Texosome-based drug delivery system for cancer therapy: from past to present
Hamideh Mahmoodzadeh Hosseini, Raheleh Halabian, Mohsen Amin, Abbas Ali Imani Fooladi
1Applied Microbiology Research Center, Baqiyatallah University of Medical Sciences, Tehran 1435916471, Iran; 2Department of Drug and Food Control, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1417653861, Iran
Introduction
The global rise of cancer outbreaks and consequent endeavor to find efficient and specific treatments are becoming hot topics in the field of cancer research.Common protocols such as chemotherapy, radiotherapy, and surgery are not efficient enough to meet all the needs in cancer eradication.Cancer recurrence, lack of sufficient efficacy on metastatic cancer, and emerging drug resistance usually occur after classic therapies.Therefore, devising new treatment strategies with both high efficiency and low toxicity is necessary and is the first priority in cancer field.Given that malfunction of the immune system,especially in a tumor microenvironment, leads to tumor growth and tumor progression1, cancer immunotherapy can be an option to stimulate the systemic immune system to combat tumor expansion2.Immune responses are modulated through immunotherapy strategies, causing specific removal of tumor cells and retarding metastases and stimulating memory immune cells against disease recurrence3,4.Recently, exosomes, which are natural nanovesicles, have been introduced as candidates for cancer immunotherapy.Exosomes are endosomal membranous vesicles with sizes ranging from 30 to 100 nm secreted by all kinds of mammalian cells into the extracellular microenvironment both in pathologic and physiologic conditions5.Moreover, exosomes have been isolated from biological fluids, such as serum and urine, and in the supernatant of cell cultures.Compared with normal cells, more exosomes are released from cancerous cells5,6(details regarding exosome properties were reviewed by Hosseini et al.5).
Exosomes exert several biological activities, including cell-cell communication and transport of genetic materials (e.g., miRNA and mRNA), alter the phenotypic characteristics of recipient cells via protein transport, and modulate the immune system7.The inherent potential of exosomes for delivering and carrying materials make them a suitable agent for drug delivery and gene therapy.In the past two decades, attempts have been made by researchers to understand the behavior of exosomes and their potency in drug delivery.To improve the exosomal drug delivery system, various manipulations have been conducted on intact exosomes, particularly on the mimetics of the exosomes(Figure 1).In this review, an overview of exosome drug delivery system is presented, and the classification of the system is explained in three categories: first-generation exosomes, in which the exosomes are applied without any manipulation;second-generation exosomes, in which biotechnological and bioengineering manipulations are applied; and third-generation exosomes, which are produced directly from cells through mimetic and synthetic methods.
Exosome biogenesis
Exosomes are endogenous vesicles budding from endosome compartment during maturation of early endosome to late endosome as multi-vesicular bodies (MVBs)8.Evidence exists that the activity of phosphatidylinositol-3 kinase (PI-3 kinase)is essential to produce MVBs and in the subsequent secretion of exosomes in mammalian cells8,9.Loss of PI-3 kinase suppresses MVB formation due to endocytic compartment swelling9,10.In general, several factors determine the fate of MVBs.These factors include cholesterol content, presence of ligand for membrane proteins, proteins involved in the endosomal sorting complex required for transport (ESCART) system, tetraspanin proteins,and presence of sphingomyelinase.The cholesterol content of MVBs could fuse with the plasma membrane and secrete exosomes (cholesterol rich manner) or be digested after fusion with lysosome in poor cholesterol content11,12.Denzer et al.8suggested that membrane proteins could incorporate into MVBs.Exosome biogenesis happens under two patterns, namely,ESCART-dependent or ESCART-independent pathways.Some accessory proteins, including Alix and vacuolar protein sorting 4 (VPS4), have been shown to be involved in the ESCART-dependent pathway11.Several processes have been explained in the ESCART-independent system.Sphingomyelinase,tetraspanin proteins, and certain regions such as endosomelike domains in the plasma membrane are involved in the independent pathway of exosome biogenesis13-15.
Finally, after the fusion of MVBs with the cellular membrane,the exosomes are secreted into the extracellular environment in both constitutive or inducible manner based on the type and condition of the cells16,17.Additionally, some members of the Rab family, such as Rab27a and b, participate in the exosome release18.Interestingly, soluble SNARE proteins can designate MVB for cellular membrane fusion19.Ubiquitination is one of the main mechanisms involved in the sorting of the endosomal proteins of MVBs.ESCRT proteins are necessary to move forward the MVB biogenesis.ESCRT-I, -II, and -III,Hrs, and Vps-27 are different kinds of protein complexes that recognize the monoubiquitinated cargos and lead them toward the MVB compartment.These complexes leave the MVB compartment with the help of adenosine triphosphatase and VPS4 after MVB formation and take part in the new cycle of cargo transportation20,21.Protein aggregation and clustering are two other mechanisms for sorting cargos.These mechanisms are independent of the monoubiquitination pathway22,23.Moreover,the passive manner for sorting cargos occurs via lipid raftenriched tetraspanins and/or cholesterol22,24.
Exosomes target and bind to recipient cells selectively.This selectivity was confirmed in a study on the exosomes from platelets and B cells25.Moreover, exosomes from B cells attached only to follicular dendritic cells (DCs)26.
Similar to other fusion processes, the regulatory role of Ca2+concentration and syntaxin-7 proteins make them plausible for exosome fusion27,28.Our findings of exosomal delivery to cells are limited.Results reported by Montecalvo et al.29pointed out the potential of exosomes to transfer their cargos into the cytosol of targets.The interaction between exosomes and recipient cells is classified into three categories: (I) fusion through a subset of integrin family or via calcium and annexin V26,30,31; (II) ligand-receptor interplay30,32; (III) endocytosis29,33,34.The environmental factors and maturation level of cells regulate the turnover of exosome-recipient cell interactions as well as their fusion.Acidic environments increase the yield of fusion.Based on that finding, the fusion turnover within tumor tissues is higher than that in normal ones due to the acidic microenvironment of tumors34,35.Furthermore, maturation of bone marrow DCs reduced the uptake of exosomes36.
Exosome structure and composition
Exosomes are bilayer membranous nanovesicles with endogenous origin.The composition profile of each exosome is closely related to the content of the cell of origin.Additionally,this profile dictates the functionality of exosomes17,37.Various common procedures, such as MALDI-TOF/Q-TOF mass spectrometry, trypsin digestion, immunoblotting, and SDSPAGE, are utilized to appraise the protein content of exosomes.Moreover, thin layer chromatography is used for lipidomics analysis of exosome lipids37-39.The most diverse compounds within exosomes are proteins.Exosomal proteins are classified into two groups; the proteins found in all exosomes irrespective to the cells they are released from and those that are exclusive to a specific exosome.The proteins that are essentially involved in the exosomal biogenesis and functions are categorized in the first group40.This type of protein includes the ones contributing to membrane fusion, cytoskeleton components, cell-signaling molecules, adhesion proteins, chaperones, metabolic enzymes,MVB-forming proteins, and tetraspanin family proteins37,41.By contrast, the specific proteins within exosomes are proteins belonging to the cells from which the exosomes originate and contribute to certain roles dependent on the original cells40.For further information, please check the ExoCarta website42.
