Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods
Bilal Yilmaz,Ulvihan Ciltas
Department of Analytical Chemistry,Faculty of Pharmacy,Ataturk University,25240 Erzurum,Turkey
Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods
Bilal Yilmaz✽,Ulvihan Ciltas
Department of Analytical Chemistry,Faculty of Pharmacy,Ataturk University,25240 Erzurum,Turkey
Diclofenac;
Sweep voltammetry;
Chromatography-mass spectrometry;
Pharmaceutical
preparation
Rapid,sensitive and specific methods were developed for the determination of diclofenac in pharmaceutical preparations by linear sweep voltammetry(LSV)and gas chromatography(GC)with mass spectrometry(MS)detection.The linearity was established over the concentration range of 5-
35 μg/mL for LSV and 0.25-5 μg/mL for GC-MS method.The intra-and inter-day relative standard deviation(RSD)was less than 4.39%and 4.62%for LSV and GC-MS,respectively.Limits of quantification(LOQ)were determined as 4.8 and 0.15 μg/mL for LSV and GC-MS,respectively.No interference was found from tablet excipients at the selected assay conditions.The methods were applied for the quality control of commercial diclofenac dosage forms to quantify the drug and to check the formulation content uniformity.
©2014 Xi’an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/3.0/).
1. Introduction
Diclofenac is a nonsteroidal anti-inflammatory drug(NSAID) that is widely prescribed for the treatment of rheumatoid arthritis,osteoarthritis,musculoskeletal injuries and post-surgery analgesia in human and veterinary medicine[1].Patients are frequently given special formulations of diclofenac or a cotreatment agent as a therapeutic strategy to attenuate the gastrointestinaltractcomplicationsthatlimitthe use of diclofenac and other NSAIDs[2,3].Many patients prescribed diclofenac for arthritis also take additional drugs for other chronic health problems such as hypertension[4].
To date,several methods for the determination of diclofenac have been reported.These include potentiometry[5-7],capillary zone electrophoresis[8],high-performance liquid chromatography (HPLC)[9-11],high-performance liquid chromatography-mass spectrometry(HPLC-MS)[12],spectrophotometry[13,14],spectrofluorometry [15,16],thin layer chromatography [17],gas chromatography[18],polarographic analysis[19],and spectroscopic methods[20-24].An extensive literature survey revealed that there were several HPLC methods for the determination of diclofenac in blood plasma,whereas there was little other work disclosed only for the quantitative determination of diclofenac in
pharmaceutical formulation samples.The reported HPLC methods were influenced by interference of endogenous substances and potential loss of drugs in the re-extraction procedure and involving lengthy,tedious and time-consuming plasma sample preparation and extraction processes and requiring a sophisticated and expensive instrumentation.
✽Corresponding author.Tel.:+90 4422315200;fax:+90 4422315201. E-mail address:yilmazb@atauni.edu.tr(B.Yilmaz).
Peer review under responsibility of Xi’an Jiaotong University.
2095-1779©2014 Xi’an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BYNC-ND license(http://creativecommons.org/licenses/by-nc-nd/3.0/).
http://dx.doi.org/10.1016/j.jpha.2014.10.005
On extensive survey of literature,no LSV method was reported till date for determination of diclofenac in pure and pharmaceutical dosage forms.The development of a new method capable of determining drug amount in pharmaceutical dosage forms is important.Electroanalytical techniques have been used for the determination of a wide range of drug compounds with the advantages that there is,in most instances,no need for derivatization and that these techniques are less sensitive to matrix effects than other analytical techniques.Additionally, application of electrochemistry involves the determination of electrode mechanism.Redox properties of drugs can give insights into their metabolic fate or their in vivo redox processes or pharmacological activity[25-28].Despite the analytical importanceoftheelectrochemicalbehaviorand oxidation mechanism of diclofenac,no report has been published on the voltammetric study of the electrochemical oxidation of diclofenac in nonaqueous media.It is well known that the experimental and instrumental parameters directly affect the electrochemical process and voltammetric response of drugs.Consequently,it would be interesting to investigate the oxidation process of diclofenac in aprotic media.
