Analytical evaluation of CaO-CO2 loop for CO2 removal
FAN Zhen, CHEN Liangyong, LIU Fang, LIU Kunlei
Analytical evaluation of CaO-CO2loop for CO2removal
FAN Zhen, CHEN Liangyong, LIU Fang, LIU Kunlei
Center for Applied Energy ResearchCAERUniversity of KentuckyLexingtonKYUSA
Post-combustion CO2removal from flue gas at power planta two-bed CaO-CO2loop (CaL) with heat provided by-oxy-fuel combustion has been investigated. Based on the principle of chemical reaction engineering, a simple analytical model with one variableonly (solids circulation ratio) has been built and used for analysis of the CaL process systematically, which includes effects from sorbent reactivity, fuel impurity and the utilization of fuel heat to looping. From the model, the minimum heat duty and the looping penalty are obtained. Both of them are impacted by fuel sulfur and ash significantly. The result also indicates that the optimum solids circulation ratiowill be located between the two minimum points. The furnace factor is important, because it can eventually reduce the fuel firing rate, fuel ash and sulfur effect, oxygen requirement and related auxiliary power, and increase steam cycle efficiency. As found, the heat duty approaches to infinite at a critical solids circulation ratioC, which is a constraint to the operableand the fuel burned. All of the effects are interconnected by the model.
CO2capture; calcium looping; reactivity; modeling
Introduction
Carbon capture can be effectively achieved by amine-based processes through gas-liquid reactions mostly operated at low temperature, or by sorbent- based processes through gas-solid reactions at high or low temperature. The latter leads to an attractive post-combustion calcium looping (CaL) process. It has been claimed[1-2]that the CaL is an excellent process and ideally it has a low energy penalty when operated at high temperature. High temperature operation makes associated heat recoverable at a high-grade compared with low temperature processes in which the released reaction heat is un-useful or less efficient due to recovery, if any, at a very low-grade.
The CaL using raw or improved limestone is based on the reversible reaction CaCO3heatCaOCO2as illustrated in Fig.1. The CO2in flue gas reacts with circulated CaO to form CaCO3at about 650℃ in carbonator. The formed CaCO3is heated to release CO2at about 900—950℃ in calciner. The released CO2at high purity is compressed for storage or utilization. One option to provide heat is to use-oxy-fuel combustion which generates high purity CO2directly (Fig.1). Heat released from carbonator at about 650℃ is applied for production of high temperature steam for power generation. Compared with MgO and ZnO, the advantage of using CaO is that it operates at the highest temperature to provide heat for power generation efficiently, which is the key for system efficiency.
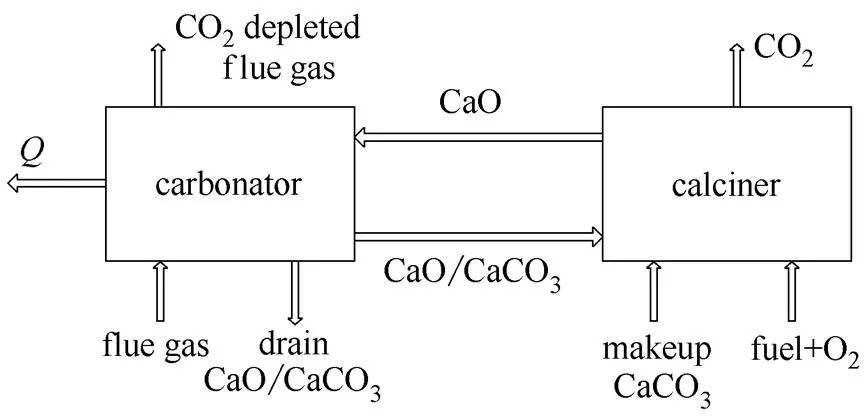
Fig.1 Illustration of calcium looping
Similar to the other CO2capture processes, the CaL requires heat and exhibits an associated looping penalty, as determined by system configuration and looping performance. The looping penalty is inherently affected by sorbent reactivity, desired CO2capture efficiency, Ca/CO2feed ratio, sorbent circulation rate, and makeup sorbent flow, as well as the associated solids flow caused by fuel-ash and formed CaSO4from fuel sulfur.