Limited research has been done on the lipidomic features of exosomes, and limited data are obtained on the lipid composition of exosomes obtained from some cells such as reticulocytes43,mast cells, DCs44, and B lymphocytes24.Sphingomyelin,lysophosphatidylcholine, saturated fatty acid, phosphatidyl ethanolamine, phosphatidylserine, phosphatidylcholine,diacylglyceride, and cholesterol are common lipids with different abundance detected in the membrane of exosomes derived from different origins37.Lysophosphatidic acid is a frequent lipid within exosomes and is necessary for exosome biogenesis and MVB formation45.Moreover, the functional units and micro-domains,i.e., lipid rafts, have been identified in exosome membranes.These units consist of major lipids, such as cholesterol and glycosphingolipids, and various proteins including Src family members and glycosyl-phosphatidylinositol-anchored proteins46.
Exosomes are considered genetic shuttles that are able to transfer genetic materials (e.g., mRNA and microRNAs) from the primary cells, conferring some new genetic and epigenetic features to the recipient cells33,47.
Texosome roles in tumor progression
Tumor microenvironment is a space that determines the fate of tumor cells through communication among resident cells in tumor tissues, such as tumor cells themselves, immune cells, and matrix cells.Figure 2 summarizes the effects of texosomes on the immune cells located in the tumor microenvironment.The release of inhibitory soluble substances suppresses the immune system located in tumor tissues and progresses the tumors48.One of the main agents contributing to this process is the exosome derived from tumor cells called texosome49,50.Convincing evidence exists for the presence of high quantity texosomes in blood and malignant effusions, which indicate the load and the stage of tumor in patients51-53.Carrying high amount of both known and unknown tumoral antigens, texosomes are offered as candidates for cancer vaccine52,54,55.However, numerous studies show the immunosuppressive effects of texosomes.
Texosomes can evade from the immune system through certain effects on both native and acquired immunity.Findings from studies on various cancer cell lines, including prostate cancer, head and neck cancer, gastric cancer, melanoma, and colorectal carcinoma, revealed that some texosomes trigger the expression of pro-apoptotic agents FasL and TRAIL56-60.These texosomes are able to interact with Fas molecules on the surface of active T cells and induce Fas/FasL apoptosis in T cells.In addition, pro-apoptotic texosomes cause CD3-ζ chain down-regulation and TCR signaling inhibition61.Increased adenosine level following dephosphorylation of 5’AMP and ATP via CD39 and CD73 happens during suppression of local immune responses62.In a study on natural killer (NK) cells,the cell function was impaired upon exposure to a texosome.This texosome prevented the expression of NK2GD in NK cells and subsequently attenuated their proliferation63,64.In another study, exposure of NK cells to texosomes containing MICA*008 alleviated the toxic effects of NK cells65.Moreover, the texosome derived from invasive murine breast tumor cells caused attenuated activation of NK cells following IL-2 secretion and decreased function of perforin/granzyme B-mediated effector66.
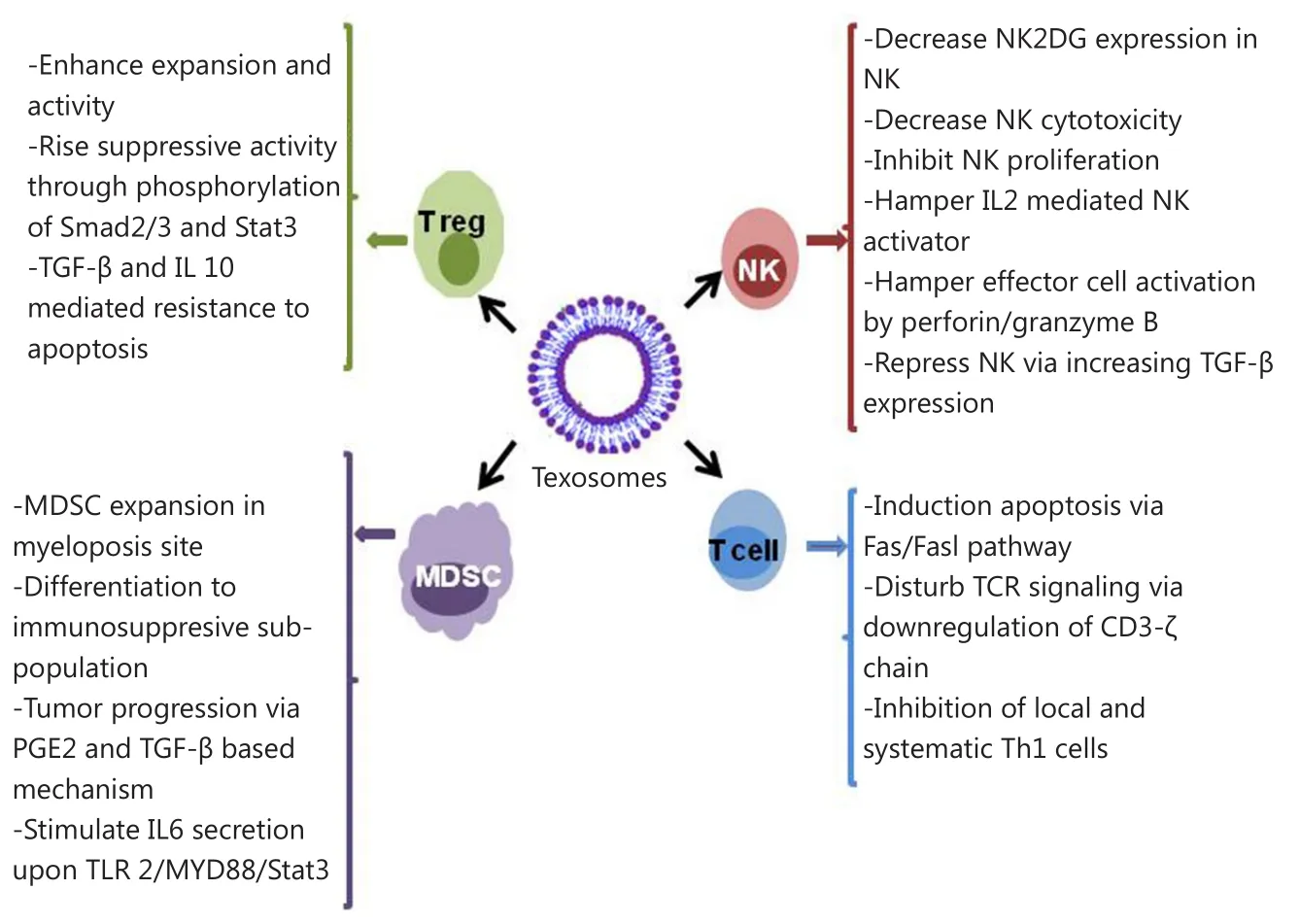
Figure 2 Effect of texosomes on the discrepant immune cells located in tumor microenvironment.
