Qualitative analysis of catechins from green tea GMB-4 clone using HPLCand LC-MS/MS
Erna Susanti,Ciptati,Retty Ratnawati,Aulanni'am,Achmad Rudijanto1DoctoralProgramofBiomedicalScience,FacultyofMedicine,BrawijayaUniversity,Jl.Veteran,Malang65145,EastJava,Indonesia
2Department of Chemistry,Faculty of Science,Bandung Institute of Technology,Bandung,Indonesia
3Biomedical Science,Faculty of Medicine,Brawijaya University,Jl.Veteran,Malang 65145,East Java,Indonesia
Qualitative analysis of catechins from green tea GMB-4 clone using HPLC
and LC-MS/MS
Erna Susanti1*,Ciptati2,#,Retty Ratnawati3,#,Aulanni'am3,#,Achmad Rudijanto3,#
1DoctoralProgramofBiomedicalScience,FacultyofMedicine,BrawijayaUniversity,Jl.Veteran,Malang65145,EastJava,Indonesia
2Department of Chemistry,Faculty of Science,Bandung Institute of Technology,Bandung,Indonesia
3Biomedical Science,Faculty of Medicine,Brawijaya University,Jl.Veteran,Malang 65145,East Java,Indonesia
ARTICLE INFO
Article history:
in revised form 13 Jul,2nd revised form 15 Jul,3rd revised form 24 Jul,4th revised form 29 Jul 2015
Accepted 30 Jul 2015
Available online 23 Oct 2015
Catechins
Green tea GMB-4 clone
LC-MS/MS
HPLC
Objective:To identify the bioactive compounds in catechins isolation and its components from green tea GMB-4 clone.
Methods:Green tea GMB-4 clones were extracted with distilled water at 90°C.Samples were eluted into the column with 10%ethanol.Subsequently,the column was eluted with 95%ethanol and evaporated separately.Green tea extract was identified by thin layer chromatography.Catechins were separated by the stationary phase in column chromatography using polyamide with 10%ethanol eluent and 95%ethanol.The results of isolations were analyzed by high performance liquid chromatographic(HPLC)and LCMS/MS.Analysis of catechins by HPLC was done by external standard.
Results:Fraction from 10%ethanol showed that four major peaks at retention time of 1.663,2.367,2.950 and 4.890,indicated the presence of four catechins components including catechin,epicatechins,gallocatechin and epigallocatechin.Whereas,fraction from 95%ethanol showed two main peaks at retention time of 5.167 and 9.82,which indicated the presence of epigallocatechin gallate(EGCG)and epicatechin gallate(ECG). EGCG(m/z 459),epigallocatechin(m/z 307),ECG(m/z 443),and epicatechin(m/z 291)were isolated and separated successfully using HPLC and LC-MS/MS.
Conclusions:The HPLC and LC-MS/MS methods were successfully tuned for the qualitative analysis of green tea extract with EGCG and ECG.Four major catechins were separated and identified by LC-MS/MS,such as EGCG,epigallocatechin,ECG and epicatechin.The result of HPLC analysis showed that EGCG and ECG were main components from catechins isolation of green tea GMB-4 clone.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2015.09.013
1.Introduction
One of potential antioxidant is catechins isolated from the tea plant(Camellia sinensis).Tea and Quinine Research Center Gambung have developed green tea GMB-4 clone with high levels of catechins(14%-16%)[1].Furthermore,green tea GMB-4 clone could be developed as a potential preventive agent for atherosclerosis.Catechins act as an antioxidant that can scavenge various free radicals.The antioxidant activity based on the structure of epimer,hydroxy phenolic and galloyl group.
Catechins with galloyl group is a natural tyrosine kinase inhibitor that may change the signaling kinases,ERK1/2,proteinkinase B(Akt),PI3K and p38 mitogen-activated protein kinase[2].Phenolic hydroxyl group of catechins can inhibit lipid peroxidation and fat hydrolysis while galloyl group contributes to the prostacyclin production,reduction of vascular cell adhesion molecule-1 expression[3].
