Effects of different concentrations of sucrose or trehalose on the postthawing quality of cattle bull semen
Reda I. El-Sheshtawy, Gamal A. Sisy, Walid S. El-Nattat
Animal Reproduction and AI Dept., National Research Center, Dokki, Egypt
Effects of different concentrations of sucrose or trehalose on the postthawing quality of cattle bull semen
Reda I. El-Sheshtawy, Gamal A. Sisy, Walid S. El-Nattat
Animal Reproduction and AI Dept., National Research Center, Dokki, Egypt
ARTICLE INFO
Article history:
Received 29 October 2014
Received in revised form 12 December 2014
Accepted 20 December 2014
Available online 20 March 2015
Trehalose
Sucrose
Extender
Cattle
Objective: To examine the effect of different concentrations of trehalose or sucrose (50 or 100 or 200 mM) on post- thawed quality of bull semen, cryo-preserved in Tris-citric acid-egg yolk-fructose (TCYF). Methods: Semen samples were diluted in TCYF extender, TCYF + trehalose (50, 100 and 150 mM/L) or TCYF + sucrose (50, 100 and 150 mM/L) to ensure 60 million motile spermatozoa mL-1, cooled slowly up to 5℃and equilibrated for 4 h. Semen was packed into 0.25 mL polyvinyl French straws. The straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen (LN2) for 10 minutes then dipped in liquid LN2. Frozen straws were thawed at 37℃for 1 min. The parameters studied were sperm motility, sperm viability, sperm abnormality, sperm membrane integrity (HOST), percent of normal intact acrosome and DNA fragmentation. Results: The output data demonstrated that addition of 50–100 mM of trehalose or sucrose/L TCYF after chilling at 5℃had significantly (P<0.0001) ameliorated motility, membrane integrity, viability, abnormal morphology, and acrosome integrity % compared to control diluted semen while 50 mM of trehalose/L, and 50-100 mM of sucrose/L to TCYF diluent had significantly (P<0.0001) improved after thawing motility (43.00,% 45.00% and 41.00%, respectively), membrane integrity (67.40%, 67.80% and 69.40%, respectively), life sperm % (70.20%, 69.40% and 71.40% respectively), and acrosome integrity percentages (56.40%, 58.80% and 55.80% respectively) compared to the control tris-base diluent, while diminishing the abnormal sperm morphology (6.20, 3.80 and 3.80 respectively) and DNA fragmentation (3.60%, 3.80% and 3.80% respectively). Besides, the addition of 100 mM of trehalose/L to tris-base diluent has also a promising effect when added to the tris-base diluent concerning the above parameters. Conclusion: It is finally concluded that the addition of 50 - 100 mM trehalose or sucrose/L to TCYF have a beneficial effect in chilling diluted bull semen, while the use of 50 mM trehalose or 50–100 mM sucrose had their benefits on freezing-thawing of extended semen.
1. Introduction
Throughout the last half century, artificial insemination (AI) used in a proper way increases the breeding capacity of the males, permitting a higher degree of selection and an extended use of animals with a high breeding value as well as reducing the risk of spreading infectious. A prerequisite for the use of AI has been the development of procedures for semen preservation.
The application of AI in animal breeding strategies has been shown to have the potential to quickly disseminate genes from the supergenetic males for improving productive traits. The quality of frozen semen is the most influencing factor for conception rate[1]. The composition of the extender in which semen is diluted before freezing is one of the most factors that influence the success of cryopreservation[2]. Trehalose and sucrose are non-penetrating disaccharidesthat seems to protect cells both by increasing the tonicity of the extender and by stabilizing the plasma membrane, possibly due to direct interaction with phospholipid polar head groups of membrane phospholipids[3]. Trehalose seems to be more efficient than other sugars for protection of spermatozoa in cryopreservation media, and many authors have reported its beneficial effect for semen cryopreservation in different species, such as bull[2, 4-6], buffalo bull[7, 8], ram[9-12], goat[13-15], rabbit semen[16]. In contrast, several studies have reported no significant positive effect of trehalose for cryopreserving spermatozoa from stallion[17], Iberian red deer[18], European brown hare[19], rooster[20] and emu[21].