On the other hand, texosomes released by melanoma and colon carcinoma impaired the differentiation of CD14 monocyte to myeloid-derived suppressor cells, which repress activity and proliferation of T cells upon TGF-β release.This type of myeloid cell was isolated from blood of patients suffering from hepatocellular carcinoma67, bladder cancer68, and multiple myeloma69.Texosomes exerted the booster impacts on the activity and expansion of T regulatory cells (Treg) through phosphorylation of Stat3 and Smad2/3.These events led to the increase of the texosomes’ suppressing activity and resistance to TGF-β and IL-10 mediated apoptosis70.
In addition to suppressing the antitumor immune responses,texosomes are involved in tumor progression owing to improving angiogenesis and remodeling the extracellular matrix upon modulation of stromal cells and metastasis71.
Texosomes contain some functional proteins and genetic materials that take part in triggering the synthesis, formation, and expansion of extracellular matrix and vasculation72.Tetraspanins are essential components of MVB biogenesis and have been known as a pro-angiogenic factor that can incite tumor growth upon systemic angiogenesis73,74.The removal of Tspan8-CD49d complex from texosomes caused stimulation of gene expression of some angiogenic factors, such as Tspan8, von Willebrand factor, VEGF receptor 2, and VEGF.This complex induces and improves the proliferation, maturation, and migration of endothelial cell progenitors75.Moreover, Notch ligand delta-like 4 carrying texosomes contribute in vasculation and angiogenesis76.Overall, the presence of angiogenic factors, such as angiogenin, FGFa, IL-6, IL-8, TIMP-1, VEGF, and TIMP-2,in texosomes induces formation and improvement of tubular for vasculation77.
On the other hand, secretion of texosomes by cancer cells can give rise to resistance to chemotherapeutic drugs such as cisplatin and vinblastine through sequestering and pumping them out of the tumor cells.Resistance can also happen through the secretion of high amount of texosomes carrying transporters of drugs such as MRP2, ATP7A and ATP7B78.Furthermore, texosome secretion negatively affects the potency of antibody-based treatment79.This phenomenon occurs due to several mechanisms.The circulating texosomes in the peripheral blood bind to and neutralize the antibodies, thereby reducing the effective dose of antibodies against a tumor tissue.These texosomes attenuate antibody-dependent cytotoxicity of immune cells.Finally, secretion of texosomes has resulted in depletion of complement factors that protect tumor cells from antibody attack and inhibit cytolysis upon complement activation80,81.
First generation of exosomal drug delivery system
Despite the tumorigenesis behavior of texosomes, these nanovesicles have properties that make them suitable for designing a noble anti-cancer vaccine.The presence of numerous broad-range tumor antigens [e.g., HER2, Mart-1, and carcinoembryonic antigen (CEA) along with MHC-peptide complexes within texosomes] confers a beneficial feature to T-cell cross-priming52,82,83.Several studies were performed based on this theory.Table 1 outlines the pioneer studies in the field of texosome-based immunotherapy.Findings of these studies were the basis for future attempts for the design of efficient drug delivery systems.
Second generation of texosome drug delivery system
In 2011, Alvarez-Erviti and his colleagues90conducted the first study on biotechnological manipulation of texosomes to make them applicable in targeted delivery of siRNA.
Texosome display strategy is another useful technology to create non-genetic engineering manipulation of texosomes for medical applications.In this strategy, a broad range of different antigens is fused with lipid bilayer compounds91,92.In this technology, antigens can be coupled with texosomes in a specific or non-specific binding manner.In specific fusion procedure,a recipient domain exists on the surface of texosomes that can attach to the desired antigen.For instance, in several studies,the presence of the C1C2 domain of lactadherin has been shown to be important for the fusion of antigens for therapeutic purposes91.
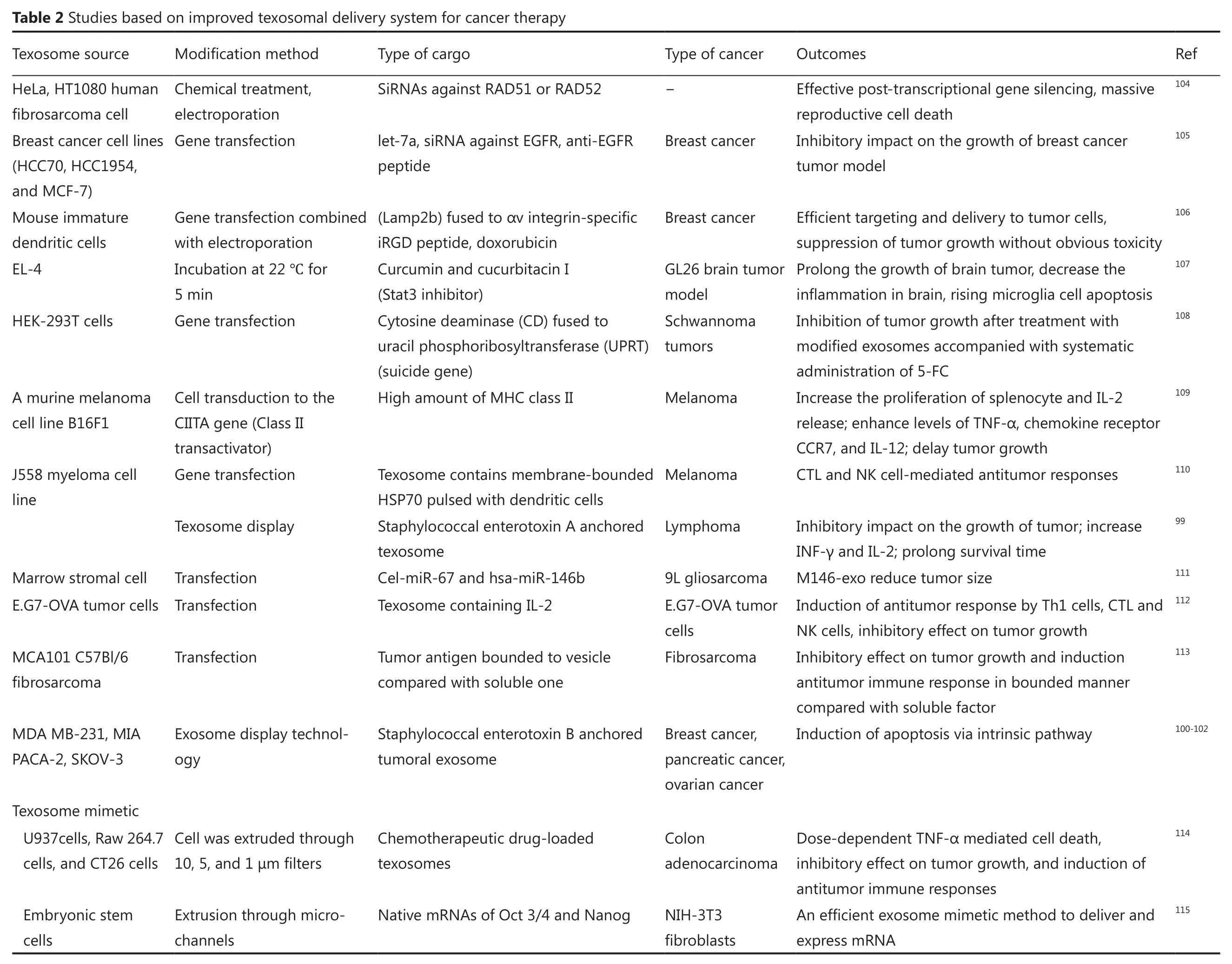
In non-specific method, the antigens are anchored in the membrane of texosomes, and the lumen is loaded with the components.Microbial metabolites and toxins, especially superantigens, can be used as cytostatic molecules in cancer prevention.Superantigens are potent T cell activators that can be suitable candidates for immunotherapy.These compounds attach to the major groove of MHC II proteins on the surface of antigen-presenting cells and enhance the proliferation and activation of T cells in a non-specific manner93.Previous researches showed that superantigens have the potential to trigger antitumor immunity94-97.Furthermore, the apoptotic features of superantigens via extrinsic pathway were reported98.Given that high amounts of tumoral antigens within texosome may induce energy in the immune system, designing a conjugate structure made up of texosomes and superantigens can activate the cytostatic events in tumor cells and can stimulate particular antitumor immune responses.The anchoring of staphylococcal enterotoxins A and B on tumor texosomes has been explained by Xiu et al.99and Mahmoodzadeh Hosseini et al.100-103.Those studies are examples of the approach that confers an antitumor activity property to the construct.Table 2 outlines the attempts using this strategy.