Catechins are classified as monomer flavan-3-ol.Flavonoids are known as the main biological active compound from green tea.Catechins levels are 60%up to 80%of the total flavonoid. The potency of catechins as antioxidant can inhibit the oxidation of low density lipoprotein,low plasma cholesterol levels and platelet aggregation[4].The C2-C3 double bond at the carbonyl of C ring and the two hydroxyl groups at positions 3-4 of B ring are main structure for the biological activity of flavonoids.The flavonoids also play a role in the inhibition of TANK-binding kinase 1 activity that related to inflammatory process[5].High contentofpolyphenolsfromgreenteaarepotentialas antioxidant.The polyphenol component can contribute to balancing the low blood pressure and reducing stroke risk andheart disease.Some studies suggested that green tea might protect from coronary heart disease by reducing blood glucose levels and body weight[6].
Catechins are a class of catechin compound group consisting of epicatechin(EC),epigallocatechin(EGC),epicatechin gallate(ECG),epigallocatechin gallate(EGCG),catechin,gallocatechin,catechin gallate and gallocatechin gallate.EGCG is a predominant catechin which has content from 48%up to 55%in total polyphenols of green tea leaves[3].
Catechins can be identified by high performance liquid chromatographic(HPLC)and LC-MS/MS instruments.A HPLC method with UV detection is commonly used for analyzing catechins,caffeine,quercetin and gallic acid[7].Pelillo et al. analyzed catechins from tea by using HPLC with UV and MS-electrospray detection[8].HPLC/MS can be used in the characterization of flavan-3-ols,flavonoids,quinic esters of caffeine,thearubigins and alkaloids of different tea[9-11].
2.Materials and methods
2.1.Chemicals and reagents
The EGCG standard was obtained from Sigma-Aldrich.The mobile phase of LC-MS/MS was 10%ammonia,99%acetic acid,98%formic acid as well as LC-MS grade methanol.For thin layer chromatography analysis,ethyl acetate,methanol and chloroform were used.Furthermore,aqua bidest,acetonitrile,methanol,and glacial acetic acid were used in HPLC analysis.
2.2.Plant material
Green tea GMB-4 clones were obtained from Tea and Quinine Research Center Gambung.Catechins were isolated from green tea GMB-4 clones in powder form.The isolation procedure was conducted on Laboratory of Chemistry,Faculty of Science,Bandung Institute of Technology,Bandung,Indonesia.
2.3.Extraction of crude catechins
A total of 25.1 g of green tea GMB-4 clones was added with 500 mL of distilled water at 90°C and filtered with combined flannel and Buchner funnel.The process was repeated twice to obtain 1.5 L sample extract[1].
2.4.Isolation of catechins
Sample extracts were eluted into column.The elution results were collected and a part of solution was persistent on the column.Furthermore,the column was eluted with 300 mL of 10% ethanol and the results were collected.The column was eluted again with 2.1 L of 10%ethanol.The elution was carried out in stages with each elution of 100 mL.The elution results were collected into different container and dried in the vacuum oven. Thus,the column was eluted with 300 mL of 95%ethanol and the results were collected.The column was eluted with 1.2 L of 95%ethanol and was carried out in stages with each elution of 100 mL.The results were collected into different container then evaporated.Identification of this extract was done by thin layer chromatography.Eluent in 10%ethanol fraction was ethyl acetate,while the eluent in 95%ethanol fraction was methanol: chloroform(1:9.4)[1].
2.5.Separation of catechins by column chromatography with polyamide stationary phase
The column was activated to obtain a better separation results.Separation of catechins with the stationary phase in column chromatography was done by polyamide with 10%ethanol eluent and 95%ethanol.Column chromatography separation with a polyamide stationary phase can separate analyzed compound based on the polarity.About 10%ethanol-water elution was used for separating catechins with a fairly high polarity,such as(+)-catechin(C),(-)-EC,(-)-EGC,and(+)-gallocatechin(GC).Whereas,elution with 95%ethanol was for desorption of EGCG and ECG from polyamide[1].