Disaccharides are effective in stabilizing biomembrane bilayers and the sperm metabolism can be better sustained in diluents containing degradable sugar[22]. Lactose, sucrose, raffinose, trehalose and dextrans are not able to diffuse across the plasma membrane, creating an osmotic pressure that induces cell dehydration and a lower incidence of intracellular ice formation. These sugars interact with phospholipids in the plasma membrane, increasing sperm survival to cryopreservation[10]. Normally there is a greater cryoprotective effect for monosaccharides than disaccharides when used in combination with Tris[9]. Last decade, trehalose is being included in ram and goat semen cryopreservation extenders. The addition of high concentrations of trehalose to sperm extender provides the best protection with regard to post-thaw motility parameters, recovery rates, thermal resistance, and acrosome integrity [13]. This disaccharide increases membrane fluidity before freezing, leading to greater resistance of spermatozoa against freezethawing damage[13]. On the other hand, addition of sucrose and trehalose for freezing of bull semen resulted in an improvement of the sperm survival[2]. This study aimed to examine the effect of different concentrations of trehalose or sucrose (50 or 100 or 200 mM) on chilled and post- thawed quality of bull semen, preserved in Tris-citric acid-egg yolk-fructose (TCYF).
2. Materials and methods
Semen collection and initial evaluation: Five mature cattle-bulls maintained at The Semen Freezing Center, General Organization for Vet. Services, Ministry of Agriculture, Abbasia, Egypt, were used for this study. Semen was collected from 5 cattle-bulls using an artificial vagina at weekly intervals for 5 weeks. The semen samples were transferred to the lab within few seconds and initially evaluated for volume (in graduated tube), concentration (Thoma rulling of the Neubaur haemocytometer), sperm motility[23], percent live sperm and hypo-osmotic swelling test[24]. The neat semen samples with more than 70% motility and 80% morphologically normal spermatozoa were admitted to freezing procedure. The ejaculates were pooled in order to have sufficient semen for a replicate and to eliminate the bull effect. The semen was given a holding time for 10 minute at 37 ℃ in a water bath before dilution.
2.1. Semen processing:
The control cryopreservation extender was Tris-citric acid-egg yolk-fructose (TCYF) diluent[25]. Semen samples were diluted in TCYF extender, TCYF + trehalose (50, 100 and 150 mM/L) and TCYF + sucrose (50, 100 and 150 mM/L) to ensure 60 million motile spermatozoa mL-1, cooled slowly up to 5 ℃ and equilibrated for 4 h. Semen was packed into 0.25 mL polyvinyl French straws (IMV, France). After equilibration periods, the straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen (LN2) for 10 minutes and were then dipped in liquid LN2.
Semen quality assessment: These assessments were undertaken on neat semen, after dilution, cooling and Freeze-thawing of bull spermatozoa. Frozen straws were thawed at 37 ℃ for 1 min. The parameters studied were sperm motility, sperm viability, sperm abnormality, sperm membrane integrity (HOS), percent of normal intact acrosome and DNA fragmentation.
2.2. Sperm motility
Sperm motility was subjectively assessed using phase contrast microscope set at magnification of × 400 and equipped with a heating plate (37 ℃). Visual motility was assessed microscopically with closed circuit television[23].
2.3. Live and abnormal spermatozoa (%)
This was evaluated using eosin-Nigrosin stained smear as described by Sidhu and Guraya [26].
2.4. Sperm membrane integrity
Sperm membrane integrity was assessed using the hypoosmotic swelling (HOS) test[24]. Two hundred spermatozoa were assessed and the percentage of spermatozoa with curled tails (swollen/ intact plasma membrane) was calculated.
2.5. Intact normal acrosome percent
Acrosome integrity was assessed by staining with giemsa[27]. Acrosomal integrity characterized by normal apical ridge was examined under oil immersion lens (× 1 000) using phase contrast microscope. Two hundred sperm were counted.
2.6. DNA fragmentation using acridine orange staining
Acridine orange staining was performed by Katayose et al.[28]. A total of 100 to 200 spermatozoa were observed and classified by type as green, red, or yellow, which is the intermediate type, based on differences in their fluorescent color.
