.Ouahouo,A.G.B.Azebaze,M.Meyer,et al.,Cytotoxic and antimicrobial coumarins from Mammea africana,Ann.Trop.Med. Parasitol.98(2004)733-739.
[14]C.Ruiz-Marcial,R.Reyes Chilpa,E.Estrada,et al.,Antiproliferative, cytotoxic and antitumour activity of coumarins isolated from Calophyllum brasiliense,J.Pharm.Pharmacol.59(2007)719-725.
[15]L.M.Bedoya,M.Beltrán,R.Sancho,et al.,4-Phenylcoumarins as HIV transcription inhibitors,Bioorg.Med.Chem.Lett.15(2005) 4447-4450.
[16]Y.Deng,R.A.Nicholson,Antifungal properties of surangin B,a coumarin from Mammea longifolia,Planta Med.71(2005)364-365. [17]L.Verotta,E.Lovaglio,G.Vidari,et al.,4-Alkyl-and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials,Phytochemistry 65(2004)2867-2879.
[18]C.K.Kokate,in:Practical Pharmacognosy,Vallabh Prakashan,Delhi, India,2000,p.218.
[19]J.B.Harborne,Photochemical Methods:A Guide to Modern Techniques of Plant Analysis,second ed.,Chapman A.&Hall,London, UK,1998,pp.4-84.
[20]J.Hudzicki,Kirby-Bauer Disk Diffusion Susceptibility Test Protocol.〈http://www.microbelibrary.org/component/resource/laboratory-test/ 3189-kirby-bauer-disk-diffusion-susceptibility-test-protocol〉(accessed 24.11.14).
[21]J.M.Andrews,BSAC standardized disc susceptibility testing method (version 3),J.Antimicrob.Chemother.53(2004)713-728.
[22]L.Barros,S.Falcão,P.Baptista,et al.,Antioxidant activity of Agaricus sp.mushrooms by chemical,biochemical and electrochemical assays, Food Chem.111(2008)61-66.
[23]M.M.Cowan,Plant products as antimicrobial agents,Clin.Microbiol. Rev.12(1999)564-582.
[24]E.U.Graefe,H.Derendorf,M.Veit,Pharmacokinetics and bioavailability of the flavonol quercetinin humans,Int.J.Clin.Pharmacol. Ther.37(1999)219-233.
[25]L.J.Cseke,A.Kirakosyan,P.B.Kaufman,et al.,Natural Products from Plants,second ed.,CRC Press,Boca Raton,USA,2006,p.17.
[26]J.J.Nah,J.H.Hahn,S.Chung,et al.,Effect of ginsenosides,active components of ginseng,on capsaicin-induced pain-related behavior, Neuropharmacology 39(2000)2180-2184.
[27]C.Rivière,V.N.T.Hong,L.Pieters,et al.,Polyphenols isolated from antiradical extracts of Mallotus metcalfianus,Phytochemistry 70 (2009)86-94.
[28]A.M.Aguinaldo,E.L.Espeso,B.Q.Guovara,Phytochemistry,in:B. Q.Guevara(Ed.),A Guide Book to Plants Screening:Phytochemical and Biological,University of Santo Tomas,Manila,Philippines, 2005,pp.121-125.
[29]M.W.Lwu,A.R.Duncan,C.O.Okunji,New antimicrobials of plant origin,in:J.Janick(Ed.),Perspectives on New Crops and New Uses, ASHS Press,Alexandria,USA,1999,pp.457-462.
[30]B.Halliwell,J.M.C.Gutteridge,The definition and measurement of antioxidants in biological systems,Free Radic.Biol.Med.18(1995) 125-126.
[31]S.Vasdev,V.D.Gill,P.K.Singal,Modulation of oxidative stressinduced changes in hypertension and atherosclerosis by antioxidants, Exp.Clin.Cardiol.11(2006)206-216.
[32]B.Uttara,A.V Singh,P.Zamboni,et al.,Oxidative stress and neurodegenerative diseases:a review of upstream and downstream antioxidant therapeutic options,Curr.Neuropharmacol.7(2009) 65-74.
[33]J.S.Rathee,S.A.Hassarajani,S.Chattopadhyay,Antioxidant activity of Mammea longifolia bud extracts,Food Chem.99(2006)436-443.
[34]R.M.Zavod,J.J.Knittel,Drug design and relationships of functional groups to pharmacological activity,in:T.L.Lemke,D.A.Williams, V.F.Roche,S.W.Zito(Eds.),Foye’s Principles of Medicinal Chemistry,sixth ed.,Lippincott Williams&Wilkins,Baltimore, USA,2008,pp.26-53.
[35]M.P.Kähkönen,A.I.Hopia,H.J.Vuorela,et al.,Antioxidant activity of plant extracts containing phenolic compounds,J.Agric.Food Chem.47(1999)3954-3962.
[36]F.Liu,T.B.Ng,Antioxidative and free radical scavenging activities of selected medicinal herbs,Life Sci.66(2000)725-735.
[37]T.Yokozawa,C.P.Chen,E.Dong,et al.,Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical,Biochem.Pharmacol.56(1998)213-222.
[38]P.Diaz,S.C.Jeong,S.Lee,et al.,Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds,Chin.Med.7(2012)1-9.
Received 7 October 2014;revised 8 January 2015;accepted 9 January 2015 Available online 19 January 2015
 Journal of Pharmaceutical Analysis2015年3期
Journal of Pharmaceutical Analysis2015年3期
- Journal of Pharmaceutical Analysis的其它文章
- Non-covalent binding analysis of sulfamethoxazole to human serum albumin:Fluorescence spectroscopy,UV-vis,FT-IR,voltammetric and molecular modeling
- Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods
- Comparison of conventional and supported liquid extraction methods for the determination of sitagliptin and simvastatin in rat plasma by LC-ESI-MS/MS
- Quality evaluation of synthetic quorum sensing peptides used in R&D
- Liquid chromatography-tandem mass spectrometry method for the estimation of adefovir in human plasma:Application to a pharmacokinetic study
- Selective extraction of dimethoate from cucumber samples by use of molecularly imprinted microspheres