Therefore,this paper describes a new LSV method for determination of diclofenac and a gas chromatography with MS detection.The LSV method was aimed at developing an easy and rapid assay method for diclofenac without any time-consuming sample preparation steps for routine analysis.GC method was attempted to demonstrate the utility of MS detection for determination of diclofenac with simple sample preparation and reasonable analysis time with high precision.In both the proposed methods,there is no need to extract the drug from the formulation excipient,thereby decreasing the error in quantization.Formulation samples can be directly used after dissolving and filtration. The developed methods were used to determine the total drug content in commercially available tablets of diclofenac.
2. Materials and methods
2.1. Chemicals
Diclofenac was obtained from Sigma(St.Louis,MO,USA). Acetonitrile and lithium perchlorate(LiClO4)were purchased from Fluka(Buchs,Switzerland).Diclomec,Dicloflam and Voltaren tablets were obtained from pharmacies(Erzurum, Turkey).
2.2. Voltammetric and chromatographic system
Electrochemical experiments were performed on a Gamry Potentiostat Interface 1000 controlled with software PHE 200 and PV 220.All measurements were carried out in a single-compartment electrochemical cell with a standard threeelectrode arrangement.A platinum disk with an area of 0.72 cm2and a platinum wire were used as the working and the counter electrodes,respectively.The working electrode was successively polished with 1.0,0.3 and 0.05 μm alumina slurries(Buehler)on microcloth pads(Buehler).After each polishing,the electrode was washed with water and sonicated for 10 min in acetonitrile.Then,it was immersed into a hot piranha solution(3:1,H2SO4,30%H2O2)for 10 min,and rinsed copiously with water.All potentials were reported versus Ag/AgCl/KCl(3.0 M)reference electrode (BAS Model MF-2078)at room temperature.The electrolyte solutions were degassed with purified nitrogen for 10 min before each experimentand bubbled with nitrogen during the experiment.
Chromatographic analysis was carried out on an Agilent 6890N gas chromatography system equipped with 5973 series mass selective detector,7673 series autosampler and chemstation (AgilentTechnologies,PaloAlto,CA).HP-5 MS column (30 m×0.25 mm,0.25 μm)was used for separation.Splitless injection was used and the carrier gas was helium at a flow rate of 1 μg/mL.The injector and detector temperatures were 250°C. The MS detector parameters were transfer line temperature 280°C,solvent delay 3 min and electron energy 70 eV.MS was run in electron impact mode with selected ion monitoring (SIM)for quantitative analysis.
2.3. Preparation of the standard and quality control(QC) solutions
For the LSV method,the stock standard solution of diclofenac was prepared in 0.1 M LiClO4/acetonitrile to a concentration of 100 μg/mL.For the GC-MS method,the stock solution of diclofenac was prepared in methanol solution to a concentration of 100 μg/mL.Standard solutions were prepared as 5-35 μg/mL (5,10,15,20,25,30 and 35 μg/mL)for LSV method and 0.25-5 μg/mL(0.25,1,2,3,4 and 5 μg/mL)for the GC-MS method. The QC samples were prepared by adding aliquots of standard working solution of diclofenac to obtain the final concentrations of 7.5,17.5 and 32.5 μg/mL for the LSV method and 0.75,2.5 and 4.5 μg/mL for the GC-MS method.
2.4. Procedure for pharmaceutical preparations
A total of 10 tablets of diclofenac(Diclomec,Dicloflam and Voltaren)were accurately weighed and powdered.For the LSV method,an amount of this powder corresponding to one tablet diclofenac content was weighed and accurately transferred into a 100 mL calibrated flask and 50 mL of 0.1 M LiClO4/acetonitrile was added and then the flask was sonicated to 10 min at room temperature.The flask was filled to volume with 0.1 M LiClO4/acetonitrile.The resulting solutions in both the cases were filtered through Whatman filter paper no.42 and suitably diluted to get a final concentration within the limits of linearity for the respective proposed method.For the GC-MS method,an appropriate volume of filtrate was diluted further with methanol so that the concentration of diclofenac in the final solution was within the working range,and then analyzed by GC-MS.
2.5. Data analysis
All statistical calculations were performed with the Statistical Product and Service Solutions(SPSS)for Windows,version 10.0. Correlations were considered statistically significant if calculated P values were 0.05 or less.

