Persistence of the reproductive toxicity of chlorpiryphos-ethyl in male Wistar rat
Augustave Kenfack, Ferdinand Ngoula, Paul Désiré Womeni Dzeufiet, Omer Bébé Ngouateu, Tsambou Megnimeza Astride Martine, Judith Kegne Chombong, Guylène Marie Zambou Zeukeng, Isabelle Leinyuy Nyuysemo, Arthenice Jemima Noumamo Guiekep, Tah Patience Nain, Pierre Kamtchouing, Joseph Tchoumboué, Narcisse Bertin Vemo
1University of Dschang, Faculty of Agronomy and Agricultural Sciences, Department of Animal Production, Dschang, Cameroon
2University of Yaoundé I, Faculty of Sciences, Department of Animal Biology and Physiology, Yaoundé, Cameroon
3Irad Bambui, Bambui, Cameroon
Persistence of the reproductive toxicity of chlorpiryphos-ethyl in male Wistar rat
Augustave Kenfack1*, Ferdinand Ngoula1, Paul Désiré Womeni Dzeufiet2, Omer Bébé Ngouateu2, Tsambou Megnimeza Astride Martine1, Judith Kegne Chombong1, Guylène Marie Zambou Zeukeng1, Isabelle Leinyuy Nyuysemo3, Arthenice Jemima Noumamo Guiekep1, Tah Patience Nain1, Pierre Kamtchouing2, Joseph Tchoumboué1, Narcisse Bertin Vemo1
1University of Dschang, Faculty of Agronomy and Agricultural Sciences, Department of Animal Production, Dschang, Cameroon
2University of Yaoundé I, Faculty of Sciences, Department of Animal Biology and Physiology, Yaoundé, Cameroon
3Irad Bambui, Bambui, Cameroon
ARTICLE INFO
Article history:
Received 20 August 2014
Received in revised form 18 September 2014
Accepted 25 September 2014
Available online 20 December 2015
Chlorpiryphos-ethyl
Male fertility
Wistar rat
Persistent effects
Objective: To study the effects of chlorpiryphos-ethyl (CE) on male fertility. Methods: Rats were gavaged daily from 30 to 120 days old with four doses of CE (10.50; 5.25; 3.50 and 0.00 mg/kg) and thereafter followed up for 90 additional days. Following this period of observation, each male rat was submitted to a fertility test with two virgin females. Male were then sacrificed and organs collected while females were followed up till delivery. Results: The weights of the testis and epididymis were lower (P<0.05) in rats treated with 10.50 mg/kg CE dose compared to controls. A decrease in the number of sperm per cauda epididymis was recorded in rat given the highest dose of CE with respect to the control value. The sperm motility was lower (P<0.05) in all CE-treated groups compared to the controls. Severe histological abnormalities were observed in testes of treated rats. The males exposed to the highest dose of CE did not produce any offspring. Conclusion: The effects of CE on reproductive system were persistent 90 days after the end of exposure.
1. Introduction
In order to react adequately to growing food demand, farmers have to increase their production. For this purpose, numerous additives including mineral and organic fertilizers are used. Farmers must also fight against weeds, mycosis and pest insects using chemicals commonly called pesticides. Although the use of pesticides is necessary, it should not be forgotten that they are harmful not only to pest organisms, but also to useful animals and human beings[1-4]. The organophosphate family of agricultural pesticides is widely used throughout the world[5-8] and it includes among others chlorpyrifos, dichlorvos, parathion, dimethoate, malathion and diazinon. Symptoms of organophosphate poisoning are generally caused by inhibition of the enzyme acetylcholinesterase which leads to the accumulation of acetylcholine in the synapses and overstimulation of cholinergic systems[2,6,9-14].
3)coord。文档包含的检索词数。一次搜索可能包含多个搜索词,而一篇文档中也可能包含多个搜索词,其包含的搜索词越多,此文档打分越高。
Exposure to organophosphates leads to the impairment of reproductive processes[15]. Thus, many reproductive toxic effects have been linked to organophosphate pesticides. Among others, we can name the decreased sperm count, an increased teratozoospermia and the diminution of the diameter of the seminiferous tubules in mice exposed to a single dose of malathion (240 mg/kg)[16]. In addition, precocious spermiation was noted after oral treatment of rats with dimethyl methyl phosphanate or trimethyl phosphanate [17]. Three-week exposure of rats to methyl parathion diminished sperm count in the epididymis and the weight of reproductive organs[18]. Further examples ofdeleterious effects of organophosphates pesticides can be added.
