Toxicity of perfluorononanoic acid and perfluorooctane sulfonate to Daphniamagna
Guang-hua Lu*,Jian-chao Liu,Li-sha Sun,Lu-jin YuanKey Laboratory of Integrated Regulation and Resources Developmentof Shallow Lakes ofMinistry of Education,College of Environment,HohaiUniversity,Nanjing 210098,PRChina Received 1 September 2013;accepted 29 July 2014 Available online 14 January 2015
Toxicity of perfluorononanoic acid and perfluorooctane sulfonate to Daphniamagna
Guang-hua Lu*,Jian-chao Liu,Li-sha Sun,Lu-jin Yuan
Key Laboratory of Integrated Regulation and Resources Developmentof Shallow Lakes ofMinistry of Education,College of Environment,HohaiUniversity,Nanjing 210098,PRChina Received 1 September 2013;accepted 29 July 2014 Available online 14 January 2015
Abstract
In order to study toxicological effects of perfluorononanoic acid(PFNA),perfluorooctane sulfonate(PFOS),and theirmixtures(PFNA/ PFOS)on Daphniamagna(D.magna),a suite of comprehensive toxicity testswere conducted,including a 48-h acute toxicity test,a 21-day chronic test,a feeding experiment,and a biomarker assay.D.magna were exposed to aqueous solutions of PFNA and PFOS(alone and in combination)at concentrations ranging from 0.008 to 5mg/L.The survival,grow th,and reproduction of D.magna w eremonitored over a 21-day life cycle.The biomarkers,including acetylcholinesterase(AChE),superoxide dismutase(SOD),and catalase(CAT)activities,were determ ined after seven days of exposure.PFOS wasmore toxic than PFNA based on the results of the acute toxicity test.Perfluorinated compounds(PFCs)inhibited both grow th and reproduction of D.magna during the testing period.The ingestion rates and the biomarkers,including AChE,SOD,and CAT activities,were significantly inhibited by PFCs inmost cases.Moreover,the combined effects related to the grow th and reproduction show ed the antagonistic effects of PFCs. ©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Toxicity effect;Perfluorononanoic acid;Perfluorooctane sulfonate;Daphniamagna;Reproduction;Ingestion rate;Biomarker
1.Introduction
Perfluorinated compounds(PFCs)w ith different functions have been extensively used in many technologies,either individually or as composites inmanufactured products(Giesy and Kannan,2002).For examples,PFCswere used as water-,oil-and grease-repellents or as surfactants(Renner,2001).In recent years,PFCs have become a focus of public health concern due to their ubiquitous presence in environmental media(air,water,soil,sediment,and house dust),food and drinking water,and in w ildlife,including fish,birds,and mammals(Mommaerts et al.,2011;Murakami et al.,2011; Rudel et al.,2011;Zhang et al.,2011;Tatum-Gibbs et al.,2011).PFCs have been detected in urban and remote areas around the world,and some researchers have indicated that PFCsaccumulate in animals,including humans(Pow ley etal.,2008;Cai et al.,2010;Leondiadis et al.,2010).
Of the PFCs,perfluorooctane sulfonate(PFOS)and perfluorononanoic acid(PFNA)have been found to be the representative PFCs in the environment and in biota(Guruge etal.,2005;Kannan etal.,2004),and PFOS is regarded as the final metabolite of many PFCs by degradation or metabolization(Olsen et al.,2004;Calafat et al.,2006).The ionic nature,high solubility,and negligible vapor pressure when PFOS and PFNA are dissolved in water,make them highly mobile,and they exist in groundwater(Plum lee et al.,2008),surfacewater(Eschauzier et al.,2010),drinking water(Flores etal.,2013),and oceanwater(Snchez-Avila etal.,2010)w ith a concentration ranging from ng/L to severalμg/L.A higher concentration of PFOS(about43.5μg/L)hasbeen detected inthe Langat River,Malaysia(Zainuddin et al.,2012).A composition profile of total PFC concentrations showed that the com position was dom inated by PFOS and PFNA in the Yodo River system of Japan(Shivakotiet al.,2011).In sediment,the contentsof PFOSwere shown to be 28-145 pg/g of the dry weightat Kogaigawa and Sakuragawa cities in Ibaraki Prefecture,in Japan(Ahrensetal.,2011).Kratzer etal.(2011)found that PFOS was the predominant compound(9.57-1 444 ng/g of wet weight),followed by PFNA(0.47-109 ng/g of wetweight),in liver tissue of grey seals(Halichoerus grypus)collected from the Baltic Sea.In addition,a study revealed that insect larvae,fish,and crabs contained levels ranging from 0.23 to 144 ng/g of wetweight of PFOS and from 0.07 to 7.5 ng/g of wet weight of PFNA(Fernandez-Sanjuan et al.,2010).PFOS is also listed as a chemical contaminant on the Final Third Drinking Water Contaminant Candidate List(CCL3)considered for future regulation(US EPA,2009).Clearly,more information is required to guide decisions about regulations of PFOS and PFNA.