Fig.1 (A)Cyclic voltammogram for the oxidation of 20 μg/mL diclofenac and(B)blank in acetonitrile containing 0.1 M LiClO4at Pt disk electrode.Scan rate:0.2 V/s.
3. Results and discussion
3.1. Method development and optimization
The electrochemical behavior of diclofenac was investigated at the Pt disc electrode in anhydrous acetonitrile solution containing 0.1 M LiClO4as the supporting electrolyte by using cyclic voltammetry(CV).
Fig.1 shows a typical cyclic voltammogram of 20 μg/mL diclofenac recorded under these conditions for describing the scan rate of 0.2 V/s.In the anodic sweep,two oxidation peaks were seen at about potentials of 0.87 and 1.27 V,respectively.
To gain a deeper insight into the voltammetric waves,the effect of scan rate on the anodic peak currents(İm)and peak potentials(Ep) was studied in the range of 0.01-1 V/s the potential scan rates in acetonitrile solution containing 20 μg/mL of diclofenac.Scan rate dependency experiments showed that the peak currents for peak varied linearly with the scan rate(ν),which points out the adsorptioncontrolled process.However,the plots of logarithm of peak currents versus logarithm of scan rates for 20 μg/mL concentration of diclofenac displayed straight lines with 0.497 slope,which are close to the theoretical value of 0.5 expected for an ideal diffusioncontrolled electrode process[29].log Im-log ν curve is more eligible for this aim;therefore,a diffusional process for peak should be considered.These results suggested that the redox species were diffusing freely from solution and not precipitating onto the electrode surface.The reason for this behavior might be due to the solubility of the intermediate species in acetonitrile or poor adherence of products on the electrode surface.
The oxidation peak potential(E)for peaks shifted toward more positive values with increasing scan rate.The relationship between the peak potential and scan rate is described by the following equation:
E=E0′+RT/[(1-α)naF]⌊0.78+In(D1/2k-1s)
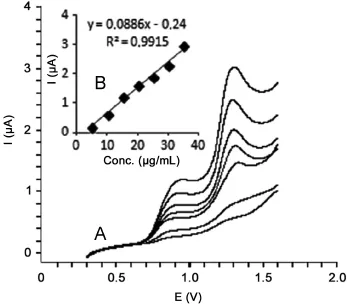
Fig.2 (A)Linear sweep voltammograms for different concentrations of diclofenac in acetonitrile solution containing 0.1 M LiCIO4(5,10, 15,20,25,30 and 35 μg/mL)and(B)mean calibration graph(n=6).
-0.5InRT/[(1-α)naF]⌋+RT/[(1-α)naF]/2Inv and from the variation of peak potential with scan rate αnacan be determined,where α is the transfer coefficient and nais the number of electrons transferred in the rate determining step.According to this equation,the plots of the peak potentials versus ln ν for oxidation peak show linear relationship.The slope indicates the value of αnais 0.38 for peak.On the base of the above results,the nais 1 and then the value of α is calculated to be 0.38,which is reasonable for the most of irreversible electrode processes.Based on the above discussions the oxidation process of diclofenac is controlled by the diffusion step and one electron and one proton are involved in the reaction.
During GC-MS method development,a capillary column coated with 5%phenyl and 95%dimethylpolysiloxane was used for separation.The injection port and detector temperature was set to 250°C.Different temperature programs were investigated to give an optimum temperature program as follows:initial temperature was 150°C,held for 1 min,increased to 220°C at 20°C/min, held for 1 min,and finally to 300°C at 10°C/min with a final maintenance of 1.0 min.The injector volume was 1 μL in splitless mode.
3.2. Method validation
To ensure optimization of the methods in light of the standardization rules,the developed methods were validated in terms of specificity,linearity,precision,accuracy,LOD,LOQ,recovery and the stability effect which was investigated by analyzing the pure diclofenac solution and drug samples[30].
3.2.1. Specificity
All the solutions were scanned from 0.5 to 1.5 V and checked for change in the peaks at respective potentials(Fig.2).
In a separate study,the specificity of the method was investigated by observing interferences between diclofenac and the excipients.For GC-MS,electron impact mode with selected ion monitoring(SIM)was used for quantitative analysis(m/z 214 for diclofenac).

Fig.3 (A)MS spectra of diclofenac and(B)chemical structure of diclofenac.
