Biomass and water partitioning in two age-related Caragana korshinskii plantations in desert steppe, northern China
RenTao Liu, Fan Zhu
1. Key Laboratory for Restoration and Reconstruction of Degraded Ecosystem in Northwestern China of Ministry of Education, Ningxia University, Yinchuan, Ningxia 750021, China
2. The Mina & Everard Goodman Faculty of Life Sciences, Bar Ilan University, Ramat Gan 5290000, Israel
Biomass and water partitioning in two age-related Caragana korshinskii plantations in desert steppe, northern China
RenTao Liu1,2*, Fan Zhu1
1. Key Laboratory for Restoration and Reconstruction of Degraded Ecosystem in Northwestern China of Ministry of Education, Ningxia University, Yinchuan, Ningxia 750021, China
2. The Mina & Everard Goodman Faculty of Life Sciences, Bar Ilan University, Ramat Gan 5290000, Israel
Understanding of biomass and water allocation in plant populations will provide useful information on their growth pattern and resource allocation dynamics. By direct measurement, the biomass and water content partitioning were compared at the aboveground, belowground and whole-plant levels for artificial Caragana korshinskii populations between 6- and 25-year-old sites in desert steppe, northern China. The biomass was mainly allocated to third-grade branches at the aboveground level, and to first- and second-grade roots at the belowground level, and to aboveground parts at the whole-plant vegetative level. Those plant parts mentioned above became the major component of biomass pool of these shrub populations. Biomass pattern changed significantly at aboveground and/or whole-plant levels (P <0.05), but not at belowground level (P >0.05) at 25-year-old site in comparison to 6-year-old site. Also, the water relations between different plant parts changed considerably at all three levels from 6- to 25-year-old sites. These results imply that biomass pattern and relative water content of plant parts are correlated with the process of plantation development. The ratio of belowground to aboveground, though below 1, increased from 6- to 25-year-old site. These results suggest that these shrub populations can adjust biomass partition and relative water content of different compartments to alter their ecological adaptive strategies during stand development in desertified regions.
shrub plantation; biomass allocation; relative water content; sandy grassland
1 Introduction
Caragana korshinskii Kom. is one of the most important plant species in semi-arid and arid regions of northern China (Wang et al., 2001; Sun et al., 2006). This species of Caragana shrubs is preferred by the local population, not only as the main source of livestock fodder, but also because it curbs desertification processes and improves soil quality (Dai, 1988; Zuo et al., 2005). Currently, there is a fairly large stand of afforested shrubland of C. korshinskii in desert steppe of Ningxia, northwestern China (Zuo et al., 2005).
Presently, the biomass of C. korshinskii populations, represented as organic matter, is the result of photosynthetic activity, and is the most important determinant of the productivity of desert steppe ecosystems (Padrón and Navarro, 2004). Thus, a better un-derstanding of biomass allocation and tissue water relations in C. korshinskii populations will provide useful information on growth patterns and resource dynamics of these populations in desert steppe. It is also a basic and necessary step for the study of biophysical properties of different components of desert steppe ecosystems, so as to fully understand ecosystem dynamics and to carry out an adequate management (Padrón and Navarro, 2004).
Recently, studies on C. korshinskii biomass in northern China have established numerical relationships between biomass data and domestic variables through regression equations (Liu and Li, 1984; Chen, 1993; Yu et al., 1993; Liu and Liu, 1994; Zhang and Yan, 2004; Fang et al., 2006; Li, 2008). The use of regression equations for biomass estimation in C. korshinskii populations has been developed in term of endogenous factors such as shrub age, height, basal diameter, crown canopy, and plant density (Chen, 1993; Yu et al., 1993; Liu and Liu, 1994; Li, 2008), and exogenous factors such as soil conditions and site factors including slop aspect and position and soil types (Liu and Li, 1984; Chen, 1993; Liu and Liu, 1994). Also, the influence of cutting (Zuo et al., 2005), clipping treatments (Fang et al., 2006) and interspecies competition (Sun et al., 2006) were discussed on the biomass accumulation and partition of C. korshinskii populations. The findings of the aforementioned studies have important implications for our understanding of biomass allocation, which was used to develop allometric models (Zhong and Yan, 2004). However, these studies did not examine biomass and water content allocation relationships of artificial C. korshinskii plantations at the above- or belowground levels, or at the whole-plant vegetative level along with plant ages.