Recent studies have indicated unintended biological activity of PFCs toward non-target organisms.The no-observed effectconcentration(NOEC)of PFOS determ ined for Daphnia magna(D.magna)is reported to be 5.3mg/L(Boudreau etal.,2003).PFOShasalso been found to decrease the heart rate of zebrafish embryos(Ding et al.,2012a).Huang et al.(2012)determined the transcription profiling of Oryziasmelastigma embryo exposed to PFOS,and indicated that the differentially expressed genes were related to neurobehavioral defects,m itochondrialdysfunction,and themetabolism of proteinsand fats.Thyroid-disrupting effects of long-term PFNA exposure have been observed on the levels of genes and proteins in zebrafish(Liu et al.,2011).However,toxicity information regarding PFNA,and especially information on its toxicity when combined w ith PFOS,is still lacking w ith regard to D. magna.D.magna,designated by the U.S.Environmental Protection Agency(U.S.EPA)asamodel organism,iswidely accepted as an indicator for assessing the toxicity of environmental contam inants because of its high rate of reproduction and sensitivity to different conditions(Ventura et al.,2010).Acute and chronic toxicity tests are simple,costeffective,and sensitive,and are aw idely acceptedmethod for toxicity determ ination,providing a fast yet accurate estimate of toxicity of a compound(Ventura et al.,2010).The feeding behavior of aquatic organisms has been studied as a physiological response to toxic effects of chem icals(Barata et al.,2008;de Schamphelaere etal.,2007).In addition,antioxidant enzymes such as catalase(CAT)and superoxide dismutase(SOD)havealso beenw idely used for both characterization of thedefensemechanismsand evaluation of the toxicity induced by oxidative stressors(Kim etal.,2012).
In order to investigate the biological effectsof PFCson D. magna after different exposure periods,we conducted a comprehensive toxicity test of PFOS,PFNA,and their mixtures(PFNA/PFOS),including a 48-h acute toxicity test and a 21-day chronic toxicity test.A feeding experiment was conducted to explore whether the two target compounds could interfere w ith food intake and ultimately cause toxicity to D. magna.Finally,the activities of acetylcholinesterase(AChE),SOD,and CAT were synchronously determ ined in order to investigate single and combined effects of the two PFCs on multiple biomarkers during a seven-day exposure period.All of the above are important for ecotoxicological risk assessments of PFOS and PFNA.
2.M aterials and methods
2.1.Chemicals
PFNA(98%purity)was purchased from the A lfa Aesar Co.,Ltd.(Los Angeles,USA).PFOS(98%purity)was purchased from the Matrix Scientific Co.,Ltd.(Basel,Sw itzerland).
The test solutionwas prepared immediately prior to use by diluting the stock solution with a daphnia culture medium(consisting of 64.75 mg/L of NaHCO3,5.75 mg/L of KCl,123.25mg/L ofMgSO4·7H2O,and 294mg/L of CaCl2·2H2O)reconstituted according to the guideline formulated by the Organization for Economic Cooperation and Development(OECD,2004).
Acetylthiocholine iodide(ATChI)and 5,5′-dithiobis(2-nitrobenzoic acid)(DTNB)were purchased from the Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Bovine serum albumin was purchased from the Shanghai Huixing Biochemistry Reagent Co.,Ltd.(Shanghai,China)w ith a purity of more than 98%.All other chemicals were of analytical grade and were obtained from the Shanghai Chemical Reagent Co.,Ltd.(Shanghai,China).
2.2.Animals
D.magna,a common zooplankton found in freshwater lakesand ponds,isone of themostsensitiveorganismsused in toxicity tests(Alberdi et al.,1996).In this study,D.magna were originally obtained from the Chinese Center for Disease Control and Prevention(Beijing,China).It was cultured in dilution water according to the International Organization for Standardization(ISO,1996).The culture medium was renewed three timesweekly.D.magna were fed daily w ith the green algae,Scenedesmusobliquus,whichwas supplied by the Wuhan Institute of Hydrobiology of the Chinese Academy of Sciences.Exposure water quality was checked daily and maintained at conditions suitable for D.magna(temperature of(20±1)°C,pH value of 7.2±0.3,and dissolved oxygen(DO)of(6.5±0.5)mg/L).A daily 16/8-h light/dark photoperiod cyclewas used throughout the experiment.
Offspring of D.magna were separated at regular intervals from culture dishes that were 3-5 weeks old and the test animalswere less than 24 h old.
2.3.Acute and chronic toxicity tests
The 48-h acute toxicity test of D.magna was performed according to OECD(2004).Six concentrations of PFC(1,3,10,30,100,and 300 mg/L)were selected.Ten neonates less than 24 h old from a designated brood were placed in a 100 m L glass beaker containing 45 m L of PFC solution for each test concentration and control group.No PFC wasadded in the control group.Test D.magna were not fed during the testing period.Each treatment was replicated three times simultaneously.Water quality parameters(pH value,temperature,conductivity,and DO)were measured at the very beginning and end of the test.The statuses of immobilization andmortality were checked at48 h,and resultswere recorded. D.magna thatwere unable to sw im w ithin 15 s after gentle agitation of the test containerwas considered to be immobile. Those animalswhose heartbeats had stopped were considered dead.The heartbeats were observed w ith a stereom icroscope w ith amagnification of four.
The effects of two kinds PFCs on the reproductive output were assessedw ith asemi-static testaccording to thestandard procedure for D.magna reproduction testing(OECD,2012). Based on the resultsof acute toxicity tests,neonates less than 24 h old wereexposed to various concentrationsof PFOSand PFNA(0.008,0.04,0.2,1,and 5 mg/L).The solutions of PFNA and PFOSwere also mixed w ith the same concentrations at 0.004,0.02,0.1,0.5,and 2.5 mg/L.Single D.magna was cultured in 100 m L glass beakers filled w ith 45 m L of solution for a 21-day period at(20±1)°C.The test was performed in 20 replicates for each exposure concentration. D.magna were fed w ith 1×106cells/m L of Scenedesmus obliquus per day for each animal.The test solution was renewed every other day.Water quality parameters were measured after changing the test solution.Survival,grow th,and reproduction(fecundity)of D.magna weremonitored for each of the 20 replicates.Grow th of the surviving adults under each treatmentw as determ ined after 21 days of exposure.The body length of each surviving animalwasmeasured from the apex of the helm et to the base of the tail spine.Days to the first pregnancy,days to the first brood,the quantity of the first brood per female,and the average number of offspring in each brood were the criteria used to evaluate the fecundity.Neonates were counted daily and were removed from the beakers.