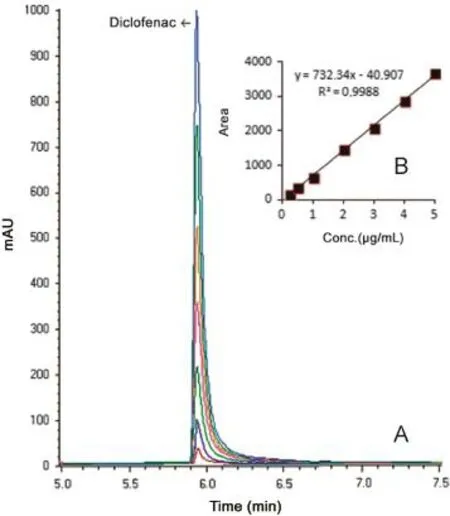
Fig.4 (A)GC-MS chromatograms of diclofenac(0.25,0.5,1,2,3,4 and 5.0 μg/mL)[Selected ion monitoring(SIM)mode,m/z 214 for diclofenac]and(B)mean calibration graph(n=6).
The mass spectra of the diclofenac are shown in Fig.3.The retention time of diclofenac in GC-MS method was approximately 5.9 min with good peak shape(Fig.4).
3.2.2. Linearity
For the LSV and GC-MS measurements,the solutions were prepared by dilution of the stock solution of diclofenac to reach a concentration range of 5-35 μg/mL(5,10,15,20,25,30 and 35 μg/mL)and 0.25-5 μg/mL(0.25,0.5,1,2,3,4 and 5 μg/mL), respectively.Calibration curves were constructed for diclofenac standard by plotting the concentration of diclofenac versus voltammogram and peak area response.The calibration curve constructed was evaluated by its correlation coefficient.The correlation coefficients(r)of all the calibration curves were consistently greater than 0.99.The regression equations were calculated from the calibration graphs,along with the standard deviations of the slope and intercept on the ordinate.The results are shown in Table 1.
3.2.3. Precision and accuracy
The precision of the LSV and GC-MS methods was determined by repeatability(intra-day)and intermediate precision(inter-day). Repeatability was evaluated by analyzing QC samples six times per day at three different concentrations.The intermediate precision was evaluated by analyzing the same samples once daily for two days.The RSD of the predicted concentrations from the regression equation was taken as precision.The accuracy of this analytic method was assessed as the percentage relative error.For all the concentrations studied,intra-and inter-day relative standard deviation values were≤4.62%and for all concentrations of diclofenac the relative errors were≤6.29%.These results are given in Table 2.
3.2.4. LOD and LOQ
For LSV measurements,LOD and LOQ of diclofenac were determined using calibration standards.The LOD and LOQ values were calculated as 3.3 σ/S and 10 σ/S,respectively,where S is the slope of the calibration curve and σ is the standard deviation of y-intercept of regression equation(n=6)[31].
For GC-MS measurements,the LOD and LOQ of diclofenac were determined by injecting progressively low concentration of the standard solution under the chromatographic conditions.The lowest concentration assayed was regarded as LOQ,where the signal/noise ratio was at least 10:1.The LOD was defined as a signal/noise ratio of 3:1.The LOD and LOQ for LSV were 1.6 and 4.8 μg/mL,for GC-MS 0.05 and 0.15 μg/mL,respectively. Among the two methods,GC-MS was more sensitive than LSV (Table 1).
3.2.5. Recovery
To determine the accuracy of the LSV and GC-MS methods and to study the interference of formulation additives,the recovery was checked at three different concentration levels. Analytical recovery experiments were performed by adding known amount of pure drugs to pre-analyzed samples of commercial dosage forms.The recovery values were calculated by comparing concentration obtained from the spiked samples with actual added concentrations.These values are also listed in Table 3.
3.2.6. Ruggedness
Determination of diclofenac using both the LSV and GC-MS methods was carried out by a different analyst on the same instrument with the same standard(Table 4).
Theresultsshowed no statisticaldifferencesbetween different operators,suggesting that the developed method was rugged.

Table 1 Linearity of diclofenac.