The CaL has been investigated experimentally at both lab and pilot scale[3-7]for sorbent development and for solids handling[1,3-12], as reviewed by Scala[2]and by Kotyczka-Moranska[12], as well as by Rubin for cost analysis[7]. Modeling works have been carried out[2,6,11]for performance evaluation. In this study, a chemical reaction kinetics and reactor based model, able to provide analytical solution, has been developed and applied for CaL process, aimed at systematical determinations of heat duty requirement, looping penalty, sorbent circulation rate, and the effect of fuel and furnace factor, as well as their relations and constraints. The simple analytical solution, in comparison with numerical solution, provides more direct relations among variables.
1 Modeling
For CaL performance analysis, an analytical model will be built in the principle of chemical reaction engineering[13], including sorbent reactivity decay, residence time distribution, sulfur and carbon capture, furnace factor, and fuel impurity.
1.1 Sorbent reactivity
Sorbent reactivity decay with the number of cycles due to loss of micro porosity has been observed experimentally, and the empirical or mechanistic models have been proposed in literatures[1,5,8-12], such as the fraction loss model on the basis of particles micro structure that provides a plausible mechanistic interpretation to the experimental data, withxfor reactivity in theth cycle,fandrfor the initial and residual reactivity[1,8]
It is assumed thatremains constant with the number of cycles. In this study for obtaining an analytical solution, the sorbent reactivity decay has been modeled by the 1st order reaction kinetics
or
Eq. (3) was well fitted by experimental data (Fig.2) for the generic limestone. It has been noted that the Eq. (3) and Eq. (2) are identical, simply linked by. But the 1st order kinetics mathematically leads to a linear model which makes analytical solution possible. Keep in mind that the kinetic parameters will change with sorbents[1,8,10].

Fig.2 Sorbent reactivity decay and data fitting
1.2 Solids cycle distribution
When sorbents recirculate, they may have different circulation history or age distribution. Reactor with an external circulation is a typical configuration, as has been discussed by Chen[13]. For a pipe reactor with external circulation, the system may behave as a plug flow for a zero circulation ratio or as a uniformly mixed flow for a high circulation ratio. For a near well-mixed reactor, such as a fluidized bed, with external circulation, it behaves as a uniformly mixed flow. Adding external circulation can essentially make the loop system approach a well-mixed flow reactor pattern even though its once-through flow may not be well mixed. Therefore for the CaL between two fluidized bed reactors, the sorbent cycle distribution can be modeled by a well-mixed flow as
Therepresents the average cycles of all sorbents. On mass balance,(circulation flow)/(drain flow),.. the solids circulation ratio.
1.3 Average reactivity
Particles with different cycles or residence time have different reactivity, fixed by Eq. (3). The average reactivityof all particles in the circulation system can be obtained by integration of all sorbent reactivity with their cycle distribution[13], that is
or
As a result for a given sorbent, theis a function ofonly. For a system simulation such as by Aspen Plus®this constraint has to be applied to include sorbent reactivity effect on the performance[6].
1.4 Looping penalty
Due to reactivity decay, sorbent makeup is required to maintain the system at the desired reactivity. There is unconverted CaO in the drain flow that results in a looping penalty, since heat provided to produce this CaO is unrecoverable. The amount of heat unrecoverable is determined by the CaO drain rate. A highmeans less solids drained and less CaO discharged. Because of this the solids drain from carbonator instead of from calciner is considered to lower the looping penalty (Fig.1).
Letfor the makeup flow (and drain flow) andif mole flow of circulated. Then formoles of CO2removed from the carbonator,moles of CaO will be drained, and the corresponding unrecoverable heat is
TheCrepresents the heat of calcination [4.0 MJ·(kg CO2)-1]. Combining Eq. (7) and Eq. (6), the looping penaltyLPdisplays as a function ofonly. Fig.3 shows a trend ofLP-for the ideal case (stoic, sorbent at the maximum capacity). Compared with the specific re-boiler duty at 3—4 MJ·(kg CO2)-1from the conventional MEA-CO2system, the CaL penalty is significantly less at a high, which is the ideal goal of the CaL for CO2removal. But at a low, the looping penalty is high and very sensitive to the.