This study also examined the intrinsic rate of population grow th(r)in order to estimate the effect of toxicant exposureson population grow th(Pestana etal.,2010;Zhao etal.,2012).Itwas calculated using the follow ing formula(Lotka,1913):

where lxis the ratio of individuals surviving to age x,mxis the age-specific fecundity(number of neonates produced per surviving female at age x),and x is in days.As r calculated based on the number of D.magna after 21 days is indistinguishable from r estimated for the entire lifespan,due to the great importance of early reproduction(van Leeuwen et al.,1985),all calculationswere based on the 21-day experim ent.
2.4.Feeding experiment
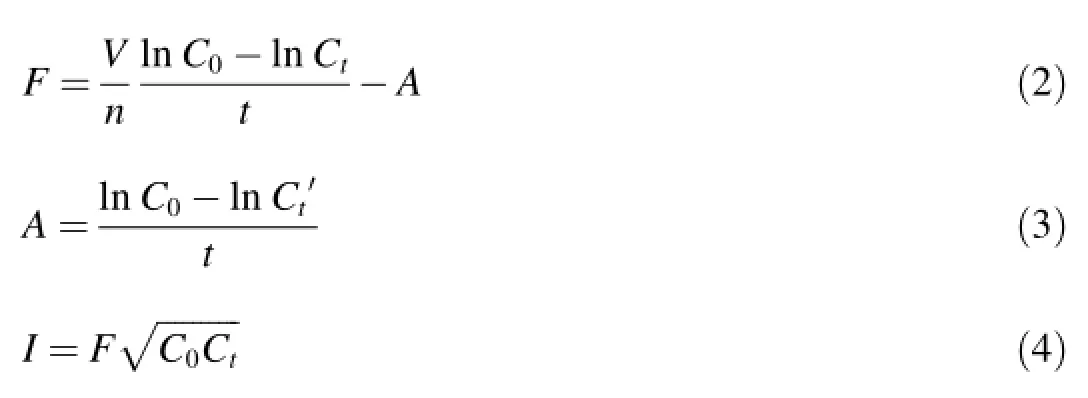
The feeding experiment was run according to a method described by Zhu et al.(2010).Filtration and ingestion rates were used asmeasures of the feeding experiment.Ten neonates less than 24 h old were placed in a 100m L glassbeakers containing 45m L of test solution in a dark time period for 5 h at(20±1)°C.During the exposed period,D.magna were fed w ith 1×106cells/m L Scenedesmusobliquus per day for each animal.Then final food concentration wasmeasured w ith a hemocytometer under an electron m icroscope w ith amagnification of 400.The filtration rate(F)was defined as the volume of medium swept clearly by a D.magna in a unit of time,and the ingestion rate(I)as the number of cells consumed by a D.magna organism during a specific time interval.Eqs.(2)through(4)from Gauld(1951)were used to calculate the average F inμL/(ind·h)and I in cells/(ind·h): where C0and Ctare the initial and final food concentrations(cells/μL)respectively,t is time(duration of the experiment in hours),n is the number of D.magna in volume V(μL),and A is a correction factor varying w ith the final concentrationafter time t.The expressionrepresents the geometric mean of food concentration during time t.
2.5.Biomarker assays
Sixty neonates less than 24 h old from a designated brood were placed in a 1 L glassbeaker for each test concentration of PFCs and control group,and the test solution was renewed daily.A ll experiments were performed in triplicate.Cultured D.magna aged seven days were used for determination of AChE,SOD,and CAT activities.
Antioxidantenzymesanalyseswere conducted according to the procedure of Barata et al.(2005).After exposure,D. magna were homogenized in 100mmol/L of phosphate buffer at a pH value of 7.4,containing 100 mmol/L of KCl and 1mmol/L of ethylene diamine tetraacetic acid(EDTA)w ith a 1:4 volume ratio,using a glasshomogenizer(Kimble Kontes,Vineland,NJ,USA).A fter centrifugation at 10 000g for 10 m in at 4°C,the supernatants were used as the enzyme extract for AChE activity determ ination.AChE activity was determined at a wavelength of 405 nm using a microplate reader(Molecular Device VersaMax,USA)w ith themethod of Guilhermino et al.(1996),by adding 50μL of homogenate and 250μL of the reaction solution(1.0m L of DTNB solution w ith the concentration of 10 mmol/L,0.2 m L of ATCh I solution w ith the concentration of 0.075 mol/L,and 30 m L of phosphate buffer)in m icro plates,and this activity wasm easured at 30°C for 3m in.AChE activity was expressed in nmol/(mg pro·m in).SOD activity was determ ined at a wavelength of 420 nm by the method of M arklund and Marklund(1974).Three hundred microlitres of Tris-HCl buffer were added to themicro plates,which werewarmed at 25°C for 10min,then 10μL of homogenate and 6μL of preheated pyrogallolwere added,and the rate of pyrogallol auto-oxidation wasmeasured for 3 min.SOD activity was expressed in U/mg pro.U was defined as the amountof enzyme required to cause 50%of inhibition of pyrogallol auto-oxidation.CAT activity was determined using ammonium molybdate(Gth,1991).Two hundred m icrolitres of the hom ogenate were incubated w ith 1 m L of substrate(65 mmol/L of hydrogen peroxide in 60 mmol/L of potassium phosphate buffer,pH value of 7.4)at 37°C for 60 s.The enzymatic reaction was term inated by adding 1m L of ammonium molybdatew ith the concentration of 32.4 mmol/L and the yellow complex of molybdate and hydrogen peroxide wasmeasured at a wavelength of 405 nm.CAT activity was expressed inμmol/(mg pro·m in).Protein concentrationswere determined at awavelength of 595 nm using a method developed by Bradford(1976),w ith bovine serum albumin as the standard.