2.7. Statistical analysis
Output data were analysed by one-way analysis of variance (ANOVA), followed by Duncan test to determine significant differences in all the parameters among all groups, with SPSS Version 11.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences with values of P<0.05 were considered to be statistically significant.
3. Results
Sucrose and trehalose concentrations affected many of the studied post-thawing parameters, yielded the highest quality semen after cooling and post-thawing, whereas higher concentrations yielded the lowest quality.
Data output in Table 1 revealed that 50–100 mM of trehalose/L or 50 - 100 mM of sucrose/L improved spermatozoa in diluted semen after cooling through ameliorating significantly (P<0.0001) the attended parameters, including their motility (82.00, 78.00, 80.00 and 82.00% respectively), their membrane integrity [HOStest] (85.00, 83.40, 89.40 and 89.60% respectively), their life% (87.40, 87.80, 90.60 and 91.00% respectively), their abnormal morphology (5.00, 5.40, 6.40 and 6.40% respectively) and their acrosome integrity (78.40, 78.00, 78.40 and 81.00% respectively), when compared to the control tris-base diluent (table 1). The addition of 200 mM/L of either trehalose or sucrose to the tris-base diluent didn’t achieve the target pursued. They lowered significantly (P<0.0001) the power of spermatozoa compared to the control tris-base diluent treatment.
By the same way, data output in table 2 confirmed that addition of 50 mM of trehalose/L, 50 or 100 mM of sucrose/ L to the tris-base diluent had significantly (P<0.0001) maintained the inseminating power of spermatozoa represented in their motility (43.00, 45.00 and 41.00%, respectively), membrane integrity (67.40, 67.80 and 69.40%, respectively), life (70.20, 69.40 and 71.40% respectively), and acrosome integrity percentages (56.40, 58.80 and 55.80% respectively) in a level higher than that of the control tris-base diluent, while diminishing the abnormal sperm morphology (6.20, 3.80 and 3.80 respectively) and DNA fragmentation (3.60, 3.80 and 3.80% respectively, P<0.0018). Besides, the addition of 100 mM of trehalose/L has also a promising effect when added to the tris-base diluent concerning the above parameters (39.00, 60.20, 61.80 and 49.00%, respectively) and 7.60 and 4.20% for abnormal sperm morphology and DNA fragmentation, respectively (table 2). Contrary to their effects on spermatozoa after cooling, the addition of 200 mM/L from sucrose had slightly increased the motility, the membrane integrity and life percentages of freeze-thawed spermatozoa. The addition of 200 mM/L from trehalose had the worst results compared to the control tris-base diluent after freeze-thawed processing (table 2).
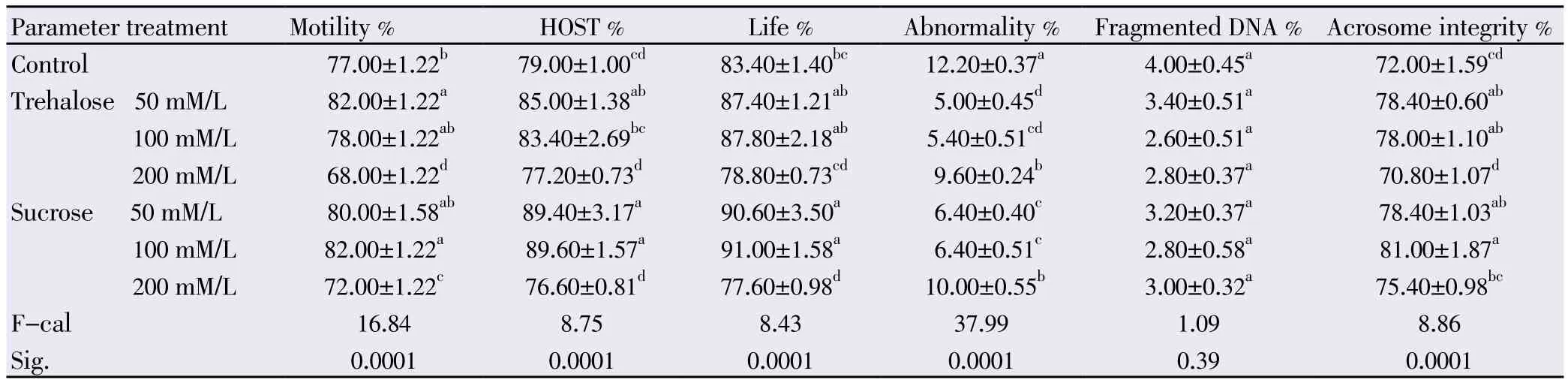
Table 1 The effect of trehalose or sucrose on bull semen after cooling.