Chlorpiryphos-ethyl (CE) is an organophosphate pesticide. It is used for crops and foodstuff protection. In Cameroon, the quantity of CE used increases yearly and often without any respect of prescribed norms. In our previous study on male rats[19], the oral administration of CE (3.50-10.50 mg/kg) resulted in a decrease in weight of reproductive organs, concentration of epididymal sperm and sperm motility at the end of the 90-day treatment. In that study, numerous histological aberrations were also recorded in the examined rats. Were those effects reversible? The aim of the present study is to evaluate the persistency of the reproductive toxicity of CE 90 days after the end of exposure in male rats.
2. Materials and methods
2.1. Animals and experimental design
Twenty four (24) male Wistar rats (28-32 days old; 28-32 g of bw) and 48 females (4 months old) were produced in Animal Physiology Laboratory, Faculty of Agronomy and Agricultural Sciences of Dschang University. Rats were housed in glass cages at room temperature and 12h-day light/dark cycle with free access to food and water.
Four doses (control, 3.50, 5.25 and 10.50 mg/kg) of chlorpiryphos-ethyl (Dursban, Chimac Agripar, Belgium) were tested on young male rats randomly distributed to four groups (n=6 per group). The control lot received distilled water. Thus, 0.583 mL of distilled water or solution containing CE was administered per kg of body weight. Animals were gavaged daily from 30 to 120 days of age and volumes of gavage solution were adjusted weekly to body weight. Ninety days following the last treatment, rats were sacrificed. Prior to sacrifice, each male and 2 females were allowed to mate for a period of 2 weeks (days 76-90 posttreatment).
2.2. Data collection
The body weight was measured on day 90 after the end of the CE exposure. Animals were then euthanized and the testes, epididymis, vas deferent, seminal vesicles and prostate were removed and weighed. The right cauda epididymis was weighed and minced in a known volume of 0.9% NaCl (36 ℃) for sperm motility and concentration evaluation. The motile and non motile sperms were counted separately with the aid of a light microscope. The sperm concentration was obtained by using the Thomas haematocytometer.
The testis was fixed in Bouin’s fluid, and washed, dehydrated in alcohol baths of ascending grade, clarified in xylen immersion, hardened in paraffin, sectioned and stained with haematoxylin and eosin. The tissue sections were observed under a light microscope (Zeiss, 400×) for changes in the seminiferous tubules and intertubular spaces. The fertility rate was calculated following the formula:
Fertility rate = number of males which procreated/total number of the examined males
2.3. Statistical analysis
Data were expressed as means ±SD and statistical analysis was performed by one way ANOVA followed by Duncan’s test at 5%.
3. Results
Except for the 5.25 mg/kg treated-group treated group, the weights of testes and epididymis (Table 1) were lower in CE-receiving rats as compared to controls, all differences were significant (P<0.05). The weight of seminal vesicles was not significantly (P>0.05) different between the control and gavaged animals, excepted in 5.25 mg/kg treated-group where it was the smallest. The weights of vas deferent and prostate did not differ (P>0.05) among doses.
The number of spermatozoa per gram and per cauda epididymis, as well as sperm motility were lower (P<0.05) in CE-treated rats compared to control animals (Table 2).
Males treated with the highest dose of CE produced no offspring (Table 3). However, fertility rate and litter size of other CE-treated rats were comparable to those of controls (P>0.05). The survival rate and the sex-ratio (males/females)decreased in a dose related-fashion, but no significant (P>0.05) difference was shown.

Table 1 Effects of chlorpyriphos-ethyl on the male reproductive organs weight (g/100 g body weight) in rat (n=6).