1.5 Ca/CO2feed ratio
For in-bed sulfur capture by limestone, capture efficiency is linked to the Ca/Sfeed mole ratio. Excess Ca/S ratio is required to reach high capture efficiency[14]. The reason for this is believed to be the effect of a dense shell of CaSO4, a resistance of SO2transmitting into particles for further reaction with CaO, and be the effect of maintaining reactivity level for capture efficiency with a reasonable reactor size[13]. Similarly, it has been found experimentally that the in-bed CO2removal is linked to the Ca/CO2feed mole ratio[4,6]. To include sorbent reactivity effect on the CO2removal efficiency,(Ca/CO2) has been used to analyze test data (Fig.4), where the performance curve will be shifted by different sorbents. The high capture efficiency will be limited by the equilibrium CO2in gas at the exit as has been analyzed in details by Dieter[6].

Fig.3 Looping penalty QLP-N and η

Fig.4 CO2 removal efficiency η-X(Ca/CO2) feed ratio
It needs to be pointed out that the excess(Ca/CO2)is determined by reactor design and operation. A plug flow reactor with fine particles can reduce reactor volume for a specified conversion, or gain in conversion for a given reactor size[13]. The same analysis has been reported[6]where the solid circulation ratio is clearly affected by the inventory,.. the reactor size.
1.6 Heat duty
Heat requirement is an important parameter for operation of CaL. Based on flow diagram (Fig.1), calciner heat duties are
Calcining circulated CaCO3:
Calcining makeup CaCO3:
Heat up circulated CaCO3:
Heat up makeup CaCO3:
For simplification makeup flow has been assumed to be pre-heated to the carbonator temperature from heat recovery. All terms added together, with(mole base), calciner heat duty becomes
Combined Eq. (6) and Eq. (8), the heat dutydisplays also as a function ofonly, impacted by. Fig.5 plots-with300℃ (calciner at 950℃ and carbonator at 650℃). Due to heating up circulated solids, heat duty becomes much higher than that of the calcinationC[4.0 MJ·(kg CO2)-1], affected by the required CO2capture efficiency, the same as the looping penalty (Fig.3).

Fig.5 Heat duty Q-N with different CO2 removal efficiency
There exists a minimum heat duty (Fig.5) at8—10, shifted a little bit with changes of CO2capture efficiency. This minimum is caused by the two opposite facts, the decrease of heat for calcination and increase of heat for heating up asincreases. High heat duty requires more oxy-fuel combustion, and results in more auxiliary power from air separation unit (ASU) and enlarged ASU and calciner size.
2 Analysis of fuel effect
When coal is fired, it introduces ash and sulfur into solids circulation, and so increases associated solids circulation rate.
2.1 Furnace factor
Furnace factor, the fraction of fuel heat input absorbed by steam/water in the furnace, has been used for boiler performance analysis, affected by the furnace exit gas temperature (FEGT), fuel and feed preheating. In general, furnace factor is about 50%—60%. The same term can be applied to calciner for the fuel heat input absorbed by sorbent in the furnace.
Fuel firing rate will be low with a high furnace factor, which can be reached by pre-heating of all feeds (flue gas, fluidization gas, fuel, oxidant, and makeup sorbent) through heat recuperation. Low-gradeheat recovery from CO2depleted flue gas and produced CO2stream to heat up boiler feed water results in shutoff of some or all of feed water heaters, and thus in reduced steam cycle efficiency, since the feed water heating by the extracted steam can improve the steam cycle efficiency over 10%. Applying pre-heating will result in remarkable gains from the reduction of fuel flow and the increase of steam cycle efficiency. Therefore pre-heating of feed is important for the design and configuration of CaL and the other chemical looping’s.
whereis the fuel flow in kg fuel·(kg CO2)-1. For oxy-fuel furnace, recycled CO2, steam, or the mixture can be used as fluidization gas, and the furnace factorcan be potentially high if less gas, from high O2% combustion, flows through the furnace. The effect of fuel on looping performance is linked by Eq. (9) for a given fuel property and impurity.