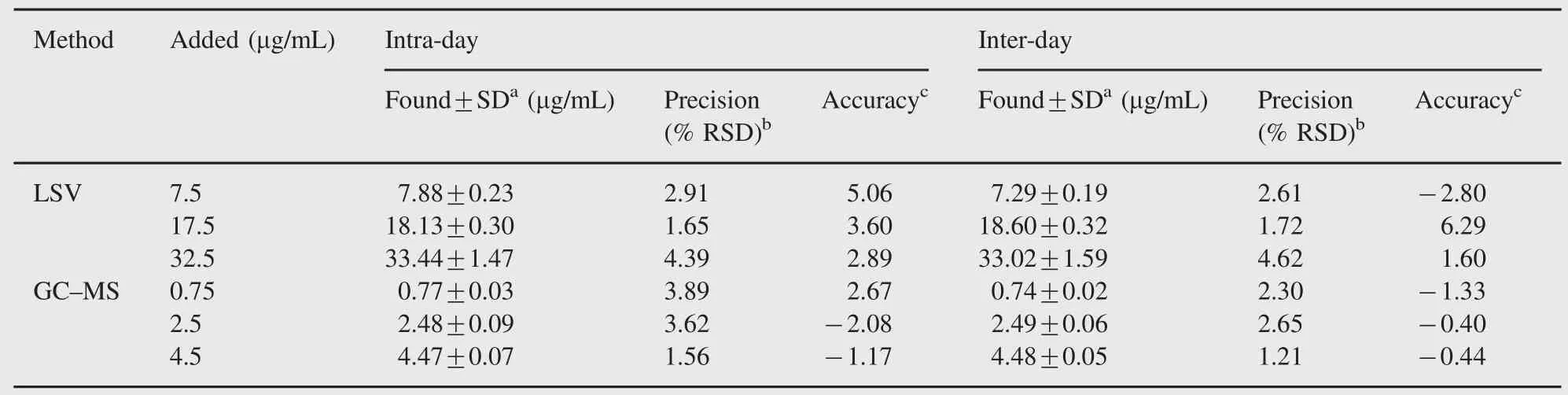
Table 2 Precision and accuracy of diclofenac.

Table 3 Recovery of diclofenac in pharmaceutical preparations.
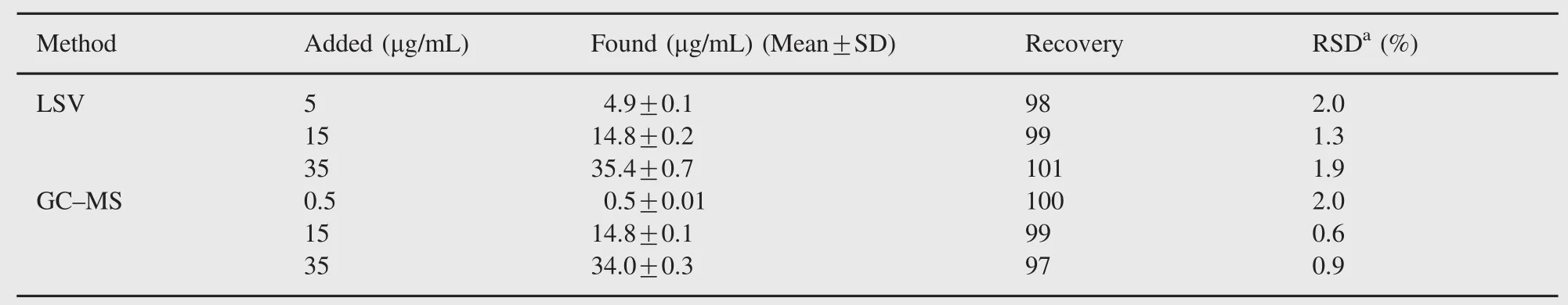
Table 4 The results of analyses of diclofenac by a different analyst.a
3.2.7. Stability
Stability studies indicated that the samples were stable when kept at room temperature,+4°C and-20°C for 24 h(short-term)and -20°C for 72 h(long-term).
The results are given in Table 5,where the percent ratios were within the acceptance range of 90-110%.
3.3. Comparison of the methods
Birajdar et al.[10]developed a reverse phase HPLC method for the simultaneous determination of rabeprazole and diclofenac in tablet.The method was based on HPLC separation of both drugs in reverse phase mode using Phenomenox C18column with WatersHPLC system by using the mobile phase composition of acetonitrile and 50 mM ammonium acetate buffer(pH 3.6)(60:40,v/v)at flow rate of 1 mL/min.Detection wavelength used was at 254 nm. Linearity was obtained in the concentration range of 1.0-3.2 μg/mL for rabeprazole and 6.0-16.0 μg/mL for diclofenac.