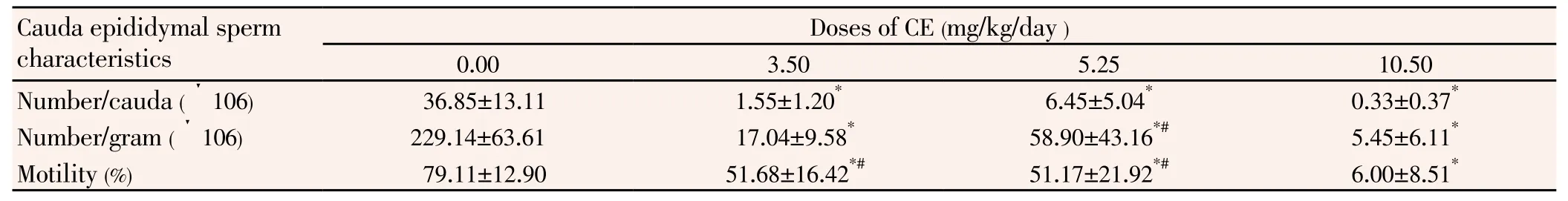
Table 2 Influence of chlorpyriphos-ethyl on cauda epididymal sperm count and motility(n=6).
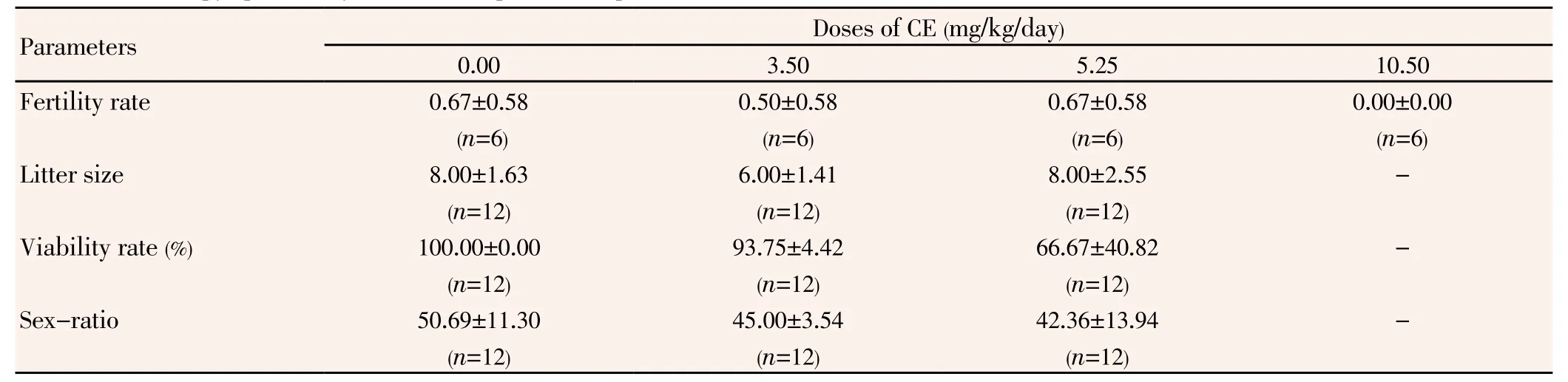
Table 3 Influence of chlorpyriphos-ethyl on the rat reproductive performances.
Typical structure of the testis was observed in control rats (Figure 1A); the seminiferous epithelium contained all generations of germinal cells corresponding to the stages of seminiferous epithelium cycle. The lumen contained normal flagellated spermatozoa. Interstitial space was also typical. In contrast, the enlargement of the interstitial spaces and the vacuolization of germinal epithelium and intertubular cells were observed in all treated animals (Figure 1B, 1C and 1D). The lumen of almost all seminiferous tubules of CE-treated rats was filled by germ cells liberated before the completion of spermatogenesis (Figures 1 C and D). The incidence of these abnormalities varied independently on the administered dose of CE.
4. Discussion
The decrease of testis and epididymis weights in CE-treated rats observed was previously recorded at the end of the exposure[19]. The diminution of testis weight could be attributed at least partly to it alteration as shown by testes histological sections. Regarding the weight of the epididymis, a close conclusion has been reported using up to 1.5 mg/kg of methyl parathion, another organophosphate pesticide[18]. The lowering of the epididymis weight resulting from the current study could be linked to that of the testis. In fact, the development of the accessory reproductive organs in male is under the control of the testis[20]. The low concentration of sperms in the epididymis could also cause the decrease of its weight.