2.6.HPLC analysis
The HPLC analysis was used as an external standard method. Eluent for HPLC analysis was used(aqua bidest:acetonitrile: methanol:glacial acetic acid=79.5:18:2:0.5).An ultrasonic bath was used to remove dissolved gases.EGCG standard solution was prepared for comparison.Furthermore,analysis of catechins from 10%ethanol fraction was done by dissolving 1.3 mg of sample in 3 mL of eluent,while the 95%ethanol fraction was prepared by dissolving 2.06 mg of the sample in 1 mL of eluent. Detection with a UV-visible detector was at 280 nm.
The freeze-dried extract,10%ethanol and 95%ethanol fraction were analyzed using HPLC to determine the amount of catechins components by comparing chromatograms of the samples with the EGCG standard chromatogram.The HPLC measurement conditions were:flow rate(1 mL/min),the wavelength(280 nm)and the pressure(3.854 psi).UV-visible was used as detector in this procedure.The peak area described the quantity of bioactive compound in the solution[1].
2.7.Preparation of sample for LC-MS/MS analysis
The daily fresh stock solution of catechins was prepared in concentration of 5.10-3mol/L.Subsequently,adequate volume of stock solution was mixed with catechins at a concentration of 5.10-4mol/L.The mixed solution was injected by initial mobile phase.The acidic pH of mobile phase enhanced stability of catechins[12].
2.8.LC-MS/MS analysis
Electrospray ionization(ESI)source was operated in positive ion detection mode.This is the parameter for positive ion detection mode(tuning range specified in the brackets):desolvation temperature was 400°C,capillary voltage was 3.2 V,extractor voltage was 2 V,RF lens(hexapole)voltage was 1.5 V,nebulisation gas flow rate was 400 L/h,cone gas flow rate was 150 L/h and cone voltage was 20 V.Selected reaction monitoring was performed with time 150 ms in a transition for positive ion detection mode:307→ 139(CE 15 eV),291→ 139(CE 15 eV),459→139(CE 20 eV),443→139(CE 15 eV).The data were analyzed using MassLynx Software version 4.1.The analytical column C18 BEH(100 mm×2.1 mm inner diameter;1.7 lm,Waters,Milford,MA,USA)was kept at constant temperature(35°C)during chromatographic separation and volume was 1.5 L.The samples were stored at 4°C and included 0.05% acetic acid eluent[12].
3.Results
3.1.HPLC analysis
Catechin analysis was evaluated by comparing to EGCG standard.The EGCG standard showed high peaks and large areas at a retention time of 5.153.The freeze-dried extract showed seven major peaks which indicated the presence of caffeine,catechin,EC,gallocatechin,EGC,EGCG and epicatechin gallate.The 10%ethanol fractions showed four major peaks at retention time of 1.663,2.367,2.950 and 4.890,which indicated the presence of four components of catechins(catechin,ECs,gallocatechin and epigallocatechin).Whereas,the 95%ethanol fraction showed two main peaks at retention time of 5.167 and 9.820,which indicated the presence of EGCG and ECG(Tables 1 and 2).
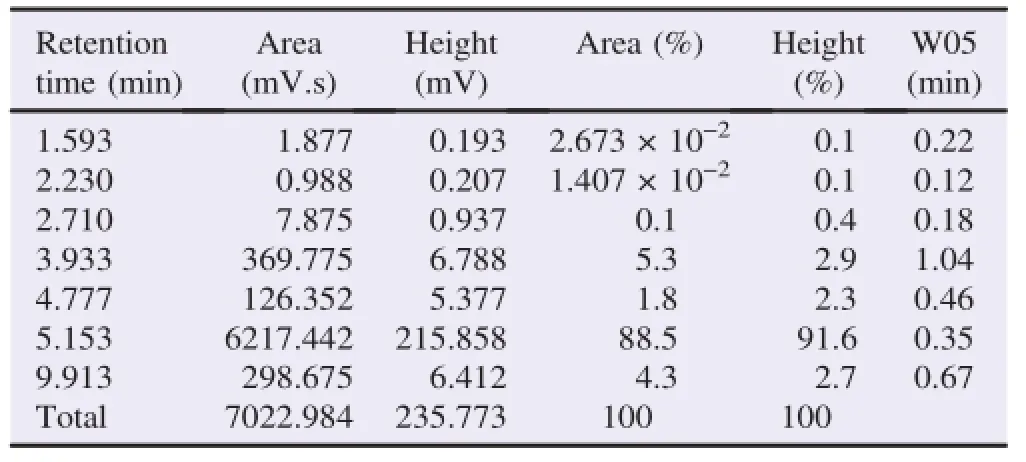
Table 1 Retention time of EGCG standard.