Methods for loading texosomes with therapeutic cargo
One strategy to alter the properties of texosomes and to give new characteristics to texosomes is the loading of differentcomponents within texosomes.Several techniques such as electroporation, transfection, and incubation are applied to load the texosomes116,117.
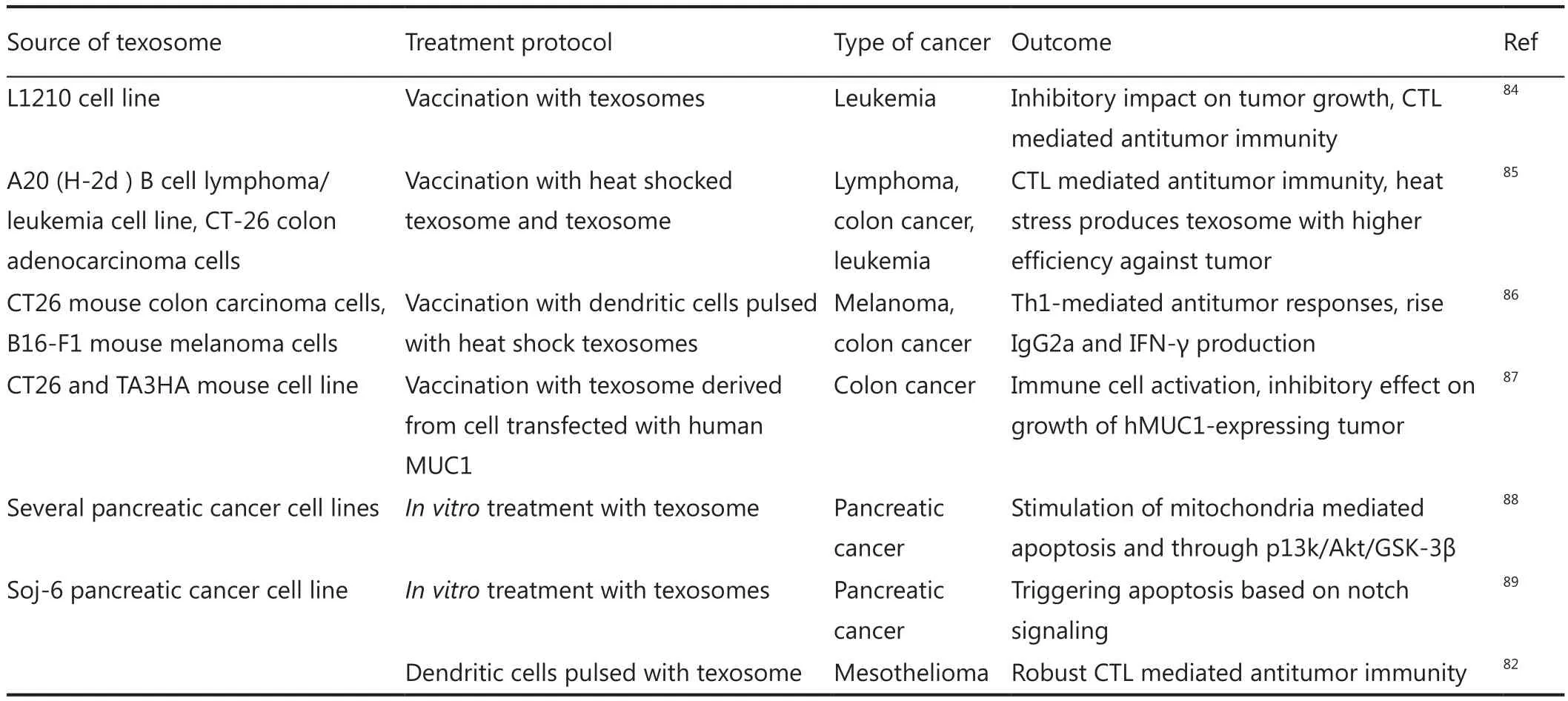
Table 1 First-generation texosome-based delivery system for cancer therapy
?
In electroporation, transient pores are created into texosomal membrane by an electrical field using 150-700 V, transferring the desired component across the membrane and reach the center of texosome lumen118.Previous studies utilized this technique for the uptake of siRNAs and doxorubicin90.Despite the success rate of this method to load various cargos, optimal parameters should be set based on the cell of origin104,106,119.In general, 0.07 to 0.5 μg/μL of texosomes is required for electroporation90,106,116,120.This method may be favorable in medical applications due to parameter control, but adverse effects may occur, such as loss of integrity of the texosome and the loaded cargo.In addition,some evidence reveals that electroporation can provoke the aggregation of both siRNAs and texosomes, which significantly reduce cargo preservation.However, optimizing the parameter and using some special media containing trehalose are able to attenuate texosome aggregates116,117.