The primary objective of the present study is to compare the distribution of biomass and water content at the aboveground and belowground levels. Within this framework, a second objective is to develop relationships for the biomass allocation and water content at the whole-plant level. Finally, we calculated the ratios of belowground to aboveground biomass at the whole-plant level with changing plant age.
2 Materials and methods
2.1Site description
The study area, about 10 km from Yanchi County (37°49′N, 107°30′E, 1,430 m elevation), is located in the middle part of Ningxia, northwestern China. This area has a temperate continental semiarid monsoonal climate. Annual precipitation is 280 mm, falling predominantly during the July–September period. Mean annual potential pan-evaporation is around 2,710 mm, about ten times greater than annual precipitation. Mean annual temperature is around 7.5 °C, and lowest and highest monthly mean temperatures are -8.7 °C in January and 22.4 °C in July. Mean annual wind velocity is around 2.8 m/s, and prevailing winds are northwest in winter and spring; blowing sand and dust in wind velocity of over 5.0 m/s occurs 323 times in one year. A wind erosion period often occurs from April to mid-June before the rainy season arrives (climate data from Yanchi Meteorological Station, 1976–2010). The zonal soils are Loessial and light Sierozem, and the azonal soils are sandy Arenosols, alkaline saline and meadow soil, all poor in fertility with loose structure and very susceptible to wind erosion. The topography is characterized by peneplain with semi-manual grasslands (Zhang et al., 2004).
Yanchi County is one of the areas of serious concern for land desertification in desert steppe of north China (Liu, 2002; Zhang et al., 2003). The desertified area increased from 1,006.11 km2in 1961 to 2,366.67 km2in 1989 (Qiao, 2006). However, since the late 1980s some successful measures to combat desertification have been implemented, such as planting indigenous shrubs and grasses adapted to sandy land, fencing grassland against grazing and using minimum tillage (Liu, 2002; Zhang et al., 2003, 2004). These measures have decreased the desertified area to 455.75 km2in 2003 (Qiao, 2006). The vegetation consists largely of low, open shrubland dominated by C. korshinskii, Salix psammophila C. Wang et Chang Y. Yang, Oxytropis aciphylla Ledeb., Astragalus adsurgens Pall., Lespedeza bicolor Turcz. and Sophora alopecuroides L., with the herbaceous stratum dominated by Stipa grandis P. A. Smirn., S. bungeana Trin., Agropyron cristatum (L.) Gaertn., Leymus secalinus (Georgi) Tzvelev, Salsola collina Pall. and Pennisetum centrasiaticum Tzvelev.
2.2Plant sampling and data collection
Two experimental sites with sandy soils were established according to stand age (6 and 25 years), and were representative of the typical stands of C. korshinskii in desert steppe. The basic information for each site such as location, plant age and height together with shrub crown, and soil physical and chemical characteristics is listed in Tables 1 and 2.
In each site, three 20m×20m C. korshinskii plots were established, with one individual representatively selected in a plot during July–August, 2011. Totally, we sampled six shrubs using the selective harvesting technique (Fang et al., 2006) at both spatial radius horizontally and soil depth vertically of 2.0 m respectively; previous studies show that 99.31% root distribution are at soil depths of 0–1.5 m (Liu and Liu,1994). Before the harvest, the plant height (m) and crown diameter (m, smallest and greatest) were measured for each shrub with a tape measure.