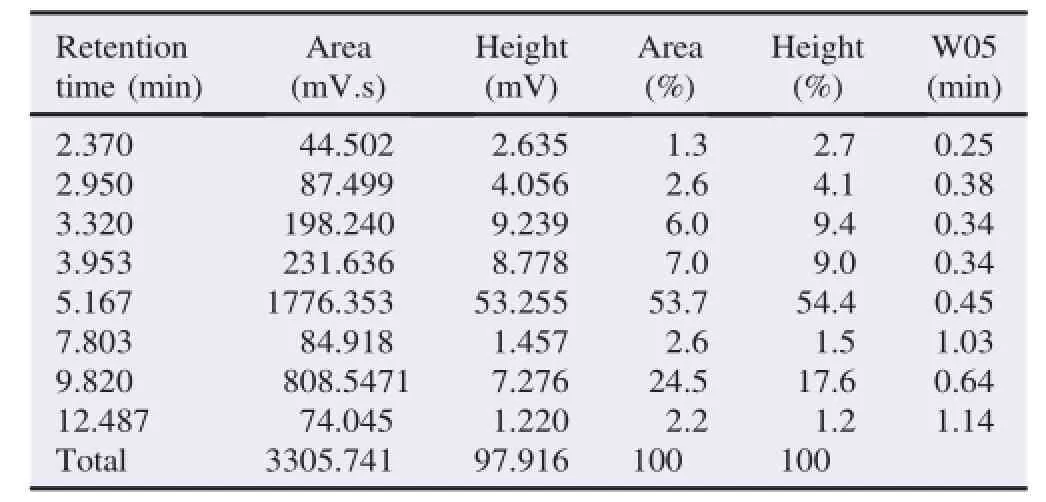
Table 2 Retention time of 95%ethanol fraction.
The determination of high peaks and large areas at the retention time was performed in order to analyze the abundant compound in the crude extract and the catechin from green tea GMB-4 clone.The HPLC profile was shown in Tables 3 and 4.
From the results of the HPLC analysis,the comparison between retention time of each reference spectra identified the active substance of ethanol fraction in green tea GMB-4 clone. Based on these references,it can be concluded active compound of 95%ethanol fraction was shown in Table 5.
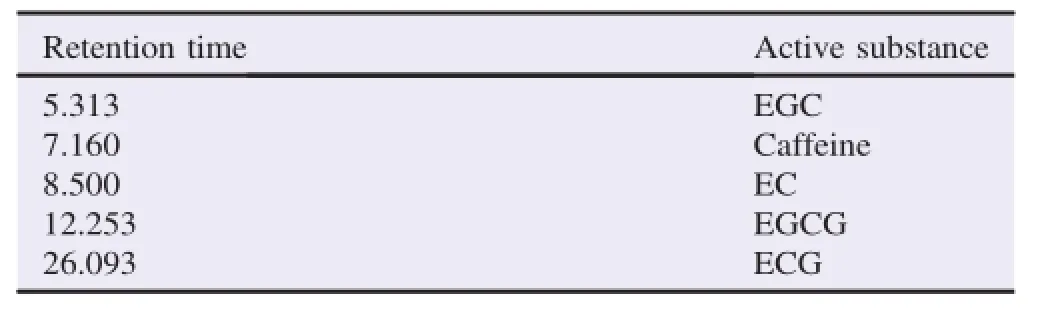
Table 5 The results of the HPLC analysis.