2.6.Statistical analysis
All data were tested for the normal distribution w ith Shapiro-Wilk's test and for homogeneity of variances w ith Levene's test.The results were expressed as the mean w ith the standard deviation(SD).In the 48-h acute toxicity test for D. magna,themedian effective concentration(EC50)andmedian lethal concentration(LC50)were calculated w ith probit analysis using SigmaPlot 12.5.To analyze reproduction,ingestion,and biomarker data,one-way analysis of variance(ANOVA)and t-testw ith Dunnett's testwere performed w ith the SPSS statistical package(SPSS Co.,Chicago,IL,USA). Tests for other types of toxicity data were also performed using SPSS.A ll differences were considered significant at P<0.05.
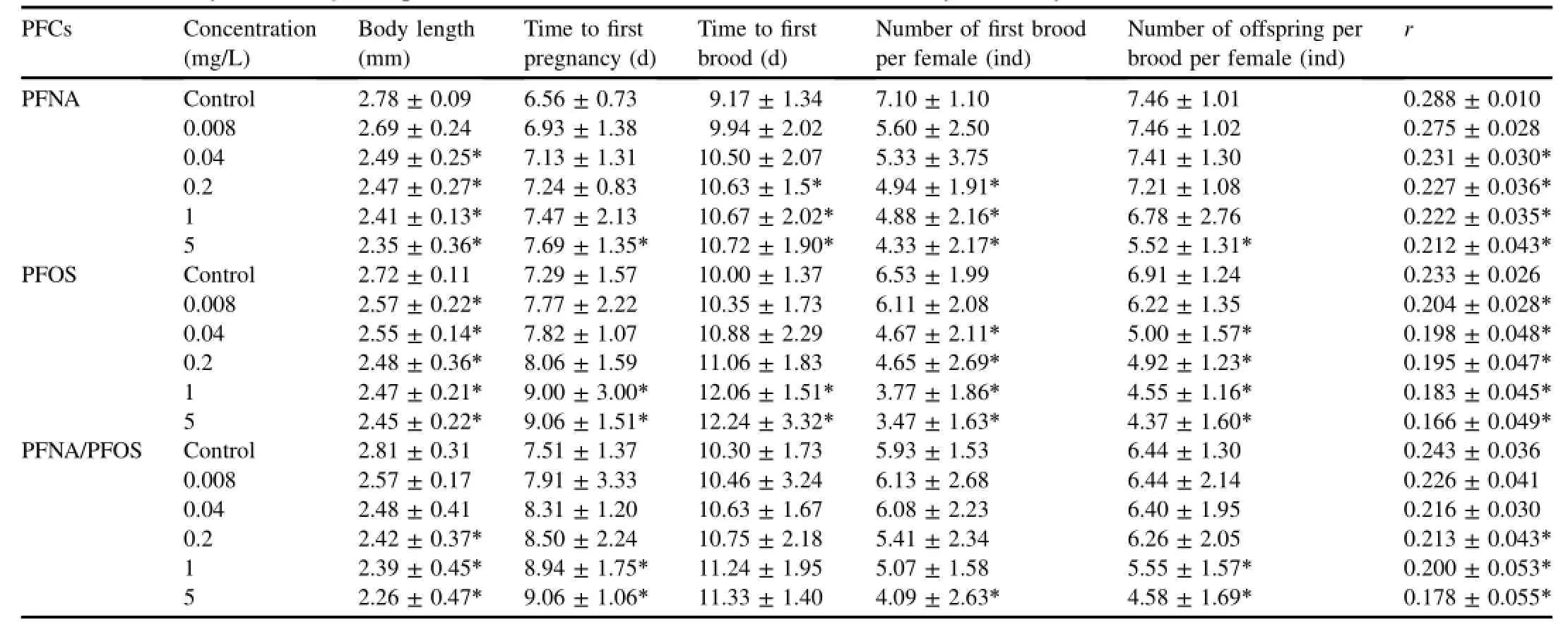
Table 1Size and fecundity of D.magna exposed to PFOS,PFNA,and PFNA/PFOS in 21-day life study.
3.Results
3.1.Acute toxicity of PFOSand PFNA
Potassium dichromatewasused asa reference chemical.The EC50 for D.magna at24 h of 0.96mg/L wasobtained for potassium dichromate,which conforms to the ISO standard(ISO,1996).Throughout the testing process,the pH values of test media ranged from 7.7 to 8.4,and the DO was 5.3 m g/L.No mortality occurred in the controlgroup in the acute toxicity test.
The immobilization and mortality of D.magna increased continuouslyw ith elevated PFC concentrations.The EC50 and LC50 values of PFCs were calculated w ith one-dimensional linear regression analysisof the negative logarithm of exposed PFC concentrations against the immobilization and mortality rates.The EC50 valuesof PFNA and PFOS for D.magna were 43.42 and 23.41mg/L,and their LC50 valueswere 80.93 and 49.27mg/L,respectively.