Table 5 Stability of diclofenac in solution at different temperatures(Recovery±RSD,%).

Fig.5 Linear sweep voltammogram of Voltaren tablet solution containing 15 and 30 μg/mL of diclofenac.
Sastry et al.[13]described a spectrophotometric method for the determination of diclofenac sodium in bulk samples and pharmaceutical preparations with p-N,N dimethylphenylenediamine as a solvent and having maximum absorbance at 670 nm.The reaction was sensitive enough to permit the determination of 2.0-24 μg/mL.
Agrawal et al.[14]described two methods for the determination of diclofenac.In the first method,diclofenac reduced iron(III)to iron(II)having a maximum absorbance at 520 nm.The reaction obeys Beer’s law for concentrations of 10-80 μg/mL.In the second method,diclofenac was treated with methylene blue in the presence of phosphate buffer(pH 6.8)and the complex was extracted with chloroform.The complex had a maximum absorbance at 640 nm and linearity was in the range of 5-40 μg/mL.
Marcela et al.[15]developed a spectrofluorometric method for the determination of diclofenac.The method was based on its reaction with cerium(IV)in an acidic solution and measurement of the fluorescence of the Ce(III)ions produced.The absorbance was measured at 356 and 250 nm with double distilled water as solvent.
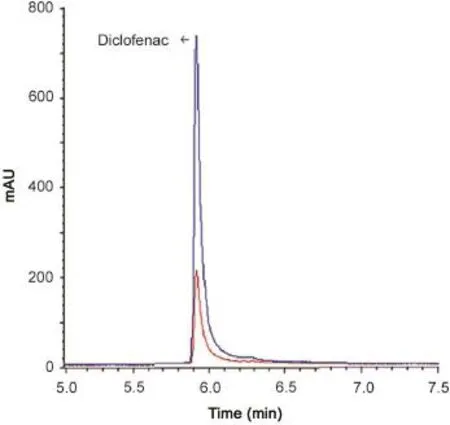
Fig.6 GC-MS chromatogram of Voltaren tablet solution containing 1 and 4 μg/mL of diclofenac.
Thongchai et al.[17]developed a high-performance thin layer chromatographic method for the determination of diclofenac sodium in pharmaceutical formulations.The drug was extracted from the sample and then various aliquots of this solution were spotted automatically by means of Camag Linomat IV on a silica gel 60 F254 aluminum plate,using a mixture of toluene:ethyl acetate:glacial acetic acid(60:40:1,v/v/v)as mobile phase.The spot areas were quantified by densitometry at 282 nm.Linear calibration curve was obtained over the range of 5-80 μg/mL.
For LSV and GC-MS measurements,calibration curves were linear over the concentration range of 5-35 and 0.25-5 μg/mL for diclofenac,which is as good as or superior to that reported in other papers[10,13-15,17].
Also,LSV and GC-MS methods were applied for the determination of diclofenac in three commercial tablets(Figs.5 and 6).
The results show the high reliability and reproducibility of two methods.The results were statistically compared using the F-test. At 95%confidence level,the calculated F-values do not exceedthe theoretical values(Table 6).Therefore,there is no significant difference between LSV and GC-MS methods.

Table 6 Comparison of the proposed and reported methods for determination of diclofenac.
USP XXIV has recommended HPLC method for analysis of diclofenac in pure and dosage forms(tablet).The method recommends use of a mobile phase of methanol and phosphate buffer(pH 2.5)at a flow rate of 1 μg/mL.
Also,the proposed LSV and GC-MS methods were compared with the HPLC method of USP XXIV[32].There was no significant difference between the three methods with respect to mean values and standard deviations at the 95%confidence level (Table 6).Therefore,this is suggested that the two methods are equally applicable.