3.2.LC-MS/MS analysis
The mass spectrum of EC was presented in Figure 1a.The peak of protonated ion[M+H]+occurred at m/z 291.The m/z signal at 291 would be a typical fragment ion detected in the mass spectrum of EC.The mass spectrum of EGC was presented in Figure 1b.The peak of protonated ion[M+H]+occurred at m/ z 307.Furthermore,the peak at m/z 443 and 459 were indicated as epicatechins gallate and epigallocatechins gallate.A typical mass spectrum of EGCG was shown in Figure 1c and d.The mass spectra showed a characteristic product ion of EGCG(m/z 459.1 was the[M+H]+ion).It was the same with mass spectra that was reported before[12].
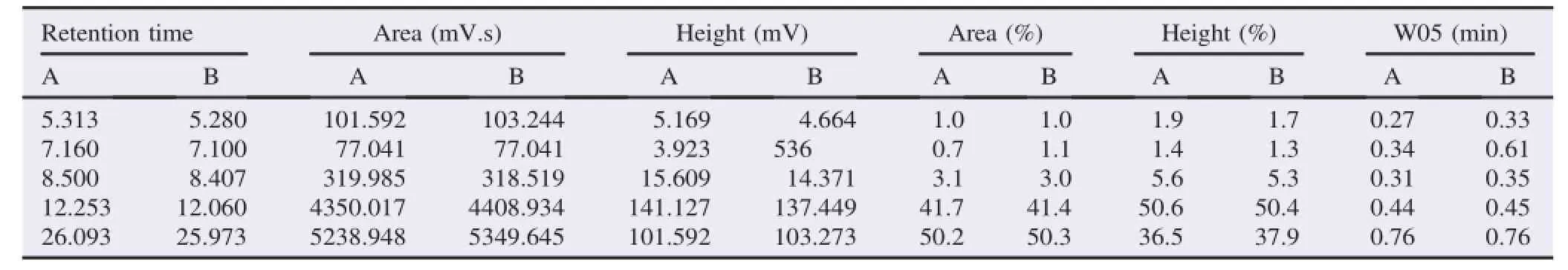
Table 3 HPLC profile of crude extract from green tea GMB-4 clone.
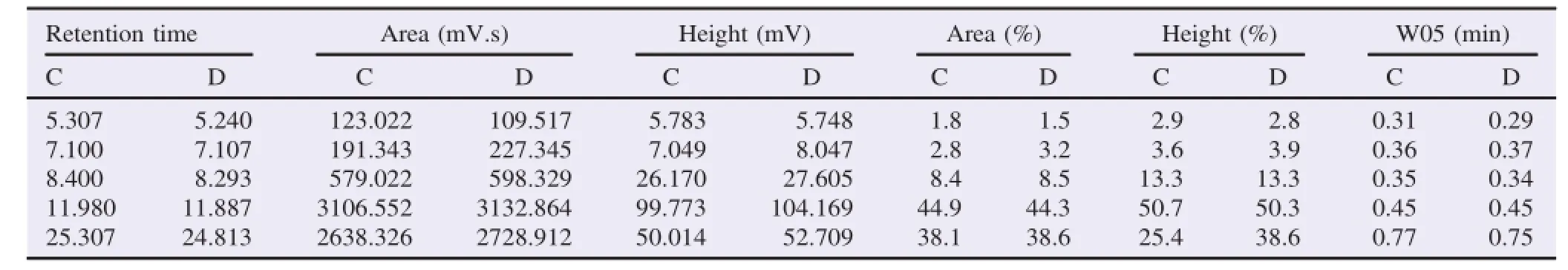
Table 4 HPLC profile of catechins isolates from green tea GMB-4 clone.
4.Discussion
SeparationofcompoundsbyHPLCdependsontheinteraction of analyte molecules with the stationary phase and the mobile phase.Optimization of HPLC means to find the most excellent conditions of separation.A simple and sensitive reversed phase HPLC method has been developed for the determination of catechin and EC in cocoa powder and chocolates[13].Other research showed that a rapid method has been developed to quantify seven catechins and caffeine in green tea(Camellia sinensis)raw material and powdered extract,and dietary supplements containing green tea extract using RP-HPLC[14].