3.2.Chronic toxicity of PFOS,PFNA,and PFNA/PFOS
The survival,body length,reproduction,and population parameters of adult D.magna were assessed after 21 days of exposure and the resultsareshown in Table1.Themortality of D.magna for all the concentrations of PFOS,PFNA,and PFNA/PFOS never exceeded 20%.No mortality occurred in the control group during the period of experiment.The body length and r of D.magna decreased significantly at the concentrations greater than or equal to 0.04 mg/L of PFNA,greater than or equal to 0.008mg/L of PFOS,and greater than or equal to 0.2 mg/L of PFNA/PFOS(P<0.05).Higherconcentrationsof PFCssignificantly prolonged the time to first pregnancy and the time to first brood of D.magna and decreased the number of offspring of first brood.Compared w ith PFNA,the reproductive toxicity of PFOS seemed to be stronger in D.magna.PFNA/PFOSsignificantly decreased the body length and r of D.magna at higher concentrationswhich were greater than or equal to 0.2 mg/L(P<0.05).However,PFNA/PFOS did not change significantly the time to first brood atall the test concentrations.
Themaximum acceptable toxicant concentration(MATC)was calculated as the geometric mean of the NOEC and the lowest observed effect concentration(LOEC).The NOEC,LOEC,and MATC values of PFNA,PFOS,and PFNA/PFOS to D.magna are shown in Table 2.
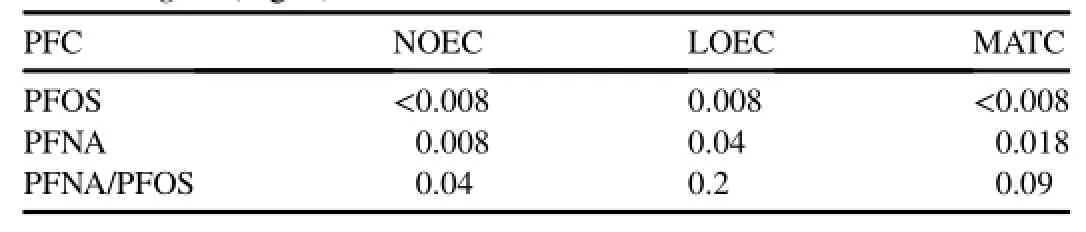
Table 2 NOEC,LOEC,and MATC values of PFOS,PFNA and PFNA/PFOS to D.magna(mg/L).
3.3.Ingestion effect
The effects of PFCs on the feeding behavior of D.magna are shown in Fig.1.The ingestion rates were significantly inhibited by PFOS and PFNA/PFOS at concentrations greater than or equal to 0.2 mg/L,while even at the lowest concentration of 0.008 mg/L,the ingestion rate was significantly inhibited by PFNA(P<0.05).The highest concentration of PFNA,PFOS,and PFNA/PFOS exposures led to maximal reduction of ingestion rates,and the inhibition rateswere 59%,55%,and 72%compared w ith control group values,respectively.The median inhibitory concentrations of ingestion(IC50)were estimated through one variable linear regression analysis,and were 10.78,14.86,and 7.48 mg/L for PFNA,PFOS,and PFNA/PFOS,respectively.The IC50 valueswere much lower than the corresponding EC50 for immobilization after 48 h of exposure.
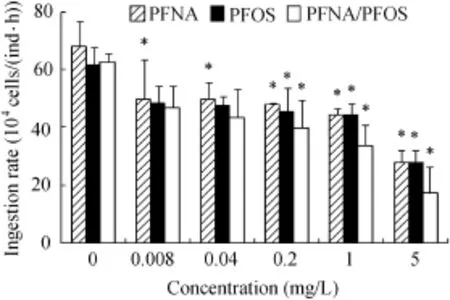
Fig.1.Ingestion rateof D.magna afterexposure to PFNA,PFOS,and PFNA/PFOS(*indicates significant difference from control group with P<0.05).
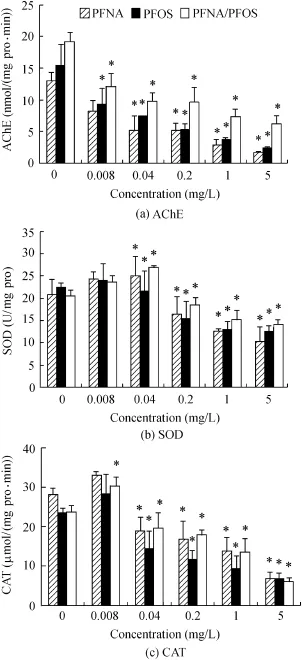
Fig.2.Biomarker responses of D.magna exposed to PFNA,PFOS,and PFNA/PFOS(*indicates significant difference from control group with P<0.05).
3.4.Biomarker response
AChE,SOD,and CAT activities in D.magna are shown in Fig.2.No mortality occurred during experiments.AChE activity was significantly inhibited by all the test concentrations of PFOSand PFNA/PFOSand concentrationsof PFNA greater than or equal to 0.04mg/L(P<0.05).The inhibition rate of AChE activity matches the concentration increase,and the maximum inhibition rates were 87%,84%,and 67%respectively,produced by the highest concentrations of PFNA,PFOS,and PFNA/PFOS.SOD activity decreased significantly at concentrations greater than or equal to 0.2m g/L(P<0.05),and the maximum inhibition rates were 51%,44%,and 32% for PFNA,PFOS,and PFNA/PFOS,respectively.However,the two lowest concentrations of PFCs increased SOD activity.The response pattern of CAT activity was sim ilar to that of SOD activity.CAT activity was significantly inhibited by all test concentrations except for the lowest concentration.The highest concentrations of PFNA,PFOS,and PFNA/PFOS induced maximal CAT activity reduction values,which were 76%,72%,and 75%,respectively.In general,the two PFCs(alone or in combination)induced similar biological responses.Furthermore,the change of enzyme activities exhibited obvious concentration dependence.