The diminution of epididymal sperm count is supported by a previous result obtained 17 days following a 3-week exposure to methyl parathion, an organophosphate pesticide [18]. The germ cells liberated into the lumen before complete spermatogenesis could be phagocytised during epididymal transit, and thus account for the observed low epididymal sperm count of treated rats[21].
The 73.11% decrease of sperm motility between control and 10.50 mg/kg is by far greater than that recorded at the end of gavage (18.66%)[19]. The weak sperm motility in CE-treated rats could also be due to the fact that a great majority of sperm was liberated prematurely from germinal epithelium of treated rats. It could also be linked to cytotoxic effects of CE.
The disorganisation of germinal epithelium was more pronounced than at the termination of the treatment[19]. These results differ from those reported for animals allowed to recover for 14 weeks after treatment with dimethyl methyl phosphanate, in which various degrees of recovery were noted in affected tubules[22]. The germ cells previouslydescribed in the lumen of the seminiferous tubules of CE-treated rats would be released from the portions of the tubule where epithelium was disorganised. It appears from testis sections that the exfoliation of germ cells start with more differentiated cells. It was stated earlier that the loss of germ cells begins with spermatids, but progressively affects preceding germ cell types, and tubules with maturation arrested at the level of spermatocytes or spermatogonia are observed[23].
The infertility of untreated females mated by males of the highest dose group was foreseeable because in that lot, no mobile spermatozoon was observed in the cauda epididymys (Table 2). The analogy between fertility rate of rats given 5.25 mg/kg and the controls was not surprising. In fact, a previous study showed that up to 90% reduction in the number of fertile sperms deposited during mating would not markedly reduce fertility, normal males of mammals ejaculating a great excess of spermatozoa[24].
The persistence of the harmful effects of CE on the male reproductive organs is not surprising since CE accumulates in the organism. However, it is not known if the effects observed 90 days after the end of the exposure are caused by the accumulated insecticide or by lesions provoked during the time of exposure.
The effects of chlorpiryphos-ethyl on male reproductive organs persisted while becoming more prominent 90 days following the last day of exposure. However, despite the lesions registered, chlorpiryphos-ethyl-treated males remained fertile.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Sebe AMD, Salim Star MD, Rana Alpay MD, Nalan Kozaci MD, Ahmet Hilal MD. Organophosphate poisoning associated with foetal death: A case study. Mount Sinai J Med 2005; 72: 354-356.
[2] Kristoff G, Cacciatore CL, Verrengia GNR, Cochón AC. Effects of the organophosphate insecticide azinphos-methyl on the reproduction and cholinesterase activity of Biomphalaria glabrata. Chemosphere 2012; 84 (5): 585-591.
[3] Jordaan MSS, Reinecke A, Reinecke AJ. Acute and sublethal effects of sequential exposure to the pesticide azinphos-methyl on juvenile earthworms (Eisenia andrei). Chemosphere 2012; 88(4): 450-458.
[4] Sheena EM, Melissa JP. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 2013; 307: 66-73.
[5] Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect 2003, 111: 536-544.
[6] Richardson JR, Chambers JE. Neurochemical Effects of repeated gestational exposure to chlorpyrifos in developing rats. Toxicol Sci 2004;77: 83-90.
[7] Richardson JR, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyriphos on cholinergic neurochemistry in developing rats. Toxicol Sci 2005; 84: 352-359.
[8] Sandra Y, Manuel G, Julio R, Gustavo FG. Semen quality in Peruvian pesticide applicators: association between urinary organophosphate metabolites and semen parameters. Environ Health 2008;7:59. doi:10.1186/1476-069X-7-59.
[9] Cox C. Chlorpyrifos, part 2: Human exposure. J Pest Reform 1994 ; 15(1): 14-28.
[10] Van Hammée ML, Wattiez C. Home use pesticides: health risks. Rhode-Saint-Genèse, Bruxelle-Belgium: Pesticides Action Network;1999, p. 84 p.