4. Conclusion
In the present work,two new methods have been developed and validated for routine determination of diclofenac in pharmaceutical preparations.Linearity range,precision,accuracy,LOD and LOQ are suitable for the quantification of diclofenac in pharmaceutical preparations.The sample recoveries in three formulations were in good agreement with their respective label claims.No extraction procedure is involved.According to the statistical comparison of the results,there is no significant difference between LSV and GC-MS methods.The proposed methods can be used for the routine quality control analysis of diclofenac in pharmaceutical preparations in a total time of 6 min.
Acknowledgments
This study was supported by a grant from Ataturk University Research Foundation(Project no.:2012/78).
[1]T.Iliescu,M.Baia,V.Miclaus.,A Raman spectroscopic study of the diclofenac sodium-betacyclodextrin interactio,Eur.J.Pharm.Sci.22 (2004)487-495.
[2]N.Gostick,I.G.James,T.K.Khong,et al.,Controlled-release indomethacin and sustained-release diclofenac sodium in the treatment of osteoarthritis-a comparative controlled clinical-trial in general-practice,Curr.Med.Res.Opin.12(1990)135-142.
[3]B.Crowley,J.J.Hamill,S.Lyndon,et al.,Controlled-release indomethacin and sustained-release diclofenac sodium in the treatment of rheumatoid-arthritis-a comparative controlled clinical-trial, Curr.Med.Res.Opin.12(1990)143-150.
[4]R.Roskar,V.Kmetec,Liquid chromatographic determination of diclofenac in human synovial fluid,J.Chromatogr.B 788(2003)57-64.
[5]M.Shamsipur,F.Jalali,S.Ershad,Preparation of a diclofenac potentiometric sensor and its application to pharmaceutical analysis and to drug recovery from biological fluids,J.Pharm.Biomed.37 (2005)943-947.
[6]A.O.Santini,H.R.Pezza,L.Pezza,Determination of diclofenac in pharmaceutical preparations using a potentiometric sensor immobilized in a graphite matrix,Talanta 68(2006)636-642.
[7]S.S.M.Hassan,W.H.Mahmoud,M.A.F.Elmosallany,et al.,Iron(II)-phthalocyanine as a novel recognition sensor for selective potentiometric determination of diclofenac and warfarin drugs,J.Pharm. Biomed.39(2005)315-321.
[8]W.Jin,J.Zhang,Determination of diclofenac sodium by capillary zone electrophoresis with electrochemical detection,J.Chromatogr. 868(2000)101-107.
[9]L.González,G.Yuln,M.G.Volonté,Determination of cyanocobalamin,betamethasone,and diclofenac sodium in pharmaceutical formulations,by high performance liquid chromatography,J.Pharm. Biomed.Anal.20(1999)487-492.
[10]A.S.Birajdar,S.Meyyanathan,B.Suresh,A RP-HPLC method for determiınatıon of diclofenac with rabeprazole in solid dosage form, Pharm.Sci.Monit.2(2011)171-178.
[11]C.Arcelloni,R.Lanzi,S.Pedercini,et al.,High-performance liquid chromatographic determination of diclofenac in human plasma after solid-phase extraction,J.Chromatogr.763(2001)195-200.
[12]M.E.Abdel-Hamid,L.Novotny,H.J.Hamza,Determination of diclofenac sodium,flufenamic acid,indomethacin and ketoprofen by LC-APCI-MS,J.Pharm.Biomed.Anal.24(2001)587-594.
[13]C.P.Sastry,T.Prasad,M.V.Suryamarayana,Spectrophotometric method for determination of diclofenac sodium in bulk samples and pharmaceutical preparations,Analyst 114(1989)513-516.
[14]Y.K.Agrawal,K.Shivramchandra,Visiblespectrophotometric method for determination of diclofenac sodium,J.Pharm.Biomed. Anal.9(1991)97-100.
[15]C.Marcela,B.Liliana,Spectroflourimetric determination of diclofenac sodium,Anal.Sci.22(2006)431-434.
[16]L.A.Carreira,M.Rizk,Y.El-Shabrawy,et al.,Europium(III)ion probe spectrofluorometric determination of diclofenac sodium,J. Pharm.Biomed.Anal.13(1995)1331-1337.
[17]W.Thongchai,B.Liawruangrath,C.Thongpoon,et al.,High performance thin layer chromatographic method for the determination of diclofenac sodium in pharmaceutical formulations,Chiang Mai J. Sci.33(2006)123-128.