The aboveground parts of each individual were grouped into four fractions: leaf, first-grade branches (older branch linked with taproot), second-grade branches (old branch on first-grade branches) and third-grade branches (new branch on second-grade branches). Likewise, at the belowground level, the roots were divided into four fractions: first-grade roots (taproot), second-grade roots (roots on first-grade roots), and third-grade roots (fine roots on second-grade roots), together with fibrous roots (all very fine roots on third-grade roots), which represented the main sources of the root system. At the whole-plant vegetative level, the individual was classified as aboveground and belowground parts (Li and Xiao, 2007; Liu et al., 2009).
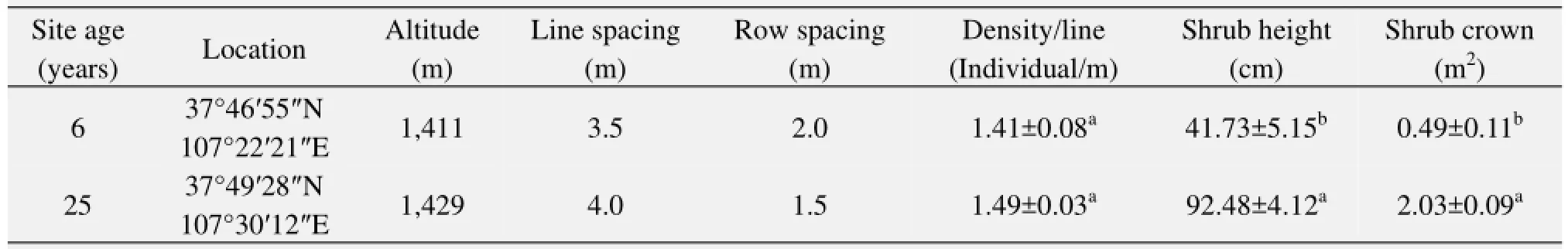
Table 1 Plot structure, plant age and characteristics of C. korshinskii communities in different habitats

Table 2 Soil properties of C. korshinskii communities in different habitats
After harvesting the shrubs, we separated their biomass into categories mentioned above and measured the total fresh weight in each category to the nearest 0.01 g. The samples were oven-dried for three days at 75 °C, and the dry weight was then recorded at room temperature.
To estimate the water content of each plant part, we used the f coefficient (Padrón and Navarro 2004): f = dry weight/fresh weight (kg/kg)
The value, 1-f, was thus equivalent to the relative water content (RWC) of the fresh biomass (Sternberg and Shoshany, 2001; Wu et al., 2008).
2.3Statistical analyses
All statistical analyses were conducted using STATISTICA v.5 and SPSS v.15.0 software. We used multiple comparisons and one-way analysis of variance (ANOVA) to compare the differences among the treatments. We used least significant difference (LSD) and nonparametric tests to determine the significance of differences among treatment means at P <0.05 following the method of Zar (1996). We also calculated means and standard errors.
3 Results
3.1Biomass and water content partitioning at the aboveground level
From Table 3, the specific gravity of biomass was significantly higher in third-grade branches than in other three parts (P <0.05) at the aboveground level, whether at 6- or 25-year-old site. Except for third-grade branches, there was a reverse biomass partition pattern from 6- to 25-year-old sites. The specific gravity of biomass was significantly higher in leaves than in second- and first-grade branches at 6-year-old site (P <0.05), but reversed at 25-year site (P <0.05).

Table 3 Specific gravity (%) of biomass at the aboveground level
Likewise, RWC was fairly high in third-grade branches than in other three parts aboveground (Table 4) at both sites. Particularly at 6-year-old site, RWC was significantly higher in third-grade branches than in other parts. Also, no significant differences were found in RWC amongst leaves, second- and firstgrade branches whether at 6- or 25-year-old site.
3.2Biomass and water content partitioning at the belowground level
Similar tendency from first-grade roots to fibrous roots was found in specific gravity of biomass between 6- and 25-year-old sites (Table 5). There was significantly higher specific gravity of biomass in first- and second-grade roots than the other two parts belowground (P <0.05). No significant differences were found in specific gravity of biomass between first- and second-grade roots, or between third-grade and fibrous roots, whether at 6- or 25-year-old sites.