[11] Ankarberg E, Fredriksson A, Eriksson P. Increased susceptibility to adult paraoxon exposure in mice neonatally exposed to nicotine. Toxicol Sci 2004, 82: 555-561.
[12]Smulders CJGM, Van Kleef RGDM, De Groot A, Gotti C, Vijverberg HPM. A non competitive, sequential mechanism for inhibition of rat α4β2 neuronal nicotinic acetylcholine receptors by carbamate pesticides. Toxicol Sci 2004; 82: 219-227.
[13] Padilla S, Marshall RS, Hunter DL, Oxendine S, Moser VC, Southerland SB, et al. Neurochemical effects of chronic dietary and repeated high-level acute exposure to chlorpyrifos in rats. Toxicol Sci 2005, 88: 161-171.
[14] Cacciatore LC, Kristoff G, Verrengia GNR, Cochón AC. Binary mixtures of azinphos-methyl oxon and chlorpyrifos oxon produce in vitro synergistic cholinesterase inhibition in Planorbarius corneus. Chemosphere 2012, 88(4): 450-458.
[15] Aguilera L, Marquetti MC, Navarro A, Bisset JA. Effects of 3 organophosphates on the reproduction of Culex quiquefasciatus Say 1823. Rev Cubana Med Trop 1994; 46:171-174.
[16] Bustos-Obregon E, Gonzalez-Hormazabal P. Effects of a single dose of malathion on spermatogenesis in mice. Asian J Androl 2003, 5: 105-107.
[17] Cho NH, Park C. Effects of dimethyl methyl phosphanate (DMMP) and trimethylphosphanate (TMP) on spermatogenesis of rat testis. Yonsei Med J 1994, 35:198-208.
[18] Prashanthi N, Narayana K, Nayanatara A, Chandra Kumar HH, Bairy K, D’souza UJ. The reproductive toxicity of the organophosphate pesticide 0, 0-dimethyl 0-4-nitrophenyl phosphorothioate (methyl parathion) in the male rat. Folia Morphol (Warsz) 2006, 65: 309-321.
[19] Kenfack A, Ngoula N, Tchoumboué J, Kamtchouing P. Influence of chlorpiryphos-ethyl in some male reproductive parameters in Albinos rats exposed post-natally. Int J Biol Chem Sci 2007; 1: 237-243.
[20] Dacheux F, Dacheux JL. The Epididymis and accessory glands. In: Thibault C, Levasseur MC (eds). Reproduction in domestic animals and human. Paris: Wiely Online Libary;2001, p. 290-315.
[21] Sutovsky P, Moreno R, Romalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependant mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci 2001, 114: 1665-1675.
[22] Chapin RE, Dutton SL, Ross MD, Sumrell BM, Lamb JC. 4th. Development and reproductive tract lesions in male F344 rats after treatment with dimethyl methylphosphanate. Exp Mol Pathol 1984, 41: 126-140.
[23] Paniagua R, Nistal M, Saez FJ, Fraile B. Ultrastructure of the aging human testis. J. Electron Microscopy Techn 1991, 19: 241-260.
[24] Amann RP. Detection of alterations in testicular and epididymal function in laboratory animals. Environ. Health Perspect 1986; 70: 149-158.
*Corresponding author: Augustave Kenfack, University of Dschang, Faculty of Agronomy and Agricultural Sciences, Department of Animal Production, Dschang, Cameroon.
Tel: (+237)95705005
E-mail: augustavekenfack@yahoo.fr
 Asian Pacific Journal of Reproduction2015年1期
Asian Pacific Journal of Reproduction2015年1期
- Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Ovine fetal sex determination using circulating cell-free fetal DNA (ccffDNA) and cervical mucous secretions
- A new treatment for premature ejaculation? Case series for a desensitizing masturbation aid
- Reproductive status of Camelus bactrianus during early breeding season in India
- Effect of frequency of collection on seminal characteristics of White Pekin duck
- Evaluation of norgestomet Crestar® on oestrus synchronization and reproductive performance of dairy cows in Algeria