Table 2 The effect of trehalose or sucrose on bull semen after freezed-thawing process.
4. Discussion
The results of the present study revealed an improving effect of trehalose and sucrose supplemented to a basic tris extender on bull semen quality (sperm motility, membrane integrity, viability, total sperm abnormalities, DNA fragmentation status and acrosome integrity) after cooling and freezing.
Our results exhibited improved sperm motility, viability, acrosome integrity, sperm membrane integrity and decreased abnormalities while, DNA status was maintained especially with adding trehalose or sucrose in concentrations 50 or 100 mM/L. These results are in accordance with the results obtained by Reddy et al. [7] in buffalo, Tonieto et al. [29] in ram, Aboagla and Terada[13] goat, Hu et al. [30] in boar.
The improved quality of cooled and post-thaw sperm on adding trehalose or sucrose to the extender is due to reducing all injury caused by ice crystallization as trehalose and sucrose are non-permeable sugars render hypertonic media decreasing intracellular freezable water[31]. Iwashi et al. [32], Aboagla and Terada[13] and Reddy et al. [7] referred this reduction in cryodamage of spermatozoa to the interaction of these sugars with phospholipids in plasma membrane and increases membrane fluidity leading to greater resistance of spermatozoa against freeze-thawing damage. While, another argument proposed that probably trehalose protects biomolecular structures through both, the replacement of water in hydrogen bonds[33] and trapping essential hydration water molecules[34]. Also, a role of viscosity in the maintenance of the biomolecular structure has been proposed[35]. Trehalose has indirect antioxidant effect by increasing the level of glutathione and reduced level of lipid peroxide[22]. Trehalose might have displayed cryoprotective effect on the functional integrity of acrosome and mitochondria that is responsible for the generation of energy from intracellular stores of ATP leading to improved post-thaw sperm motility[7].
Trehalose is able to protect the integrity of cells against a variety of environmental stresses such as dehydration, heat, cold and oxidation[36]. It had the remarkable stabilizing properties due to the formation of a non-hygroscopic glass state and protected protein and lipids membranes from degradation during the freeze–drying process. Furthermore, trehalose had been extensively used to improve sperm quality parameters in semen cryopreservation and its protective effects significantly improved the freezability of goat spermatozoa due to increase in membrane fluidity resulting from the depression of membrane transition temperature, allowing the sperm membrane to tolerate lowtemperature effects[5, 13]. The extender containing trehalose improved antioxidant action and reduced the oxidative stress provoked by cryopreservation in bull[5, 6], buffalo bull[8], ram[5, 11, 12, 22] noticed that the extender supplemented with 100 mM trehalose did not affect superoxide dismutase (SOD) levels but catalase (CAT) and glutathione peroxidase (GSH-Px) activities were greater with the supplementation of trehalose at 100 and 200 mM. Sitaula et al. [4] studied the effect of sorbitol and trehalose on sperm motility following partial dehydration and showed a much improved bovine sperm motility in the presence of sorbitol and trehalose. Tuncer et al. [6] evaluated the effects of the addition of different sugars (25 mM raffinose, 25 mM sucrose, and 25 mM trehalose) on bull spermatozoa cryopreserved in a commercial extender (Optidyl) supplemented with 3 mM glutamine on semen parameters, fertilizing ability and superoxide dismutase (SOD) activity. They found that the supplementation of additives did not provide an effect on the level of post-thaw sperm CASA progressive motilities, the sperm motion characteristics, HOST, percentages of sperm motility, acrosomal membrane integrity, and plasma membrane integrity in comparison with the control group. Also, the supplementation of additives did not provide any significant difference on the level of SOD activity[37-39]. However, Khalili et al. [40] obtained