Some transfection reagents such as HiPerFect and Lipofectamine 2000 are commercially available for loading siRNA into texosomes104,119.However, the efficacy of these reagents is lower than that of electroporation; therefore, it may not be an appropriate method for therapeutic purposes104,119.Another method for loading the therapeutic compounds is the isolation of texosomes from transfected cells containing overexpressed proteins of choice or miRNAs.These special products will be packaged into the secreted exosomes105,111.This strategy could be useful for tumor therapy to suppress certain oncogenes.The transfection-based method of loading cargo is an appropriate process.However, this method is not favorable for medical use when the individual donor cell was applied because the processes of achieving potent engineered cells are labor-intensive and time-consuming.An efficient transfected cell should produce texosomes bearing both targeting properties and containing high quality/quantity cargo.
Finally, certain incubation procedure can be utilized to load the desired cargo into texosomes.A study used this method to load curcumin into the lumen of texosomes for 5 min at 22 ℃, inducing significant anti-inflammatory impacts in diseased models107,121.Curcumin can alter the fluidity of exosomal lipid bilayer and can facilitate movement of the cargo into the lumen122,123.Interestingly,1 and 2 h incubation at 37 ℃ were successful for loading miR-150 and doxorubicin, respectively114,124.The size of the cargo is a key factor for its loading and movement across the membrane and has an impact on the efficacy of this method.In addition to loading technique, purification protocols improve the quantity and quality of texosome-based drug delivery systems for therapeutic purposes125.Limited research is available on the methods for loading cargos within texosomes.Therefore, novel procedures to improve loading efficiency are necessary for medical use.
Third generation: texosome mimetics
Along with biotechnological strategies, synthesizing texosomes opened a new avenue to design an efficient texosomal drug delivery system named texosome mimetics.The idea of texosome mimetics has originated from the fact that numerous compounds existing in texosome structures, such as proteins and lipids, are unnecessary for special practical purposes.On the other hand,some components carried by natural texosomes are incompatible with therapeutic and medical purposes, and even some adverse effects may happen.Texosome mimetics provides the possibility to select special functional lipid, protein, and nucleic acid, such as siRNAs and miRNAs, as cargos according to desired purposes.Some structural similarities exist between texosomes and liposomes.Both of them have a spherical shape with size of lower than 100 nm, and their contents have been surrounded by lipid bilayer; thus, the principles involved in liposome preparation could be beneficial to texosome mimetics.These principles can provide a new field to generate efficient non-viral drug delivery systems126,127.The small size of texosomes allows them to penetrate the tissues and deliver the cargo (e.g., drugs) efficiently without systemic side effects.On the other hand, texosome mimetics improves the pharmacokinetic parameters, such as bioavailability, metabolism, and exertion.Mimetic texosomes can be classified based on the functional cargos and targeting components such as adhesion molecules or special ligands or receptors114.Cell extrusion by serial filtration is a procedure to synthesize exosome mimetics with structural properties relatively equivalent to natural texosomes.Data of several studies on texosome mimetics showed no side effects associated with different cell sources.Therefore, the application of nonautologous texosome mimetics is feasible for treating different diseases.In addition, similar to natural texosomes, texosome mimetics have the potential of loading and carrying several drugs(particularly chemotherapeutic and herbal drugs) to target cells without adverse effects on healthy cells.Surprisingly, all sorts of modifications discussed in the biotechnological manipulations and texosome display in the former sections can be applied in texosome mimetics to design new structures.This possibility is useful for dual targeting the structures needed for angiogenesis in cancer therapy.For this purpose, a drug delivery system with dual targeting (i.e., one toward cancerous cell and the other toward tumor endothelium) should be created to enhance the efficacy of antitumor activity, especially in drug-resistant tumors.
Advantage of texosomal drug delivery system
Texosomes have specific characteristic of carrying functional materials within the body, which makes the texosomal delivery system a relatively new discipline for specific and efficient therapy.Texosomes are stable in blood circulation, especially against the activity of coagulant substances and complementary systems90,128, and their autologous usage is due to lack of immunogenicity15.In spite of considerable advantage over other delivery systems, the lower yield of texosome production for clinical application is an obstacle.Native texosomes form complex structures with unknown pharmaceutical properties129,130.Given that various endogenous vesicles from different cell types are present in biological fluids, the isolation of a specific population of the vesicle (e.g., texosome) is a difficult process or is even impossible.On the other hand, exogenous texosomes purified from a cell line are immunogens and trigger unpleasant immune response and adverse effects131.
To overcome the described disadvantages, texosome mimetics strategy was invented.In this strategy, texosomes are produced in large scale, which is desirable for preclinical or clinical applications.The yield of texosome production is 100 times higher than those of convenient purification methods.Texosome mimetics is a controllable process.The product is well characterized with explicit and acceptable pharmaceutical properties, and the impact of each substance can be studied132.The information concerning probable biological activities of functional proteins and lipids assembled in texosome mimetics is not accessible in the literature.
Clinical trial
To date, few clinical trials have been conducted against cancer therapy.As outlined in Table 3, the first two accomplished studies on melanoma stage IIIb/IV133and non-small cell lung cancer III/IV134used the exosomes derived from the DCs of each patient.The exosomes were modified to present tumor antigens and were reinjected to the same patients.In another study on colorectal cancer stage III/IV, purified exosomes from ascites of each patient were administered along with GMCSF54.All these phase I clinical trials emphasized on the desired immunostimulatory effects of exosome-based drug delivery systems in some patients, with no or minimal side effects.Recently, two clinical trials have been conducted based on herbal exosomes in colorectal cancer and head and neck cancer patients(Table 3).Overall, findings from previous trials confirm the feasibility of exosome in cancer therapy as a safe and specific approach.
Conclusion
Recently, the trend in cancer therapy has shifted toward the design of biologically stable and safe delivery systems compatible with humans.The application of texosomes for safe and efficient immunotherapy opened a new window to cancer treatment.Despite the potential tumorigenesis properties of texosomes, the presence of a broad range of both known and unknown tumor antigens allowed the induction of significant antitumor immune responses.Moreover, texosome-baseddelivery system is stable without side effects.To improve this system, biotechnological approaches have been used to design a potent immunostimulatory delivery system capable of targeting properties.Mimetic technics allowed the engineering of texosome-based delivery systems in large scale to be used in medical applications.However, several subjects are still unclear in this discipline.In summary, our knowledge about the structure and function of the components, texosome mimetics, and their effects on each other is limited.Furthermore, the selection of proteins and lipids used in texosome assemblies is the key point in the field.Further studies are needed to address the current challenges of designing an efficient delivery system.