2.2 Sulfur from flue gas
Sulfur in flue gas or in fuel will be captured in CaL. Sulfationwill release heat, more than the heat of calcination. The formed CaSO4, co-existent in particles with CaO/CaCO3, needs to be drained out to avoid accumulation. Since the capture of CO2and SO2shares the same sorbent, letto share the same capacity, and letSO2/CO2for SO2added to the loop. For the ideal case for both CO2and SO2, then a mass balanceleads to
The same calculation procedures for heat duty and looping penalty can be performed by substitutingwithCinto Eq. (7) and Eq. (8), but the term () in Eq. (8) needs to be expanded as () to cover theXand the ratio ofC,m(CaSO4)/C,m(CaO)3.6. Fig.6 and Fig.7 display the results with(for3.2% and60% in coal), and the impact of flue gas SO2on the performance, where it seems pretty high on the heat duty at high.
2.3 Sulfur from fuel
Sulfur from fuel to calciner is fuel and heat duty dependent, and behaves differently. Eqs. (8)—(10) can be applied for the heat duty, withlinked to the fuel-S by

Fig.6 Looping penalty QLPaffected by flue gas SO2 and η
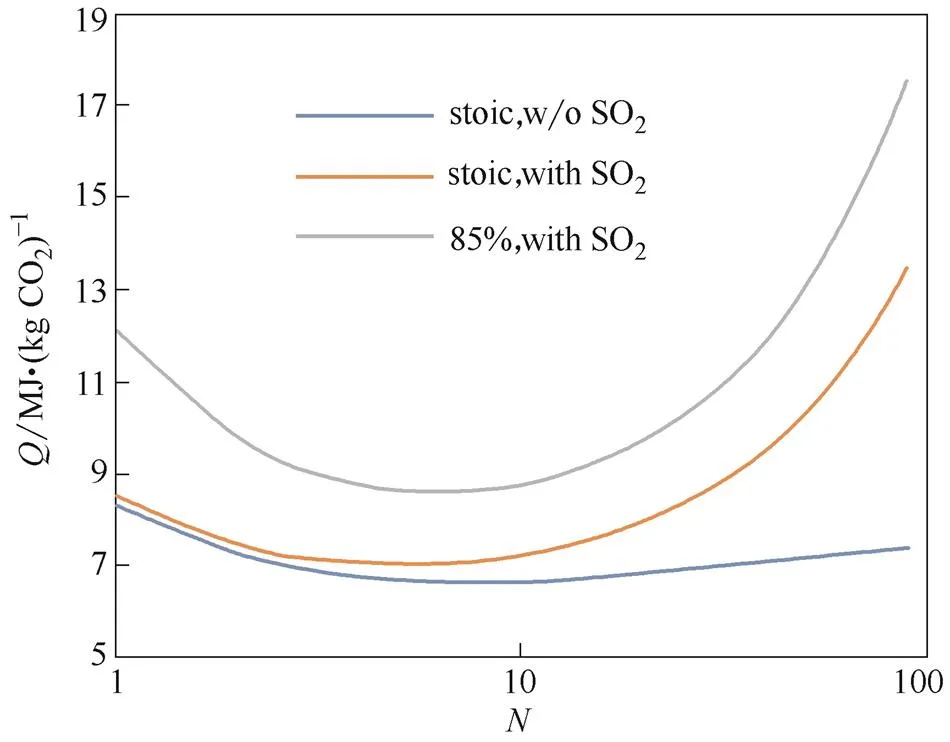
Fig.7 Heat duty Q affected by flue gas SO2 and η
In Eq. (11),is for the sulfur in fuel. A solution can be obtained directly from Eqs. (8)—(11) or simply by iteration. Fig.8 shows the calculated results and a comparison of different sulfur carried into CaL, with fuel LHV26 MJ·(kg coal)-1, furnace factor0.6, fuel-S3.2% and 6.4% in coal respectively. Sulfur shows almost no impact on heat dutywhenis small (Fig.8), but it becomes significant and even infinite asrises. As reported in Ref. [5], the0.045 for0.11 and0.067 for0.06, indicating a significant amount of associated solids flow.

Fig.8 Calciner heat duty Q affected by different sulfur input
2.4 Ash from fuel
Ash is expected to have the same impact on the looping performance as the fuel-S from associated solids circulation point of view. For simplicity, let CaCO3represent for the property of ash andAfor ash in solids per mole of Ca circulated, then, to cover sulfur and ash from fuel, the term () in Eq. (8) becomes (). Without ash separation from sorbent, theAis constrained by
TheAfor ash andfor sulfur have to be co-iterated for a solution. Fig.10 displays the result for a case of 3.2% fuel-S and 10% fuel-ash. As a trend, the criticalCreduces from>100 (stoic case) toonly (coal case), indicating a significant impact of fuel-ash and fuel-S on the heat duty. It is noted that fuel-ash has a big impact on theCcompared with fuel-S (Fig.9 and Fig.10) due to the fact that fuel-S can provide heat from sulfation. The criticalCoccurs basically at the same point for the same impact from fuel (Fig.9 and Fig.10). For a better CaL performance, sulfur should be pre-removed or not introduced.