4.Discussion
In the acute toxicity test,our results showed that PFOS is m ore toxic than PFNA to D.magna.These results are comparablew ith those of a previous study by Zheng et al.(2011),which reported that the values of 48-h EC50 of PFNA and PFOS for D.magna were42.84 and 33.76mg/L,respectively.Ji etal.(2008)also reported that the valueof 48-h EC50 of PFOS was17.95mg/L for Moinamacrocopa,whereas M.macrocopa was generally much more sensitive to PFOS than D.magna. Thevalueof 48-h EC50 of PFNAwas151mg/L for D.magna,as reported by Ding etal.(2012a).The value of 96-h EC50 of PFOSwas reported to be 78.13 mg/L,while that for 120 h was 76.53 mg/L for zebrafish embryo(Ding et al.,2012b).However,for marine shrimp,Mysidopsis bahia,the value of 96-h LC50 of PFOSwas reported to be 3.5 mg/L(Beach et al.,2006),significantly lower than that for the freshwater organisms mentioned above.Different culture conditions and test proceduresmay partly account for the differencesbetween the reported data in the same species(Ding et al.,2012a).
The use of chronic toxicity data,especially the chronic toxicity of PFNA/PFOS,in environmental protection is an important part of an integrated environm entalmonitoring and assessment strategy(Dao et al.,2010;Yi et al.,2010).As far aswe know,till now there have been no published data on the response patterns of D.magna exposed to PFC mixtures,although there are some data on single PFC.Boudreau et al.(2003)investigated the acute and chronic toxicity of PFOS to D.magna,and found that PFOS exposure for 21 days could cause significant inhibition of grow th and reproduction,and even causemortality.
Reproduction,and in particular r,has been recommended as a superior laboratory toxicological endpoint compared to the acute mortality,because it com bines lethal and sublethal effects into onem eaningfulmeasure(Zhao et al.,2012;Pestana etal.,2010).In this study,parameter r and body length of D.magna were confirmed to bemore sensitive endpoints in the 21-day chronic toxicity test.Pane et al.(2004)recommended the use of r to estimate the chronic toxic effectdue to thesensitivity of r in chronic toxicity tests.Thus,r valueswere used to estimate theMATC values for PFCs.The reproduction LOEC value of PFNA was approximately five times higher than that of PFOS.The fact that PFOSwas found to bemore toxic than PFNA in this study is not surprising,because this finding is sim ilar to the results of other studies that have evaluated the toxicity of PFOS and other PFCs in aquatic organisms(Ji et al.,2008;Ding et al.,2012a).However,the reproduction NOEC and LOEC values of PFNA/PFOS were allapproximately five times higher than those of a single dose of PFNA or PFOS.A similar result was also found in the MATC.The MATC value of PFNA/PFOS was the largest,followed by the values of PFNA and PFOS,which suggested that the inhibition effects of PFNA/PFOS on grow th were lower than those of the corresponding individual exposures,suggesting that an antagonistic effect appeared to exist.Wei etal.(2009)investigated the combined effectsof six PFCson primary cultured hepatocytes from a rare minnow(Gobiocypris rarus),and found that the gene expression related to fatty acid biosynthesis and transport showed the antagonistic effects of PFCs.Although the possible mechanisms of combined effects of PFCs have not been previously reported,the present study indicates that the PFOS and PFNA mixture can elicit effects differing from exposure to a single PFC.Therefore,the effectsof PFC combination in the assessmentof PFC exposurew ith regard to D.magna require further study.
Inhibition of the ingestion rateby PFNA,PFOS,and PFNA/ PFOS was observed,and showed obvious concentration dependence in the present study.Toxicants that affect the movementof appendagesand the coordination of the nervous system may reduce ingestion rates(Yi et al.,2010).In addition,PFCs could accumulate w ithin the gastrointestinal tract(Kim et al.,2010),which may interfere w ith normal food intake and cause the toxicity observed in D.magna.These findingssuggest thatasensitive feeding response is required to evaluate the effect of low-level PFCs.
AChE activity plays an important role in many physiological functions,when the activity of the enzyme decreases,neuronal andmuscle injurymay occur(Dettbarn etal.,2006). A significant AChE activity decrease by 20%ormore can be considered a clear toxicological effect of xenobiotic exposure,and cause reductions in the feeding activity and sw imm ing rate(USEPA,2000).Xuereb etal.(2009)also found that the feeding rate and locomotive impairment in Gammarus fossarum were directly correlated to AChE inhibitions w ith the correlation coefficientof higher than 50%for chlorpyrifosand methomyl.In the present study,the AChE activity and the ingestion rate of D.magna were both obviously inhibited in a concentration-dependent manner.Thus,the inhibition of AChE activity that resulted from exposure to the two PFCs may cause disruption of the nervous system.