Reversed phase of HPLC has the same column with other phase method,but the silica is modified to make it non-polar by attaching long hydrocarbon chains to its surface-typically with either8or18carbonatomsinthem.Apolarsolventisusedsuchas methanol.In this case,it will be of high attraction between the polar solvent and polar molecules in the mixture when passing through the column.But,the attraction between hydrocarbon chain and the polar molecule will be low.Polar molecules in the mixture will flow with the solvent normally.Non-polar compounds in the mixture will tend to form attractions with the hydrocarbon groups because of van der Waals dispersion forces. Theywillalsobelesssolubleinthesolventbecauseitneedstolose hydrogen bond interaction.It makes the flow in the column run slowly.Itmeansthepolarmoleculeswillpassthroughthecolumn more quickly[14,15].The results of HPLC analysis indicate that EGCG and ECG are the main components of catechins from green tea GMB-4 clone with retention time at 12.253 and 26.093.
A mass spectrophotometer was run by ionizing the molecules,to separate and identify the ions and their mass to charge ratio.Two main keys in this process are a source of ions forming and the analysis period separating the formed ions.LC-MS used different ion sources depending on the components.Ionized molecules on a spectrophotometer under vacuum condition were used electron ionization.Atmospheric pressure ionization technique is widely used for LC-MS analysis.Analyte ions separate mechanically and electrostatically from neutral molecules. Common atmospheric pressure ionization is ESI,atmospheric pressure chemical ionization and atmospheric pressure photo ionization[16].The ESI-MS method was used to determine the bioactive compounds in this extract.The presence of these compounds were confirmed based on the comparison of m/z values from the MS2 spectra with the literature.A positive ionization mode was used for the identification of catechins.
Identification of peaks of catechins isolates in green tea were carried out by comparing mass spectra of the standard data and literature.The results are consistent with research conducted by Araya-Farias et al.Catechins content in green tea extract show that M[+H]+m/z for GC(307.06),EGC(307.09),EC(291.11),EGCG(459.09),GCG(459.10)and ECG(443.11).Other supporting data are as follows:M[H+]+m/z GC(307.08),EGC(307.08),C(291.10),EC(291.12),EGCG(459.10),GCG(459.07),ECG(443.08)and CG(443.08)[15].However,ECG and CG had identical[M+H]+ions at 443.08 and one peak only can be observed in MS spectra.It can be concluded that separation and identification of green tea compounds by LCMS/MS were successful for identifying four major catechins(EGCG,EGC,ECG,and EC).
Stereoisomer of catechins can not be determined using conventional LC-MS/MS but should use mass spectrometer with hydrogen/deuterium exchange reactions which are known to be very sensitive probe of molecular structure,especially the conformation.The analysis of mass spectrometer can distinguish between catechin and EC,gallocatechin gallate and EGCG,gallocatechin and EGC,catechin gallate and ECG[17].
The HPLC and LC-MS/MS methods were successfully tuned for the qualitative analysis of green tea GMB-4 clone extract and its components.The methods provided excellent resolution and separate catechins group completely.Four major catechins were successfully separated and identified as follows:EGCG,EGC,ECG and EC.The results of HPLC analysis indicate that EGCG and ECG are the main components of catechins group from green tea GMB-4 clone.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by a grant of Directorate of General Higher Education,Ministry of Education and Culture of Indonesia for the“BPPDN”scholarship.The authors would like to thank Tea and Quinine Research Center Gambung and Polinema Malang for providing HPLC and LC-MS/MS facilities.
[1]Bailey DT,Yuhasz RL,Zheng B.Method for isolation of caffeinefree catechins from green tea.US Patent 6210679.2001 Apr 3.
[2]Stangl V,Dreger H,Stangl K,Lorenz M.Molecular targets of tea polyphenols in the cardiovascular system.Cardiovasc Res 2007;73(2):348-58.
[3]Babu PV,Liu D.Green tea catechins and cardiovascular health:an update.Curr Med Chem 2008;15(18):1840-50.
[4]Kim HS,Quon MJ,Kim JA.New insights into the mechanisms of polyphenols beyond antioxidant properties;lessons from the green tea polyphenol,epigallocatechin 3-gallate.Redox Biol 2014;2:187-95.