Table 6 presents the different tendency in RWC from first-grade roots to fibrous roots between 6- and 25-year-old sites. At 6-year-old site, there was a significant decrease trend in RWC from first-grade roots through second-grade and third-grade roots to fibrous roots (P <0.001). While at 25-year-old site, RWC was significantly higher in third-grade roots than in second-grade roots, with the intermediate values in the first-grade and fibrous roots.

Table 4 Values of 1-f (1- dry weight/fresh weight) at the aboveground level

Table 5 Specific gravity (%) of biomass at the belowground level

Table 6 Values of 1-f (1- dry weight/fresh weight) at the belowground level
3.3Biomass and water content partitioning at the whole-plant level
There was a similar tendency from aboveground to belowground in specific gravity of biomass between 6- and 25-year-old sites (Figure 1a). The specific gravity of biomass was remarkably higher in aboveground parts than in belowground parts at both sites, particularly at 6-year-old site where there was a significant difference (P <0.05).
For relative water content tendency (Figure 1b), water content relations of aboveground to belowground were quite different between 6-year-old site and 25-year-old site. At 6-year-old site, RWC was significantly higher aboveground than belowground (P <0.0001), whereas there was little difference in RWC between aboveground and belowground at 25-year site.
3.4Ratios of belowground to aboveground biomass
Figure 2 shows that the mean ratio of belowground to aboveground biomass was less than 1, whether at 6- or 25-year-old site (P <0.05) (i.e., the shrubs had a higher proportion of aboveground biomass). Also, the ratio of belowground to aboveground was considerably higher at 25-year-old site than at 6-year-old site (1.3 times higher), though no significant differences were found (P >0.05).
4 Discussion
Our paper represents the first study that provides data on biomass and water content partitioning in artificial populations of shrub species in desert steppe. Although our sample size was small because of the amount of labor required to excavate the shrubs, itwas necessary to carry on this research into the growth patterns and resource dynamics during stand development (King et al., 2007; Liu et al., 2009) in desert steppe.
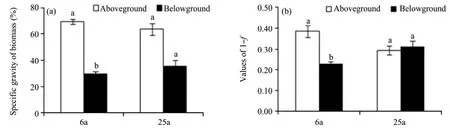
Figure 1 Specific gravity of biomass (a) and values of 1-f (1- dry weight/fresh weight) (b) at the whole-plant level. Bars for a given biomass fraction labeled with different letters at the same site differed significantly (P <0.05). 6a and 25a were 6- and 25-year-old sites, respectively

Figure 2 Ratios of belowground to aboveground biomass. Bars for a given ratio labeled with same letters represent no significance (P >0.05). 6a and 25a were 6- and 25-year-old sites, respectively
At the aboveground level, the biomass was mainly allocated to third-grade branches regardless of shrub age, which was in agreement with previous research (Liu and Liu, 1994). Third-grade branches with main biomass as a considerable carbon stock indicated the necessity of generation of sprout tillers (Liu et al., 2009), which represented a hierarchy in the priority of biomass partitioning to new branches (Burkes et al., 2003). The observed pattern of biomass allocation to third-grade branches might also represent an important response to increasing water demand (Li and Xiao, 2007). In this study, there was a remarkably higher RWC in third-grade branches than in other three parts at both sites, especially at 6-year site where prioritizing water allocation to peripheral branches could be beneficial for the plant to sprout and photosynthesize in these perennial shrubs (James et al., 2005; Wu et al., 2008).
From 6- to 25-year-old sites, there was a reverse pattern of biomass allocation, except for third-grade branches discussed above. At 6-year site, higher gravity of biomass in leaves suggested a higher ability to maximize the shrub's resource acquisition for more photosynthetic products (Schierenbeck et al., 1994), which is associated with their capacity to become established under a variety of competitive conditions and poor habitats (Schweitzer and Larson, 1999). After 25-year establishment, the shrub partitions a larger proportion of biomass into branch support (i.e., branch diameter growth of first- and second-grade branches), because the branches were longer, and more foliage was located further from the stem (Ford, 1985). The transition of biomass to branch support was also correlated with increased water demands as the shrub grew (Wu et al., 2008). The relative water content was higher at 25-year-old site than at 6-year-old site, whether in leaves or in any branch parts at the aboveground level (Table 4), which provided the possibility for biomass partition into old branches through transforming system within the plants (Cambui et al., 2011).