Fig.9 Looping penalty QLPfor cases with fuel-S/ash

Fig.10 Heat duty Q for cases with fuel-S/ash
In general ash is not mixed with sorbent particles chemically. The difference in density and particle size can be used to separate ash out from sorbent to reduce its content in circulation, and to improve the looping performance. However, ash may deposit on the surface of sorbent, and may react with sorbent at high temperature to lower the sorbent reactivity, which needs to be further investigated.
2.5 Makeup flow
Based on modeling, the sorbent makeup flow can be expressed as
Fig.11 shows sorbent makeup flow for different cases, where obviously it is high at a lowand even higher for a coal with high sulfur and ash, but it could be low for an ideal case at a high, determined by the sorbent property, mainly the residualr. That is a key parameter in sorbent research and improvement[2,4-5,9].
As indicated by Fig.11, the sorbent makeup flow is influenced significantly by fuel ash and sulfur due to the reduced critical circulation ratioCand thus a limited operable. For the fuel used in this study with the generic limestone, it results with>0.3, which means that for each mole of CO2removed, 0.3 mol of CaCO3as makeup has to be added and calcined. This seems too high for commercial application. Therefore use of solid fuel such as coal as heat support for CaL is limited.

Fig.11 Sorbent makeup flow for difference cases
2.6 Heat recovery
It has been claimed that the heat of reaction from carbonator can be applied to generate power by producing high temperature steam[1-2]. The two-bed CaL is considered as a circulating fluidized bed boiler (CFB), where heat from oxy-fuel combustion is transferred by solids circulation from calciner to carbonator through CaL (as a kind of chemical looping). As discussed previously, the heat from fuel at calciner is released at a high bed temperature about 900—950℃ but it is recovered at carbonator at a relatively low bed temperature about 650℃, constrained by the approach to the equilibrium capture efficiency[6]. The higher the capture efficiency, the lower the bed temperature will be required.
The bed temperature of 650℃ seems low as a CFB boiler for the advanced such as the USC steam cycle. The bed temperature of a conventional CFB boiler is at about 850—900℃[14], determined by fuel. This 200—250℃ temperature difference leads to a low driving force for heat transfer in furnace. As a result, the required heat transfer surface area will increase remarkably, depending upon the steam pressure, or the water evaporation temperature. The same applies to the steam superheat and reheat, which requires heat at high temperature. Adding more surfaces into CFB is a challenge for the boiler design. Of course, circulated hot solids can provide part of high-grade heat if an external fluidized bed heat exchanger (FBHX) is installed between two beds. In case the calciner is used to provide heat for steam by more oxy-fuel combustion, the operable solid circulation ratiomay be further reduced if coal is fired.
Nevertheless, heat recovery from CaL as a boiler is challenging and costly, especially for the advanced steam cycle and increased high level heat duty for steam superheat and reheats. For application of chemical looping system including CaL for power generation, the bed temperature is the key for the reachable plant efficiency.
3 Discussions
As analyzed, thefor a given sorbent is a function of operation parameteronly, that is the solids circulation ratio. On the other side, the required solids circulation ratiocan be calculated based on the given sorbent and the desired average sorbent reactivity. It is a trade-off betweenand. A low sorbent conversioncan be compensated by a high solids circulation rate. This compensation however leads to a decrease in performance due to the looping penalty and calciner heat duty that results in more oxy-fuel firing and more auxiliary power.
Makeup and drain flow are required for steady state operation to avoid accumulation and to compensate sorbent loss. From this study, the drain CaO, calcined from the feed CaCO3, causes a looping penalty due to unrecoverable energy loss, especially at a low. As analyzed, this loop penalty decreases significantly just after a few cycles, but could be significantly high at a highwhen sulfur and ash from fuel are added into looping.