CAT plays a critical role in dismutation of the hydrogen peroxide,whereas SOD dismutates the superoxide anion radical(Kim etal.,2012).The SOD-CAT system provides the firstdefense line againstoxygen toxicity and isusually used as a biomarker to indicate reactive oxygen species(ROS)production(Li et al.,2011).However,information on the response of the antioxidant defense system in D.magna exposed to PFCs is still lacking.The changes in the SOD and CATactivitieswere approximately the sameas those observed by Qu et al.(2010),where the toxic effects of different concentrations(0.1 mg/L to 200 mg/L)of PFOS on wheatwere investigated.Their results showed that low concentrations of PFOS(0.1 m g/L to 10 mg/L)induced SOD and peroxidase(POD)activities in wheat roots and leaves,and highconcentrations of PFOS(200 mg/L)inhibited SOD and POD activities.Pollution induces the expression of antioxidant enzymes that allow organisms to partially or totally overcom e stress resulting from exposure to an unsafe environment. However,the decrease of SOD and CAT activitiesmay have been due to excessiveROSproduction(Xu etal.,2013).These results indicate that treatmentw ith PFOS,PFNA,and PFNA/ PFOS resulted in an increase of ROS.ROS,in turn,stimulated the response of antioxidant defenses and resulted in impaired physiological functions.
5.Conclusions
Our study demonstrated the acute and chronic toxicities of two PFCs(single or in combination),and PFOSwas found to be more toxic than PFNA.Parental exposure of D.magna transferred adverse effects to offspring.The body length and r of D.magna were confirmed to bemore sensitive grow th and reproduction parameters for PFCs.In addition,an inhibition of the feeding rate was found in D.magna.AChE,SOD,and CATactivities in D.magna werealso significantly inhibited by the two PFCs in a concentration-dependentmanner.The inhibition action attributed to the two PFCs showed positive correlations between the biom arker responses and the effects on grow th and feeding rates,which suggested that physiologicaland biochem icalendpointsneed to be incorporated into the current PFC regulations.The results of the present study should help increase the base of know ledge on risk assessment of PFCs,and additional research is required to assess the potential adverse impacts of these compounds on aquatic systems.
References
Ahrens,L.,Yeung,L.W.Y.,Taniyasu,S.,Lam,P.K.S.,Yamashita,N.,2011. Partitioning of perfluorooctanoate(PFOA),perfluorooctane sulfonate(PFOS)and perfluorooctane sulfonam ide(PFOSA)between water and sediment.Chemosphere 85(5),731-737.http://dx.doi.org/10.1016/ j.chemosphere.2011.06.046.
Alberdi,J.L.,Saenz,M.E.,Di Marzio,W.D.,Tortorelli,M.C.,1996. Comparative acute toxicity of two herbicides,paraquat and glyphosate,to Daphniamagna and D.spinulata.Bull.Environ.Contam.Toxicol.57(2),229-235.http://dx.doi.org/10.1007/s001289900180.
Barata,C.,Varo,I.,Navarro,J.C.,Arum,S.,Porte,C.,2005.Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphniamagna exposed to redox cycling compounds.Comp.Biochem. Physiol.,Part C:Toxicol.Pharmacol.140(2),175-186.http://dx.doi.org/ 10.1016/j.cca.2005.01.013.
Barata,C.,A lanon,P.,Gutierrez-A lonso,S.,Riva,M.C.,Fernandez,C.,Tarazona,J.V.,2008.A Daphnia magna feeding bioassay as a cost effective and ecological relevant sublethal toxicity test for environmental risk assessment of toxic effluents.Sci.Total Environ.405(1-3),78-86. http://dx.doi.org/10.1016/j.scitotenv.2008.06.028.
Beach,S.A.,New sted,J.L.,Coady,K.,Giesy,J.P.,2006.Ecotoxicological evaluation of perfluorooctanesulfonate(PFOS).Rev.Environ.Contam. Toxicol.186,133-174.http://dx.doi.org/10.1007/0-387-32883-1_5.
Boudreau,T.M.,Sibley,P.K.,M abury,S.A.,2003.Laboratory evaluation of the toxicity of perfluorooctane sulfonate on Selenastrum capricornutum,Chlorella vulgaris,Lemna gibba,Daphniamagna,and Daphnia pulicaria. Arch.Environ.Contam.Toxicol.44(3),307-313.http://dx.doi.org/ 10.1007/s00244-002-2102-6.
Bradford,M.M.,1976.A rapid and sensitivemethod for the quantitation of m icrogram quantities of protein utilizing the principle of protein-dye binding.Anal.Biochem.72(1-2),248-254.http://dx.doi.org/10.1016/ 0003-2697(76)90527-3.
Cai,Y.Q.,Wang,J.M.,Shi,Y.L.,Pan,Y.Y.,Cai,Y.Q.,2010.Perfluorooctane sulfonate(PFOS)and other fluorochem icals in viscera and muscle of farmed pigs and chickens in Beijing.Chin.Sci.Bull.55(31),3550-3555. http://dx.doi.org/10.1007/s11434-010-4098-y.
Calafat,A.M.,Needham,L.L.,Kuklenyik,Z.,Reidy,J.A.,Tully,J.S.,Aguilar-Villalobos,M.,Naeher,L.P.,2006.Perfluorinated chem icals in selected residentsof the American continent.Chemosphere63(3),490-496.http:// dx.doi.org/10.1016/j.chemosphere.2005.08.028.
Dao,T.S.,Do-Hong,L.C.,W iegand,C.,2010.Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring.Toxicon 55(7),1244-1254.http://dx.doi.org/10.1016/j.toxicon.2010.01.014.
de Schamphelaere,K.A.C.,Forrez,I.,Dierckens,K.,Sorgeloos,P.,Janssen,C.R.,2007.Chronic toxicity of dietary copper to Daphnia magna.Aquat.Toxicol.81(4),409-418.http://dx.doi.org/10.1016/ j.aquatox.2007.01.002.
Dettbarn,W.D.,M ilatovic,D.,Gupta,R.C.,2006.Oxidative stress in anticholinesterase-induced excitotoxicity.In:Gupta,R.C.(Ed.),Toxicology of Organophosphate and Carbamate Compounds.Academ ic Press,Burlington,pp.511-532.