[18]A.Siou,F.Pommier,J.Godbillon,Determination of diclofenac in plasma and urine by capillary gas chromatography-mass spectrometry with possible simultaneous determination of deuteriumlabeled diclofenac,J.Chromatogr.571(1991)87-100.
[19]M.T.Xu,L.F.Chen,J.F.Song,Polarographic behaviors of diclofenac sodium in the presence of dissolved oxygen and its analytical application,Anal.Biochem.329(2004)21-27.
[20]R.L.De Souza,M.Tubino,Spectrophotometric determination of diclofenac in pharmaceutical preparations,J.Braz.Chem.Soc.16 (2005)1068-1073.
[21]S.Agatonovic-Kustrin,L.J.Zivanovic,M.Zecevic,et al.,Spectrophotometric study of diclofenac-Fe(III)complex,J.Pharm.Biomed. Anal.16(1997)147-153.
[22]A.A.Matin,M.A.Farajzadeh,A.Joyuban,A simple spectrophotometric method for determination of sodium diclofenac in pharmaceutical formulations,IL Farmaco 60(2005)855-861.
[23]C.S.P.Sastry,A.S.R.Prasad-Tipirneni,M.V.Suryanarayana,Spectrophotometric analysis of some anthranilic acid-derivatives and their pharmaceutical preparations,Microchem.J.39(1989)277-289.
[24]M.M.Sena,F.Chaudhry,Z.C.H.Collins,et al.,N-way PLS applied to simultaneous spectrophotometric determination of acetylsalicylic acid,paracetamol and caffeine,J.Pharm.Biomed.Anal.36(2004) 743-749.
[25]G.B.El-Hefnawey,I.S.El-Hallag,E.M.Ghoneim,et al.,Voltammetric behavior and quantification of the sedative-hypnotic drug chlordiazepoxide in bulk form,pharmaceutical formulation and human serum at a mercury electrode,J.Pharm.Biomed.Anal.34 (1)(2004)75-86.
[26]P.Corti,G.Corbini,P.Gratteri,et al.,Determination of some quinolones in tablets,human plasma and urine by differential-pulse polarography,Int.J.Pharm.11(1994)83-87.
[27]A.Radi,T.Elmogy,Differential pulse voltammetric determination of carvedilol in tablets dosage form using glassy carbon electrode,IL Farmaco 60(2005)43-46.
[28]S.A.Ozkan,B.Uslu,Z.Senturk,Electroanalytical characteristics of amisulpride and voltammetric determination of the drug in pharmaceuticals and biological media,Electroanalysis 16(2004)231-237.
[29]E.Laviron,L.Roullier,C.Degrand,A multilayer model for the study of space distributed redox modified electrodes.2.Theory and application of linear potential sweep voltammetry for a simple reaction,J.Electroanal.Chem.112(1980)11-23.
[30]The European Agency for the Evaluation of Medicinal Products.ICH Topic Q2B Note for Guideline on Validation of Analytical Procedures:Methodology GPMP/ICH/281/95,1996.
[31]B.Yilmaz,D.Ekinci,Voltammetric behavior of carvedilol in nonaqueous media and its analytical determination in pharmaceutical preparations,Rev.Anal.Chem.30(2011)187-193.
[32]The United States Pharmacopeia XXIV.United States Pharmacopoeial Convention Inc.,Rockville 2000.
Received 22 July 2014;revised 2 October 2014;accepted 15 October 2014 Available online 28 October 2014
 Journal of Pharmaceutical Analysis2015年3期
Journal of Pharmaceutical Analysis2015年3期
- Journal of Pharmaceutical Analysis的其它文章
- Non-covalent binding analysis of sulfamethoxazole to human serum albumin:Fluorescence spectroscopy,UV-vis,FT-IR,voltammetric and molecular modeling
- Comparison of conventional and supported liquid extraction methods for the determination of sitagliptin and simvastatin in rat plasma by LC-ESI-MS/MS
- Quality evaluation of synthetic quorum sensing peptides used in R&D
- Extraction,characterization and biological studies of phytochemicals from Mammea suriga
- Liquid chromatography-tandem mass spectrometry method for the estimation of adefovir in human plasma:Application to a pharmacokinetic study
- Selective extraction of dimethoate from cucumber samples by use of molecularly imprinted microspheres