the highest post-thawingquality when combining nearly 200 mM of trehalose (198.24 mM) and 8% glycerol. This suggests both that there may be important differences between species regarding the optimal trehalose/sucrose concentrations. This could explain, apart from species differences, why several studies have reported non-positive effects of trehalose and sucrose and even negative effects at some concentrations [22, 38, and 39]. The antioxidant characteristics of some disaccharides as trehalose may be related to its effectiveness in membrane cryopreservation[5, 6]. Trehalose has indirect antioxidant effect by increasing the level of glutathione and reduced level of lipid peroxide[22]. Chhillar et al. [41] reported that both trehalose and taurine decreased H2O2and MDA in frozen- thawed bull semen to the levels of fresh semen, and Badr et al. [8], reported similar results in buffalo semen. Therefore, the effect of trehalose on the oxidative stress concomitant to sperm cryopreservation seems to vary with species, and possibly with the application of different protocols. Also, trehalose might have displayed cryoprotective effect on the functional integrity of acrosome and mitochondria that is responsible for the generation of energy from intracellular stores of ATP leading to improved post-thaw sperm motility.
Ours results revealed that trehalose and sucrose at high concentration (200 mM/L) reduced sperm membrane integrity. These results are in close relation to that of Fernandez –Santosa et al. [18] who proved that membrane integrity and mitochondrial status after thawing depend on osmolarity as low osmolarity (hyposmotic extenders) produce a higher percentage of spermatozoa with intact spermatozoa membrane. Jafaroghli et al. [42] showed that ram sperm can tolerate hyperosmotic diluents at a range of sugar concentration (50–100 mM/L) with improved post-thaw semen quality.
In conclusion, the addition of 50 mM trehalose or sucrose /L or 100 mM trehalose or sucrose/L to TCYF have a beneficial effect in chilling diluted bull semen, while the use of 50 mM trehalose or 50-100 mM sucrose had their benefits on freezing-thawing of extended semen.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are greatly indebted to the National Research Center for sponsoring this work through the project entitled:“ Conservation of the genetic resources of local male breeds using natural additives for semen extenders”, also thanks are due to the team assistants in the project for their help, also thanks are due to Dr. Gharieb A. El-Morsy and all staff of Artificial Insemination Center and Dr. Abd El-Hamid El-Sokary in the General Organization for Veterinary Services, Ministry of agriculture, Egypt, for their kind assistance during this study.
[1] Saacke RG, Nadir S, Dalton JC, Bame JH, DeJarnette JM, Degelos S, et al. Accessory sperm evaluation and bull fertility: an update. Proc. 15th Tech. Conf. Artif. Insem. and Reprod. Nat’l. Assoc. Animal Breeders, Columbia, MO, 1994,p. 57-67.
[2] Woelders H, Matthijs A, Engel B. Effects of trehalose and sucrose, osmolality of the freezing medium and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiol 1997; 35: 93-105.
[3] Crowe JH, Crowe LM, Carpenter JF, Wistrom CA. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J 1987; 242: 1-10.
[4] Sitaula R, Elmoazzen H, Toner M, Bhowmick S. Desiccation tolerance in bovine sperm: a study of the effect of intracellular sugars and the supplemental roles of an antioxidant and a chelator. Cryobiol 2009; 58(3):322-330.
[5] Hu JH, Zan LS, Zhao XL, Li QW, Jiang ZL, Li YK, et al. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J Anim Sci 2010; 88: 1657-1662.
[6] Tuncer PB, Sarıözkan S, Bucak MN, Ulutas PA, Akalın PP, Büyükleblebici S, et al. Effect of glutamine and sugars after bull spermatozoa cryopreservation. Theriogenol 2011; 75: 1459-1465.
[7] Reddy NSS, Mohanarao GJ, Atreja SK. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim Reprod Sci 2010; 119: 183-190.
[8] Badr MR, Mary GA, Hassan MH. Effect of trehalose on cryopreservation, oxidative stress andDNA integrity of buffalo spermatozoa. J Reprod Infert 2010; 1(2): 50-57.