[5]Perron NR,Brumaghim JL.A review of the antioxidant mechanisms of polyphenol compounds related to iron binding.Cell Biochem Biophys 2009;53(2):75-100.
[6]Chacko SM,Thambi PT,Kuttan R,Nishigaki I.Beneficial effects of green tea:a literature review.Chin Med 2010;5:13.
[7]Savic IM,Nikolic VD,Savic IM,Nikolic LB,Stankovic MZ. Development and validation of a new RP-HPLC method for determination of quercetin in green tea.J Anal Chem 2013;68(10):906-11.
[8]Pelillo M,Bonoli M,Biguzzi B,Bendini A,Toschi TG,Lercker G. An investigation in the use of HPLC with UV and MS-electrospray detection for the quantification of tea catechins.Food Chem 2004;87(3):465-70.
[9]Beretta G,Furlanetto S,Regazzoni L,Zarrella M,Facino RM. Quenching ofα,β-unsaturated aldehydes by green tea polyphenols:HPLC-ESI-MS/MS studies.J Pharm Biomed Anal 2008;48(3):606-11.
[10]Clifford MN,Stoupi S,Kuhnert N.Profiling and characterization by LC-MSn of the galloylquinic acids of green tea,tara tannin,and tannic acid.J Agric Food Chem 2007;55(8):2797-807.
[11]Del Rio D,Stewart AJ,Mullen W,Burns J,Lean ME,Brighenti F,et al.HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea.J Agric Food Chem 2004;52(10): 2807-15.
[12]Sp´aˇcil Z,Nov´akov´a L,Solich P.Comparison of positive and negative ion detection of tea catechins usingtandem mass spectrometry and ultra high performance liquid chromatography.Food Chem 2010;123:535-41.
[13]Gottumukkala RV,Nadimpalli N,Sukala K,Subbaraju GV. Determination of catechin and epicatechin content in chocolates by high-performance liquid chromatography.Int Sch Res Not 2014;http://dx.doi.org/10.1155/2014/628196.
[14]Roman MC.Determination of catechins and caffeine in Camillia sinensis raw materials,extracts,and dietary supplements by HPLC-UV:single-laboratory validation.J AOAC Int 2013;96(5): 933-41.
[15]Araya-Farias M,Gaudreau A,Rozoy E,Bazinet L.Rapid HPLCMS method for the simultaneous determination of tea catechins and folates.J Agric Food Chem 2014;62:4241-50.
[16]Savi′c IM,Nikoli′c VD,Savi′c IM,Nikoli′c LB,Jovi′c MD,Jovi′c MD.The qualitative analysis of the green tea extract using ESI-MS method.Adv Technol 2014;3(1):30-7.
[17]Niemeyer ED,Brodbelt JS.Isomeric differentiation of green tea catechins using gas-phase hydrogen/deuterium exchange reactions. J Am Soc Mass Spectrom 2007;18(10):1749-59.
15 Jun 2015
Erna Susanti,Doctoral Program of Biomedical Science,Faculty of Medicine,Brawijaya University,Jl.Veteran,Malang 65145,East Java,Indonesia.
Tel:+6282335223211
E-mail:rna_far@yahoo.co.id
Peer review under responsibility of Hainan Medical University.
#These authors contributed equally to this work.
 Asian Pacific Journal of Tropical Biomedicine2015年12期
Asian Pacific Journal of Tropical Biomedicine2015年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Effect of sucrose and potassium nitrate on biomass and saponin content of Talinum paniculatum Gaertn.hairy root in balloon-type bubble bioreactor
- The frequency and antimicrobial resistance patterns of nosocomial pathogens recovered from cancer patients and hospital environments
- Seroepidemiology of Toxoplasma infection in blood donors in Jahrom District,Southern Iran
- Evaluation of febrile neutropenic patients hospitalized in a hematology clinic
- Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs
- Cytotoxic activity of crude extracts and fractions from Premna odorata(Blanco),Artocarpus camansi(Blanco)and Gliricidia sepium(Jacq.)against selected human cancer cell lines