Associated solids circulation increases with fuel sulfur and ash, which induces a big impact on the looping performance. Both the heat duty and looping penalty increase with fuel sulfur and ash at a high, and cause a critical solids circulation ratioC, at which the heat duty and the looping penalty approach to infinite because of too much associated solids flow induced from fuel, and in turn a positive feedback to the requirement of fuel flow for heating up. The highest operableis therefore constrained by this criticalC. Eventually the operable rangereduces when coal is fired.
For a better efficiency it is important to increase the furnace factorby pre-heating up of all feeds through recuperation with their hot exit streams. The advantages of this pre-heating are:
(1)Fuel firing rate is directly reduced, and so the effect of fuel impurity reduced (Fig.12 & Fig.13);
(2)Steam cycle efficiency is maintained due to no shutting off of feed water heaters;
(3)Oxygen requirement and the related auxiliary power for oxy-fuel firing are reduced, the same for the ASU size and thus the equipment cost;
(4)The combined system for CO2removal by CaL and by oxy-fuel CFB becomes more efficient due to a reduced sharing of oxy-fuel CFB.

Fig.12 Effect of furnace factor α on fuel flow

Fig.13 Effect of furnace factor α on heat duty
As a result, when integrated with CaL power plant efficiency could be boosted by a few points. On the other side as a CFB boiler, the low bed temperature of carbonator may limit its application and efficiency, such as for the USC steam cycle.
Based on the present analysis, the sorbent reactivity may be better defined as g-CO2/g-sorbent for a fair comparison of different sorbents. For example, dolomite has near the same performance as limestone when counted in g-CO2/g-sorbent base, as can be seen from the data in reference[10].
It needs to be pointed out that performance curves shown in this study will shift with sorbent, and so as to conclusions. The present study is just for analysis of system integration, understanding of complex process, and thorough insight into the feasibility, gain and constraint, as well as ways of improvement.
4 Conclusion
On the basis of the principle of chemical reaction engineering and the use of the 1st order kinetics for the decay of sorbent reactivity, a simple analytical model with only one variable(solids circulation ratio) for a two-bed CaO-CO2loop system with-oxy-fuel combustion has been built and applied for the CaL process analysis systematically, which includes effects from fuel and the utilization of fuel heat to sorbent in calciner.
From the model, the minimum requirement of heat duty and the minimum looping penalty are obtained analytically. It can be concluded that the solids circulation ratiowill be constrained by the critical solids circulation ratioC, which is affected by fuel impurity. The higher the fuel ash and sulfur, the lower the criticalCwill be. Therefore fuel with high sulfur and ash is not suitable for CaL. The CaL performance is also impacted by the required CO2capture efficiency due to the required excess Ca/CO2feed ratio.
It is important to have a high furnace factorto directly reduce fuel flow, fuel ash and sulfur effect, oxygen and related auxiliary power requirement, and to maintain the steam cycle efficiency by keeping all feed water heaters on. In this way, the CaL efficiency can be boosted by a few points.
The analysis and results from this study are helpful in reaching some insights for the development of CaO-CO2looping process for CO2removal.
List of acronyms

CAER——center for applied energy research CaL——calcium looping Cp,m——molar heat capacity at constant pressure, kJ·mol-1·℃-1 F——fuel flow rate, kg·(kg CO2)-1 HC——heat of calcination, MJ·(kg CO2)-1 HS——sensible heat, MJ·(kg CO2)-1 k,f——sorbent reactivity kinetic parameters m——makeup or drain flow, mole per mole Ca circulation N——average number of cycles, circulation ratio n——nth cycles Q,QLP——heat duty, looping penalty, MJ·(kg CO2)-1 T,δT——temperature, temperature difference, ℃ USC——ultra-supercritical X——average sorbent reactivity in loop XA,XC,XS——mole fraction of ash, CaCO3 and CaSO4 in loop x,xf,xr——sorbent, initial, and residual reactivity α——furnace factor, fraction of fuel heat absorbed in furnace β——SO2/CO2 ratio η——CO2 removal efficiency
[1] Abanades J, Alvarez D. Conversion limits in the reaction of CO2with lime [J]., 2003, 17: 308-315.
[2] Fabrizio Scala. Fluidized Bed Technologies for Near-Zero Emission Combustion and Gasification [M]. Amsterdam: Elsevier, 2013.