[9] Molinia FC, Evans G, Casares PI, Maxwell WM. Effect of monosaccharides and disaccharides in tris based diluents on motility, acrosome integrity and fertility of pellet frozen ram spermatozoa. Anim Reprod Sci 1994; 36: 113-122.
[10] Aisen EG, Medina VH, Venturino A. Cryopreservation and postthawed fertyility of ram semen frozen in different trehalose concentrations. Theriogenol 2002; 57: 1801-1808.
[11] Bucak M, Tekin N. Protective effect of taurine, glutathione and trehalose on the liquid storage of ram semen. Small Rum Res 2007a; 73: 103-108.
[12] Bucak MN, Atessahim A, Varisli A, Yüce A, Tekin N, Akcay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ramsemen microscopic and oxidative stress parameters after freezethawing process. Theriogenol 2007b; 67: 1060-1067.
[13] Aboagla EM, Terada T. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol Reprod 2003; 69: 1245-1250.
[14] Aboagla EM, Terada T. Effects of the supplementation of trehalose extender containing egg yolk with sodium dodecyl sulfate on the freezability of goat spermatozoa. Theriogenol 2004; 62: 809-818.
[15] Tuncer PB, Umut TD, Serhat B, Taner Ö¸ Erdem CK, Halil E, et al. Effects of different doses of trehalose supplementation in egg yolk extender in frozen–thawed Angora buck semen. Small Rum Res 2013; 113:383-389.
[16] Dalimata AM, Graham JK. Cryopreservation of rabbit spermatozoa using acetamide in combination with trehalose and methyl cellulose. Theriogenol 1997; 48: 831-841.
[17] Squires EL, Keith K, Graham JK. Evaluation of alternative cryoprotectants for preserving stallion spermatozoa. Theriogenology 2004; 62:1056-1065.
[18] Fernandez-Santosa MR, Martinez-Pastor F, Garcia-Macia V, Estes MC, Soler AJ, Paz P, et al. Extender osmolality and sugar supplementation exert a complex effect on the cryopreservation of Iberian reed deer (cervus elaphus hispanicus) epididymal spermatozoa. Theriogenology 2007; 67: 738-753.
[19] Kozdrowski R. The effect of trehalose on post-thaw viability and fertility of European brown hare (Lepus europaeus Pallas, 1778) spermatozoa. Anim Reprod Sci 2009; 116: 326-334.
[20] Madeddu M, Berlinguer F, Pasciu V, Succu S, Satta V, Leoni GG, et al. Differences in semen freezability and intracellular ATP content between the rooster (Gallus gallus domesticus) and the Barbary partridge (Alectoris barbara). Theriogenology 2010; 74: 1010-1018.
[21] Sood S, Malecki IA, Tawang A, Martin A. A survival of emu (Dromaius novaehollandiae) sperm preserved at subzero temperatures and different cryoprotectant concentrations. Theriogenology 2012; 78: 1557-1569.
[22] Aisen EG, Quintana M, Medina V, Morillo H, Venturino A. Ultramicroscopic and biochemical changes in ram spermatozoa cryopreserved with trehalose based hypertonic extenders. Cryobiology 2005; 50: 239-249.
[23] Graham EF, Schmehl MKL, Maki-Laurila M. Some physical and chemical methods of evaluating semen. In: Proc. 3rd NAAB Tech. Conf. Artif. Insemin. Reprod., 12–14 April Milwaukee, WI. National Association of Animal Breeders, Columbia, MO, 1970,p. 44-48.
[24] Jeyendran RS, Vander Ven HH, Perez Pelaez M, Crabo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70: 219-228.
[25] Foote RH. Fertility of bull semen at high extension rates in Tris buffered extenders. J Dairy Sci 1970; 53: 1475-1477.
[26] Sidhu KS, Guraya SS. Buffalo bull semen morphology, biochemistry, physiology and methodology. Ludhiana:USA Publishers and Distributors;1985,p. 152-154.
[27] Watson PF. Use of giemsa stain to detect changes in the acrosome of frozen ram spermatozoa. Vet Record 1975; 97: 12-15.