At the belowground level, there was a similar pattern of biomass partition between 6-year and 25-year sites, which represented an adaptation to similar soil conditions (Li and Xiao, 2007) (no significant differences found in soil bulk density, and content of very fine sand and clay silt between 6-year and 25-year sites). There was a significantly higher biomass partition to first-grade and second-grade roots, which might be related to the functions of an important carbon sink that could be used for smaller side roots (King et al., 2007) and asexual reproduction after cutting (Fang et al., 2006; Liu et al., 2009). The relative water content changed considerably in different root parts from 6- to 25-year-old sites. This provided an important insight into the distribution of water content within the roots. In general, RWC was an identical index of drought resistance of plants (Padrón and Navarro, 2004) in desert steppe. These findings indicate that the Caragana shrubs have higher drought resistance at 25-year-old site than at 6-year-old site. Further, the higher RWC in third-grade roots at25-year-old site reflects an adaptation to the arid conditions due to the higher capacity of water and soil conservation (Liu and Li, 1984; Fang et al., 2006). These results also suggest that this shrub is an ecologically important species for vegetation restoration and desertification control in desert steppe (Liu and Li, 1984; Chen, 1993; Liu and Liu, 1994).
At the whole-plant level, the considerably higher gravity of biomass in aboveground parts at both sites indicates that the biomass was mainly concentrated in the aboveground plant parts. As a result, the ratio of belowground to aboveground in our shrubs was below 1, which is not consistent with previous results that the below- to aboveground biomass ratio of desert and shrubland species is usually higher than 1 (Jackson et al., 1996; Mokany et al., 2006), but is consistent with research in Mu Us desert of northern China (Li and Xiao, 2007). The lower values of below- to aboveground biomass ratio at both sites are related to the growth properties of this shrub (Li and Xiao, 2007). In this species, individual plant growth of belowground organs was much slower than aboveground growth (Niu, 1998; Zhu et al., 2011). One important reason was the aboveground compensatory growth due to grazing on peripheral branches and leaves by sheep. Also, the ratio of belowground to aboveground increased from 6- to 25-year-old site. Obviously, the below- to aboveground biomass ratio was affected by plant age and size (Schmid, 2002; Mokany et al., 2006), though no significant differences in each parts were found between 6- and 25-year-old site, whether at aboveground, belowground, or whole-plant levels. Furthermore, plants with more roots could explore the soil more efficiently and therefore grow and survive better under water-stress conditions (Fernández et al., 2002), especially after establishment for 25 years. Our results were in line with similar studies about environmental stresses on other species (Fernández et al., 2002; Li and Xiao, 2007).
5 Conclusion
This study has important implications for the dynamics of biomass and water content at aboveground, belowground and whole-plant levels for two age-related artificial C. korshinskii populations in desert steppe by direct measurement. Third-grade branches at aboveground level, first- and second-grade roots at belowground level, and the aboveground parts at whole-plant level, became the major component of biomass pool of these shrub populations. Significant influences of stand age were found on biomass allocation at the aboveground and whole-plant levels, but not at the belowground level. Also, RWC changed considerably at these three levels from 6- to 15-year-old sites, which implies that RWC functioned differently during stand development. Furthermore, the ratio of belowground to aboveground, though below 1, increased from 6- to 25-year-old site. These results suggest how this shrub species alter their ecological adaptive strategies through adjustments in biomass and relative water content of different compartments at different stages of stand development.
Acknowledgments:
The authors are grateful to the anonymous reviewers for their critical review and comments on drafts of this manuscript. This research was in part financially supported by the National Natural Science Foundation of China (No. 41101050), the National Science and Technology Support Program (2010BAC07B03) of China and the Projects of the National Basic Research Program of China (No. 2009CB421303).