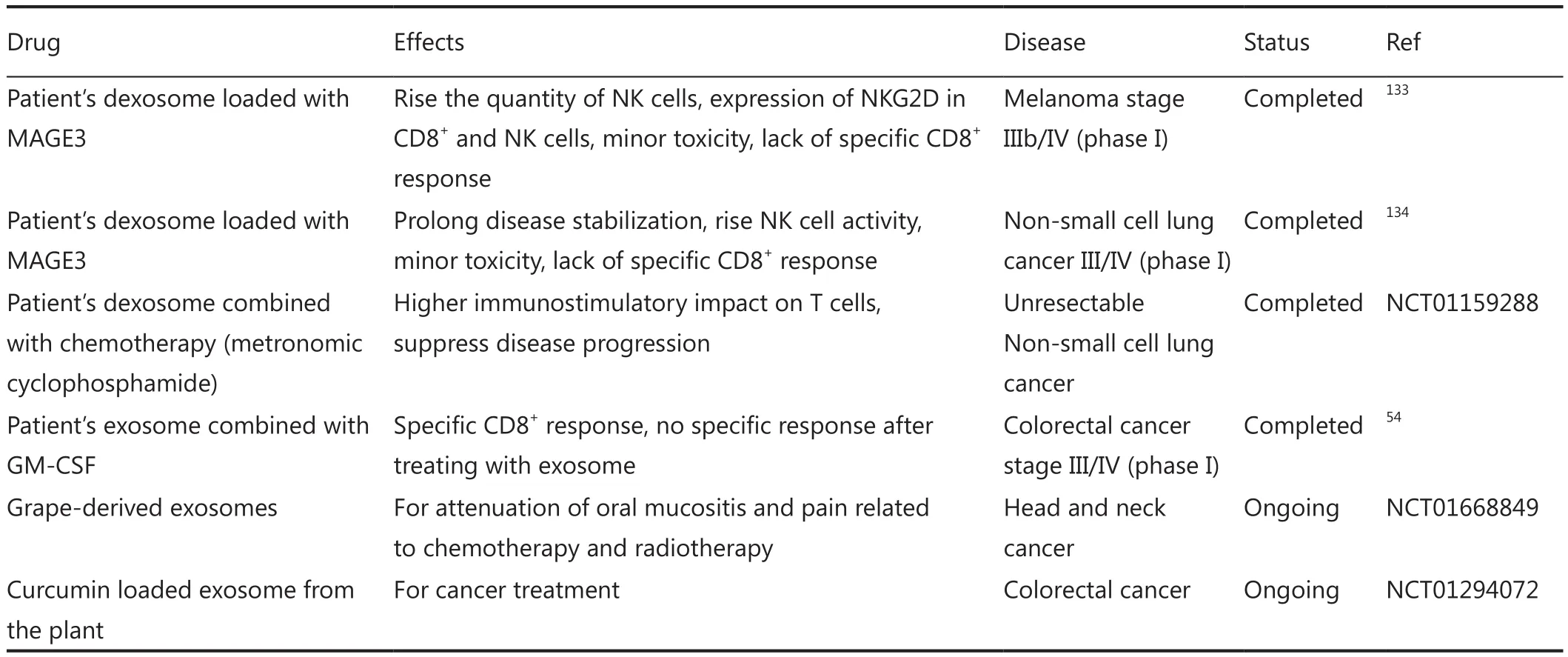
Table 3 Clinical trials based on exosome delivery system for cancer therapy.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Caspi RR.Immunotherapy of autoimmunity and cancer: the penalty for success.Nat Rev Immunol 2008;8:970-976.
2.Paulis LE, Mandal S, Kreutz M, Figdor CG.Dendritic cell-based nanovaccines for cancer immunotherapy.Curr Opin Immunol 2013;25:389-395.
3.Klippstein R, Pozo D.Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies.Nanomedicine 2010;6:523-529.
4.Mellman I, Coukos G, Dranoff G.Cancer immunotherapy comes of age.Nature.2011;480:480-489.
5.Hosseini HM, Fooladi AA, Nourani MR, Ghanezadeh F.The role of exosomes in infectious diseases.Inflamm Allergy Drug Targets 2013;12:29-37.
6.Imani Fooladi AA, Mahmoodzadeh Hosseini H.Biological functions of exosomes in the liver in health and disease.Hepat Mon 2014;14:e13514.
7.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T,Duroux M.A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy.Biochim Biophys Acta 2014;1846:75-87.
8.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ.Exosome: from internal vesicle of the multivesicular body to intercellular signaling device.J Cell Sci 2000;113:3365-3374.
9.Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, et al.Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity.Curr Biol 1999;9:55-58.
10.World Health Organization.Cholera, 2009.Wkly Epidemiol Rec 2010;85:293-308.
11.Raposo G, Stoorvogel W.Extracellular vesicles: exosomes,microvesicles, and friends.J Cell Biol 2013;200:373-383.
12.Möbius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM,Shimada Y, Fujimoto T, et al.Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O.J Histochem Cytochem 2002;50:43-55.
13.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al.Ceramide triggers budding of exosome vesicles into multivesicular endosomes.Science 2008;319:1244-1247.
14.Simons M, Raposo G.Exosomes--vesicular carriers for intercellular communication.Curr Opin Cell Biol 2009;21:575-581.
15.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ.Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane.J Cell Biol 2006;172:923-935.
16.Record M, Subra C, Silvente-Poirot S, Poirot M.Exosomes as intercellular signalosomes and pharmacological effectors.Biochem Pharmacol 2011;81:1171-1182.
17.Théry C, Ostrowski M, Segura E.Membrane vesicles as conveyors of immune responses.Nat Rev Immunol 2009;9:581-593.
18.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al.Rab27a and Rab27b control different steps of the exosome secretion pathway.Nat Cell Biol 2010;12:19-30; sup pp 1-13.
19.Bobrie A, Colombo M, Raposo G, Théry C.Exosome secretion:molecular mechanisms and roles in immune responses.Traffic 2011;12:1659-1668.
20.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD.Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting.Dev Cell 2002;3:271-282.
21.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD.Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body.Dev Cell 2002;3:283-289.
22.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M.Lipid raft-associated protein sorting in exosomes.Blood 2003;102:4336-4344.
23.Vidal M, Mangeat P, Hoekstra D.Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation.J Cell Sci 1997;110:1867-1877.
24.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, et al.Proteomic and biochemical analyses of human B cell-derived exosomes.Potential implications for their function and multivesicular body formation.J Biol Chem 2003;278:10963-10972.
25.Lösche W, Scholz T, Temmler U, Oberle V, Claus RA.Plateletderived microvesicles transfer tissue factor to monocytes but not to neutrophils.Platelets 2004;15:109-115.
26.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C,Geuze HJ.Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface.J Immunol 2000;165:1259-1265.
27.Rodríguez A, Webster P, Ortego J, Andrews NW.Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells.J Cell Biol 1997;137:93-104.
28.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW.Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts.J Cell Biol 2000;148:1141-1149.
29.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al.Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes.Blood 2012;119:756-766.
30.Köppler B, Cohen C, Schlöndorff D, Mack M.Differential mechanisms of microparticle transfer toB cells and monocytes:anti-inflammatory propertiesof microparticles.Eur J Immunol 2006;36:648-660.
31.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, HallettMB.Adhesion and signaling by B cell-derived exosomes: the role of integrins.FASEB J 2004;18:977-979.