[28] Katayose H, Yanagida K, Hashimoto S, Yamada H, Sato A. Use of diamide-acridine orange fluorescence staining to detect aberrant protamination of human-ejaculated sperm nuclei. Fertil Steril 2003; 79 (Suppl. 1): 670-676.
[29] Tonieto RA, Goularte KL, Gastal GDA, Schiavon RS, Deschamps JC, Lucia JrT. Cryoprotectant effect of trehalose and low-density lipoprotein in extenders for frozen ram semen. Small Rum Res 2010; 93: 206-209.
[30] Hu JH, Li QW, Li G, Jiang ZL, Bu SH, Yang H, et al. The cryoprotective effect of trehalose supplementation on boar spermatozoa quality. Anim Reprod Sci 2009; 112: 107-118.
[31] Bakás LS, Disalvo EA. Effect of encapsulated Ca2+on the surface properties of curved phosphatidylcholine bilayers. Biochim Biophys Acta 1991; 1065: 114-120.
[32] Iwashi H, Obuchi K, Fuji S, Komatsu Y. The correlative evidence suggesting that trehalose stabilizes membrane structure in the yeast Saccharomyces cerevisiae. Cell Mol Biol 1995; 41: 763-769.
[33] Chen T, Fowler A, Toner M. Brief communication, literature review: supplemented phase diagram of the trehalose-water binary mixture. Cryobiol 2000; 40: 277-282.
[34] Patist A, Zoerb H. Preservation mechanism of trehalose in food and biosystems. Colloid Surface B 2005; 40: 107-113.
[35] Sampedro JG, Uribe S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition: The role of viscosity. Mol Cell Biochem 2004; 256-257: 319-327.
[36] Chen Q, Haddad GG. Review: Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol 2004; 207: 3125-3129.
[37] Najafi A, Zhandi M, Towhidi A, Sharafi M, Akbari SA, Khodaei MM, et al. Trehalose and glycerol have a dose-dependent synergistic effect on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiol 2013; 66:275-282.
[38] Atessahin A, Mustafa N, Bucak MN, Tuncer UB, Kızıl M. Effects of anti-oxidant additives on microscopic and oxidative parameters of Angora goat semen following the freeze–thawing process. Small Rum Res 2008a; 77: 38-44.
[39] Atessahin A, Bucak MN, Tuncer PB, Kızıl M. Effects of anti-oxidant additives on microscopic and oxidative parameters of Angora goat semen following the freeze–thawing process. Small Rumin Res 2008b; 77: 38-44.
[40] Khalili B, Farshad A, Zamiri M, Rashidi A, Fazeli P. Effects of sucrose and trehalose on the freezability of markhoz goat spermatozoa. Asian-Aust J Anim Sci 2009; 22(12): 1614-1619.
[41] Chhillar S, Singh VK, Kumar R, Atreja SK. Effects of taurine or trehalose supplementation on functional competence of cryopreserved karan fries semen. Anim Reprod Sci 2012; 135: 1-7.
[42] Jafaroghli M, Khalili B, Farshad A, Zamiri MJ. The effect of supplementation of cryopreservation diluents with sugars on the postthawing fertility of ram semen. Small Rum Res 2011; 96: 58-63.
*Corresponding author: Walid S. El-Nattat, Animal Reproduction and AI dept., Veterinary Research Division, 13 National Research Centre, Dokki, Giza, Egypt. Postal code: 12622.
Tel: +202-01006212295; +202-26853508;2 02 33371635
Fax : 202-37601877
E-mail: elnattat@gmail.com
 Asian Pacific Journal of Reproduction2015年1期
Asian Pacific Journal of Reproduction2015年1期
- Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Effect of seasons on semen production, effect of melatonin on the liquid storage (5 ℃) with correlated study of birth rate in mithun (Bos frontalis)
- Variations in semen characteristics rams of Ouled Djellal breed have received an important dietary supplement after regular and intensive collection
- Effects of long term storage of semen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa
- Effect of different thawing procedures on the quality and fertility of the bull spermatozoa
- Naloxone affects reproductive system in a rat model with polycystic features