Burkes EC, Will RE, Barron-Gafford GA, et al., 2003. Biomass partitioning and growth efficiency of intensively managed Pinus taeda and Pinus elliottii stands of different planting densities. Forestry Science, 2: 224–234.
Cambui CA, Svennerstam H, Gruffman L, et al., 2011. Patterns of plant biomass partitioning depend on nitrogen source. PLoS ONE, 6: 1–7.
Chen YM, 1993. A study and investigation on biomass of Caragana korshinskii forest in Loess Hilly Region. Shaanxi Forestry Science and Technology, 4: 23–26, 48.
Dai FH, 1988. Vegetation in Ningxia. Yinchuan, China: Ningxia People's Press.
Fang XW, Wang WP, He XQ, et al., 2006. A study on vegetative compensatory growth of shrub, Caragana korshinskii, under different clipping treatments in disturbance environment. Journal of Plant Ecology, 5: 810–816.
Fernández RJ, Wang MB, Reynolds JF, 2002. Do morphological changes mediate plant responses to water stress? A steady-state experiment with two C4grasses. New Phytologist, 155: 79–88.
Ford ED, 1985. Branching, crown structure and control of timber production. In: Cannell MGR, Jackson JE (eds.). Attributes of Trees as Crop Plants. Institute of Terrestrial Ecology, Abbots Ripton, UK, pp. 228–252.
Jackson RB, Canadell J, Ehleringer JR, et al., 1996. A global analysis of root distributions for terrestrial biomes. Oecologia, 108: 389–411.
James JJ, Tiller RL, Richards JH, 2005. Multiple resources limit plant growth and function in a saline-alkaline desert community. Journal of Ecology, 93: 113–126.
King JS, Giardina CP, Pregitzer KS, et al., 2007. Biomass partitioning in red pine (Pinus resinosa) along a chronosequence in the Upper Peninsula of Michigan. Canadian Journal of Forestry Research, 37: 93–102.
Li CP, Xiao CW, 2007. Above- and belowground biomass of Artemisia ordosica communities in three contrasting habitats of the Mu Us desert, Northern China. Journal of Arid Environments, 70: 195–207.
Li Y, 2008. Study on the ecophysiological characteristics of shrubs in Loess Plateau of China. Master Thesis of Gansu Agricultural University, Lanzhou, China.
Liu M, 2002. Present situation, causes and countermeasures of sandy desertification in Yanchi Grassland. Pratacultural Science, 6: 5–6.
Liu PH, Li SY, 1984. Investigation on the relationships between biomass and site conditions and plant density of Caragana korshinskii in Yulin regions of northern Shaanxi, China. Shaanxi Forestry Science and Technology, 1: 21–26.
Liu RT, Bi RC, Zhao HL, 2009. Biomass partitioning and water content relationships at the branch and whole-plant levels and as a function of plant size in Elaeagnus mollis populations in Shanxi, North China. Acta Ecologica Sinica, 29: 139–143.
Liu ZD, Liu ZW, 1994. Study on biomass of Hippophae rhamoidesl and Caragana microphylla on Loess Plateau. Acta Agriculturae Boreall-Occidentalis Sinica, 2: 92–96.
Mokany K, Raison RJ, Prokushkin AS, 2006. Critical analysis of root: shoot ratios in terrestrial biomass. Global Change Biology, 12: 84–96.
Niu XW, 1998. Biological characters of cultivars in Caragana. Acta Agriculturae Boreall-Sinica, 4: 122–129.
Padrón E, Navarro RM, 2004. Estimation of above-ground biomass in naturally occurring populations of Prosopis pallid (H. & B. ex. Willd.) H. B. K. in the north of Peru. Journal of Arid Environments, 56: 283–292.
Qiao F, 2006. The studies of desertification monitoring: taking Yanchi County in Ningxia Hui Autonomous Region as example. Dissertation of Beijing Forestry University, China.