32.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al.B lymphocytes secrete antigen-presenting vesicles.J Exp Med 1996;183:1161-1172.
33.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO.Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells.Nat Cell Biol 2007;9:654-659.
34.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A,et al.Microenvironmental pH is a key factor for exosome traffic in tumor cells.J Biol Chem 2009;284:34211-34222.
35.Diao J, Ishitsuka Y, Lee H, Joo C, Su Z, Syed S, et al.A single vesicle-vesicle fusion assay for in vitro studies of SNAREs and accessory proteins.Nature Protocols 2012;7:921-934.
36.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al.Endocytosis, intracellular sorting,and processing of exosomes by dendritic cells.Blood 2004;104:3257-3266.
37.Schorey JS, Bhatnagar S.Exosome function: from tumor immunology to pathogen biology.Traffic 2008;9:871-881.
38. Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al.Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles.J Immunol 2001;166:7309-7318.
39. Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al.Molecular characterization of dendritic cellderived exosomes.Selective accumulation of the heat shock protein hsc73.J Cell Biol 1999;147:599-610.
40. Théry C, Zitvogel L, Amigorena S.Exosomes: composition,biogenesis and function.Nat Rev Immunol 2002;2:569-579.
41.Mignot G, Roux S, Thery C, Ségura E, Zitvogel L.Prospects for exosomes in immunotherapy of cancer.J Cell Mol Med 2006;10:376-388.
42.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ.ExoCarta 2012:database of exosomal proteins, RNA and lipids.Nucleic Acids Res 2012;40:D1241-D1244.
43.Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A.Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”.J Cell Physiol 1989;140:455-462.
44.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF,et al.Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization.Biochem J 2004;380:161-171.
45.Chu Z, Witte DP, Qi X.Saposin C-LBPA interaction in lateendosomes/lysosomes.Exp Cell Res 2005;303:300-307.
46.Echarri A, Muriel O, Del Pozo MA.Intracellular trafficking of raft/caveolae domains: insights from integrin signaling.Semin Cell Dev Biol 2007;18:627-637.
47.Abujamra AL, Spanjaard RA, Akinsheye I, Zhao X, Faller DV, Ghosh SK.Leukemia virus long terminal repeat activates NFkappaB pathway by a TLR3-dependent mechanism.Virology 2006;345:390-403.
48.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al.Immune resistance orchestrated by the tumor microenvironment.Immunol Rev 2006;213:131-145.
49.van Niel G, Porto-Carreiro I, Simoes S, Raposo G.Exosomes:a common pathway for a specialized function.J Biochem 2006;140:13-21.
50.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al.Tumour-released exosomes and their implications in cancer immunity.Cell Death Differ 2008;15:80-88.
51.Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M,et al.Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage.Gynecol Oncol 2011;122:437-446.
52.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al.Malignant effusions and immunogenic tumour-derived exosomes.Lancet 2002;360:295-305.
53.Taylor DD, Gercel-Taylor C.MicroRNA signatures of tumorderived exosomes as diagnostic biomarkers of ovarian cancer.Gynecol Oncol 2008;110:13-21.
54.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al.Phase I clinical trial of autologous ascites-derived exosomes combined with GMCSF for colorectal cancer.Mol Ther 2008;16:782-90.
55.Zhang Y, Luo CL, He BC, Zhang JM, Cheng G, Wu XH.Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma.Int J Oncol 2010;36:133-140.
56.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al.Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis.Blood Cells Mol Dis 2005;35:169-173.
57.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ,Whiteside TL.Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes.J Immunol 2009;183:3720-3730.
58.Martínez-Lorenzo MJ, Anel A, Alava MA, Piñeiro A, Naval J,Lasierra P, et al.The human melanoma cell line MelJuSo secretes bioactive FasL and APO2L/TRAIL on the surface of microvesicles.Possible contribution to tumor counterattack.Exp Cell Res 2004;295:315-329.
59.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al.Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape.Gastroenterology 2005;128:1796-1804.
60.Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ, et al.The role of cbl family of ubiquitin ligases in gastric cancer exosome-induced apoptosis of Jurkat T cells.Acta Oncol 2009;48:1173-1180.
61.Taylor DD, Gerçel-Taylor C.Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects.Br J Cancer 2005;92:305-311.
62.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z.Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production.J Immunol 2011;187:676-683.
63.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z.Human tumor-derived exosomes down-modulate NKG2D expression.J Immunol 2008;180:7249-7258.
64.Clayton A, Tabi Z.Exosomes and the MICA-NKG2D system in cancer.Blood Cells Mol Dis 2005;34:206-213.
65.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al.Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function.J Immunol 2006;176:1375-1385.
66.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M.Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1.Haematologica 2011;96:1302-1309.
67.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, et al.A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells.Gastroenterology 2008;135:234-243.
68.Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P.Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma.J Int Med Res 2011;39:1381-1391.
69.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al.Increased level of both CD4+FOXP3+regulatory T cells and CD14+HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma.Scand J Immunol 2010;72:540-547.
70.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M,Whiteside TL.Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg).PLoS One 2010;5:e11469.
71.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C.Microvesicles: mediators of extracellular communication during cancer progression.J Cell Sci 2010;123:1603-1611.
72.Hood JL, San RS, Wickline SA.Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis.Cancer Res 2011;71:3792-3801.
73.Richardson MM, Jennings LK, Zhang XA.Tetraspanins and tumor progression.Clin Exp Metastasis 2011;28:261-270.
74.Gesierich S, Berezovskiy I, Ryschich E, Zöller M.Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029.Cancer Res 2006;66:7083-7094.
75.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A,Trendelenburg M, et al.Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation.Cancer Res 2010;70:1668-1678.
76.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al.New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes.Blood 2010;116:2385-2394.
77.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al.Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers.Nat Cell Biol 2008;10:1470-1476.
78.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al.Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells.Mol Cancer Ther 2005;4:1595-1604.
79.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F,Campiglio M, et al.Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy.J Cell Physiol 2012;227:658-667.
80.Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al.Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC.Cancer Immunol Immunother 2011;60:639-648.
81.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, et al.Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3.Proc Natl Acad Sci U S A 2011;108:15336-15341.
82.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al.Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming.Nat Med 2001;7:297-303.
83.Napoletano C, Rughetti A, Landi R, Pinto D, Bellati F, Rahimi H, et al.Immunogenicity of allo-vesicle carrying ERBB2 tumor antigen for dendritic cell-based anti-tumor immunotherapy.Int J Immunopathol Pharmacol 2009;22:647-658.
84.Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R, et al.Exosomeloaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma.J Neurooncol 2011;104:659-667.
85.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, et al.Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells.Eur J Immunol 2006;36:1598-1607.
86.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW.MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models.Cancer Lett 2009;275:256-265.
87.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, et al.Exosomes: a new delivery system for tumor antigens in cancer immunotherapy.Int J Cancer 2005;114:613-622.