[3] Chang M H, Chen W C, Huang C M, Liu W H, Chou Y C, Chang W C, Chen W, Cheng J Y, Huang K E, Hsu H W. Design and experimental testing of 1.9 MWthcalcium looping pilot plant// GHGT-12 [C]. Austin, TX, 2014.
[4] Sakadjian B B, Wang W K, Li S G, Ramkumar S, Gumuluru S, Fan L S, Statnick R M. Sub-pilot demonstration of the CCR process: high temperature CO2capture sorbents for coal fired power plants//the 34th International Clearwater Conference [C]. Clearwater, FL, 2009.
[5] Diego M E, Arias B, Grasa G, Abanades J C, Diaz L, Lorenzo M, Sanchez-Biezma A. Cacium looping with enhanced sorbent performance: experimental testing in a large pilot//GHGT-12 [C]. Austin, TX, 2014.
[6] Dieter H, Beirow M, Schweitzer D, Hawthorne C, Scheffknecht G. Efficiency and flexibility potential for calcium looping CO2capture//GHGT-12 [C]. Austin, TX, 2014.
[7] Mantripragada H C, Rubin E S. Calcium looping cycle for CO2capture: performance, cost and feasibility analysis//GHGT-12 [C]. Austin, TX, 2014.
[8] Fennell P S, Pacciani R, Dennis J S, Davidson J F, Hayhurst A N. The effects of repeated cycles of calcination and carbonation on a variety of different limestones, as measured in a hot fluidized bed of sand [J]., 2007, 21: 2072-2081.
[9] Fennell P S, Davidson J F, Dennis J S, Hayhurst A N. Regeneration of sintered limestone sorbents for the sequestration of CO2from combustion and other systems [J].., 2007, 80 (2): 116-119.
[10] Lisbona P, Martinez A, Lara Y, Romeo L M. Integration of carbonate CO2capture and coal-fired power plants, a comparative study for different sorbents [J]., 2010, 24: 728-736.
[11] Abanades J C, Anthony E J, Lu D Y, Salvador C, Alvarez D. Capture of CO2from combustion gases in a fluidized bed of CaO [J].., 2004, 50: 1614-1622.
[12] Kotyczka-Moranska M, Tomaszewicz G, Labojko G. Comparison of different methods for enhancing CO2capture by CaO based sorbent [J]...., 2012, 48 (1): 77-90.
[13] Chen Gantang (陈甘棠). Chemical Reaction Engineering (化学反应工程) [M]. Beijing: Chemical Industry Press, 1981.
[14] Cen Kefa (岑可法). Theory Design and Operation of CFB Boiler (循环流化床锅炉理论设计与运行) [M]. Beijing: China Electric Power Press, 1997.
氧化钙-二氧化碳循环系统脱碳的分析研究
范镇,陈良勇,刘方,刘坤磊
(美国肯塔基大学应用能源研究中心, KY 40511,美国)
采用燃料氧燃烧直接供热的氧化钙-二氧化碳双床循环系统已经被研究开发,用于从电厂尾气中脱碳。本研究基于实验和化学反应工程原理建立了分析模型,用于系统地研究循环特征,氧化钙活性衰减,燃料及其炉内热利用率的影响。基于模型推导获得了重要参数:最小循环热损失和最小热需求量,以及对应的固体循环比。它们都受供热燃料灰分和含硫量的影响,也受脱碳率的影响。显然最佳固体循环比介于二者之间。另一个重要参数是燃料在炉内的热利用率。高的热利用率不仅降低燃料需求量,降低其灰分和含硫的影响,降低氧的需求量及其辅助功,而且提高蒸汽循环的发电效率。一个发现是热需求量在临界固体循环比接近无穷大,这就限制了固体循环比的可操作范围, 以及燃料的灰分和含硫量。建立的分析模型和推导直接提供了这些变量之间的关系和范围。
CO2捕集;钙循环;反应性;模型
10.11949/j.issn.0438-1157.20150631
TH 09
A
0438—1157(2015)08—3233—09
刘坤磊。
范镇(1957—),博士。
2015-05-21.
Prof. LIU Kunlei, kunlei.liu@uky.edu
2015-05-21收到初稿,2015-05-28收到修改稿。