Schierenbeck KA, Mack RN, Sharitz RR, 1994. Effects of herbivory on growth and biomass allocation in native and introduced species of Lonicera. Ecology, 75: 1661–1672.
Schmid I, 2002. The influence of soil type and interspecific competition on the fine root system of Norway spruce and European beech. Basic Applied Ecology, 3: 339–346.
Schweitzer JA, Larson KC, 1999. Greater morphological plasticity of exotic honeysuckle species may make them better invaders than native species. Journal of Torrey Botanic Society, 126: 15–23.
Sternberg M, Shoshany M, 2001. Aboveground biomass allocation and water content relationships in Mediterranean trees and shrubs in two climatological regions in Israel. Plant Ecology, 157: 171–179.
Sun ZR, Wang WQ, Zhai MP, 2006. Effects of interspecies competition on biomass accumulation and allocation of Glycyrrhiza uralensis and Caragana microphylla. Scientia Silvae Sinica, 6: 1–6.
Wang QZ, Li QF, Cui J, et al., 2001. Path-coefficient analysis of seed yield with main agronomic characters in Caragana korshinskii. Grassland China, 3: 35–37.
Wu FZ, Wei KB, Li FL, et al., 2008. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environmental Experimental Botany, 63: 248–255.
Yu HB, Wang BJ, Shi ZG, et al., 1993. Biomass and utilization of Caragana microphylla in the arid regions of central Gansu. Ecology and Forest Research, 2: 19–23.
Zar JH, 1996. Biostatistical Analysis (third ed.). Prentice-Hall Inc., NJ, USA.
Zhang KB, Li R, Hou RP, 2004. Study on plant diversity of different control measures of desertification in Yanchi County, Ningxia. Science of Soil Water Conservation, 4: 66–72.
Zhang KB, Wang JL, Li R, et al., 2003. Study on the land desertification and its control in the crop-grazing crisscross area of China: taking Yanchi County in Ningxia Hui Autonomous Region as example. Science of Soil Water Conservation, 1: 85–90.
Zhong YS, Yan AL, 2004. Modification and application of the PRESS in the multiplicative regression model. Journal of Northwest Forestry University, 3: 168–170.
Zhu YL, Wang S, Lin YG, et al., 2011. Development of Caragana microphylla seedling root system in Hilly Regions of Loess Plateau. Bulletin of Soil Water Conservation, 2: 232–237.
Zuo Z, Guo YZ, Zhang QY, et al., 2005. Technical research on cutting and utilization of Caragana microphylla. Contemporary Animal Husbandry, 6: 31–34.
Liu RT, Zhu F, 2015. Biomass and water partitioning in two age-related Caragana korshinskii plantations in desert steppe, northern China. Sciences in Cold and Arid Regions, 7(3): 0238-0244. DOI: 10.3724/SP.J.1226.2015.00238.
*Correspondence to: RenTao Liu, Key Laboratory for Restoration and Reconstruction of Degraded Ecosystem in Northwestern China of Ministry of Education, Ningxia University, Yinchuan, Ningxia 750021, China. Tel: +86-951-4967201; Fax: +86-951-4967201. E-mail: liubarilanu@gmail.com
June 22, 2014 Accepted: September 28, 2014
 Sciences in Cold and Arid Regions2015年3期
Sciences in Cold and Arid Regions2015年3期
- Sciences in Cold and Arid Regions的其它文章
- Comparing the seasonal variation of parameter estimation of ecosystem carbon exchange between alpine meadow and cropland in Heihe River Basin, northwestern China
- Comparative studies on leaf epidermal micromorphology and mesophyll structure of Elaeagnus angustifolia L. in two different regions of desert habitat
- Elemental composition and its environmental significance for the varicolored hills in the northern foothills of the Qilian Mountains of Sunan Yugur Autonomous County, China
- The characteristics of oasis urban expansion and drive mechanism analysis: a case study on Ganzhou District in Hexi Corridor, China