88.Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V,et al.Human tumor nanoparticles induce apoptosis of pancreatic cancer cells.FASEB J 2008;22:3358-3369.
89.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A.Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles.Int J Cancer 2009;125:1016-1026.
90.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, MJ W.Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes.Nat Biotechnol 2011;29:341-345.
91.Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, et al.Exosome Display technology: applications to the development of new diagnostics and therapeutics.Blood Cells Mol Dis 2005;35:158-168.
92.Otzen DE, Blans K, Wang H, Gilbert GE, Rasmussen JT.Lactadherin binds to phosphatidylserine-containing vesicles in a two-step mechanism sensitive to vesicle size and composition.Biochim Biophys Acta 2012;1818:1019-1027.
93.Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J.Interaction of Staphylococcus aureus toxin” superantigens” with human T cells.Proc Natl Acad Sci U S A 1989;86:8941-8945.
94.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, et al.V beta-specific stimulation of human T cells by staphylococcal toxins.Science 1989;244:811-813.
95.Imani Fooladi AA, Sattari M, Reza Nourani M.Synergistic effects between Staphylococcal enterotoxin type B and Monophosphoryl lipid A against mouse fibrosarcoma.J BUON 2010;15:340-347.
96.Fooladi AA, Sattari M, Nourani MR.Study of T-cell stimulation and cytokine release induced by Staphylococcal enterotoxin type B and monophosphoryl lipid A.Arch Med Sci 2009;5:335-341.
97.Fooladi AA, Sattari M, Hassan ZM, Mahdavi M, Azizi T, Horii A.In vivo induction of necrosis in mice fibrosarcoma via intravenous injection of type B staphylococcal enterotoxin.Biotechnol Lett2008;30:2053-2059.
98.Higgs BW, Dileo J, Chang WE, Smith HB, Peters OJ, Hammamieh R, et al.Modeling the effects of a Staphylococcal Enterotoxin B(SEB) on the apoptosis pathway.BMC Microbiol 2006;6:48.
99.Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X.Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes.J Mol Med (Berl)2007;85:511-521.
100.Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J,Nourani MR, Mahdavi M.Exosome/staphylococcal enterotoxin B, an anti tumor compound against pancreatic cancer.J BUON 2014;19:440-448.
101.Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J,Nourani MR, Davaran S, Mahdavi M.Staphylococcal entorotoxin B anchored exosome induces apoptosis in negative esterogen receptor breast cancer cells.Tumour Biol 2014;35:3699-3707.
102.Mahmoodzadeh Hosseini H, Soleimanirad J, Mehdizadeh Aghdam E, Amin M, Imani Fooladi AA.Texosome-anchored superantigen triggers apoptosis in original ovarian cancer cells.Med Oncol 2015;32:409-415.
103.Imani Fooladi AA, Halabian R, Mahdavi M, Amin M,Mahmoodzadeh Hosseini H.Staphylococcal enterotoxin B/texosomes as a candidate for breast cancer immunotherapy.Tumour Biol 2015.[Epub ahead of print].
104.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV,Filatov MV.Exosomes are natural carriers of exogenous siRNA to human cells in vitro.Cell Commun Signal 2013;11:88.
105.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al.Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells.Mol Ther 2013;21:185-191.
106.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al.A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy.Biomaterials 2014;35:2383-2390.
107.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al.Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain.Mol Ther 2011;19:1769-1779.
108.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S,Erkan EP, et al.Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth.MolTher 2013;21:101-108.
109.Lee YS, Kim SH, Cho JA, Kim CW.Introduction of the CIITA gene into tumor cells produces exosomes with enhanced antitumor effects.Exp Mol Med 2011;43:281-290.
110.Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al.Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heatshocked tumour cells expressing cytoplasmic HSP70.J Cell Mol Med 2010;14:2655-2666.
111.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O,et al.Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth.Cancer Lett 2013;335:201-204.
112.Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, et al.Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha.PLoS One 2009;4:e4942.
113.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, et al.Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses.Cancer Res 2008;68:1228-1235.
114.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al.Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors.ACS Nano 2013;7:7698-7710.
115.Jo W, Jeong D, Kim J, Cho S, Jang SC, Han C, et al.Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers.Lab Chip 2014;14:1261-1269.
116.Hood JL, Scott MJ, Wickline SA.Maximizing exosome colloidal stability following electroporation.Anal Biochem 2014;448:41-49.
117.Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC,Hendrix A, Wood MJ, et al.Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles.J Control Release 2013;172:229-238.
118.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH.Gene transfer into mouse lyoma cells by electroporation in high electric fields.EMBO J 1982;1:841-845.
119.Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E,Sunnerhagen P, et al.Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes.Nucleic Acids Res 2012;40:e130.
120.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, et al.Exosome-mediated delivery of siRNA in vitro and in vivo.Nat Protoc 2012;7:2112-2126.
121.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al.A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes.MolTher 2010;18:1606-1614.
122.Jaruga E, Sokal A, Chrul S, Bartosz G.Apoptosis-independent alterations in membrane dynamics induced by curcumin.Exp Cell Res 1998;245:303-312.
123.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A.Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin.J Am Chem Soc 2009;131:4490-4498.
124.Bryniarski K, Ptak W, Jayakumar A, Püllmann K, Caplan MJ, Chairoungdua A, et al.Antigen-specific, antibodycoated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity.J Allergy Clin Immunol 2013;132:170-181.
125.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al.Standardization of sample collection, isolation and analysis methods in extracellular vesicle research.J Extracell Vesicles 2013;2.
126.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al.Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic.Crit Rev Ther Drug Carrier Syst 2009;26:523-580.
127.Fenske DB, Cullis PR.Liposomal nanomedicines.Expert Opin Drug Deliv 2008;5:25-44.
128.Clayton A, Harris CL, Court J, Mason MD, Morgan BP.Antigenpresenting cell exosomes are protected from complementmediated lysis by expression of CD55 and CD59.Eur J Immunol 2003;33:522-531.
129.Taylor DD, Gercel-Taylor C.Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments.Semin Immunopathol 2011;33:441-454.
130.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C.Exosome/microvesicle-mediated epigenetic reprogramming of cells.Am J Cancer Res 2011;1:98-110.
131.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, et al.Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs.J Transl Med 2011;9:47.
132.Jang SC, Gho YS.Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics.Nanomedicine 2014;9:177-180.
133.Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al.Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial.J Transl Med 2005;3:10.
134.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al.A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer.J Transl Med 2005;3:9.
 Cancer Biology & Medicine2015年3期
Cancer Biology & Medicine2015年3期
- Cancer Biology & Medicine的其它文章
- Ultrasensitive detection of DNA and protein markers in cancer cells
- Human induced pluripotent stem cells labeled with fluorescent magnetic nanoparticles for targeted imaging and hyperthermia therapy for gastric cancer
- Single-cell analyses of circulating tumor cells
- Reality of evidence-based practice in palliative care
- Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors
- Advances in immunotherapy for treatment of lung cancer