Ding,G.H.,Fromel,T.,van den Brandhof,E.J.,Baerselman,R.,Peijnenburg,W.J.G.M.,2012a.Acute toxicity of poly-and perfluorinated compounds to two cladocerans,Daphniamagna and Chydorussphaericus. Environ.Toxicol.Chem.31(3),605-610.http://dx.doi.org/10.1002/ Etc.1713.
Ding,G.H.,Zhang,J.,Chen,Y.H.,Luo,G.Y.,Mao,C.H.,2012b.Acute toxicity effect of PFOS on zebrafish embryo.Adv.Mater.Res.356-360(2012),603-606.http://dx.doi.org/10.4028/www.scientific.net/AMR.356-360.603.
Eschauzier,C.,Haftka,J.,Stuyfzand,P.J.,de Voogt,P.,2010.Perfluorinated compounds in infiltrated River Rhine water and infiltrated rainwater in coastal dunes.Environ.Sci.Technol.44(19),7450-7455.http:// dx.doi.org/10.1021/es100471z.
Fernandez-Sanjuan,M.,Meyer,J.,Damasio,J.,Faria,M.,Barata,C.,Lacorte,S.,2010.Screening of perfluorinated chemicals(PFCs)in various aquatic organisms.Anal.Bioanal.Chem.398(3),1447-1456.http:// dx.doi.org/10.1007/s00216-010-4024-x.
Flores,C.,Ventura,F.,Martin-Alonso,J.,Caixach,J.,2013.Occurrence of perfluorooctanesulfonate(PFOS)and perfluorooctanoate(PFOA)inNESpanish surface waters and their removal in a drinking water treatment plant that combines conventionaland advanced treatments in parallel lines.Sci.Total Environ.461,618-626.http://dx.doi.org/10.1016/j.scitotenv.2013.05.026.
Gauld,T.,1951.The grazing rate ofmarine copepods.J.Mar.Biol.Assoc.U. K.29(3),695-706.http://dx.doi.org/10.1017/S0025315400052875.
Giesy,J.P.,Kannan,K.,2002.Peer reviewed:perfluorochem ical surfactants in theenvironment.Environ.Sci.Technol.36(7),146-152.http://dx.doi.org/ 10.1021/es022253t.
Guilhermino,L.,Lopes,M.C.,Carvalho,A.P.,Soared,A.M.V.M.,1996.Inhibition of acetylcholinesterase activity as effect criterion in acute tests w ith juvenile Daphnia magna.Chemosphere 32(4),727-738.http:// dx.doi.org/10.1016/0045-6535(95)00360-6.
Guruge,K.S.,Taniyasu,S.,Yamashita,N.,Wijeratna,S.,Mohotti,K.M.,Seneviratne,H.R.,Kannan,K.,Yamanaka,N.,M iyazaki,S.,2005.Perfluorinated organic compounds in human blood serum and sem inal plasma: a study of urban and rural teaworker populations in Sri Lanka.J.Environ. Monit.7(4),371-377.http://dx.doi.org/10.1039/B412532k.
Huang,Q.S.,Dong,S.J.,Fang,C.,Wu,X.L.,Ye,T.,Lin,Y.,2012.Deep sequencing-based transcriptome profiling analysis of Oryziasmelastigma exposed to PFOS.Aquat.Toxicol.120-121,54-58.http://dx.doi.org/ 10.1016/j.aquatox.2012.04.013.
International Organisation for Standardisation(ISO),1996.Water Quality-Determ ination of the Inhibition to the M obility of Daphnia magna Straus(Cladocera,Crustacea):Acute Toxicity Test(ISO 6341).British Standards Institute,London.
Ji,K.,Kim,Y.,Oh,S.,Ahn,B.,Jo,H.,Choi,K.,2008.Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwatermacroinvertebrates(Daphniamagna and Moinamacrocopa)and fish(Oryzias latipes).Environ.Toxicol.Chem.27(10),2159-2168.http://dx.doi.org/ 10.1897/07-523.1.
Kannan,K.,Corsolini,S.,Falandysz,J.,Fillmann,G.,Kumar,K.S.,Loganathan,B.G.,Mohd,M.A.,Olivero,J.,Van Wouwe,N.,Yang,J.H.,A ldous,K.M.,2004.Perfluorooctanesulfonate and related fluorochem icals in human blood from several countries.Environ.Sci.Technol.38(17),4489-4495.http://dx.doi.org/10.1021/Es0493446.
Kim,K.T.,Klaine,S.J.,Cho,J.,Kim,S.H.,Kim,S.D.,2010.Oxidative stress responses of Daphnia magna exposed to TiO2nanoparticles according to size fraction.Sci.Total Environ.408(10),2268-2272.http://dx.doi.org/ 10.1016/j.scitotenv.2010.01.041.
Kim,S.,Kim,W.,Chounlamany,V.,Seo,J.,Yoo,J.,Jo,H.-J.,Jung,J.,2012. Identification of multi-level toxicity of liquid crystal display wastewater toward Daphnia magna and Moina macrocopa.J.Hazard.M ater. 227-228,327-333.http://dx.doi.org/10.1016/j.jhazmat.2012.05.059.
K ratzer,J.,Ahrens,L.,Roos,A.,Backlin,B.M.,Ebinghaus,R.,2011. Tem poral trends of poly fluoroalkyl compounds(PFCs)in liver tissue of grey seals(Halichoerus grypus)from the Baltic Sea,1974-2008.Chemosphere 84(11),1592-1600.http://dx.doi.org/10.1016/ j.chemosphere.2011.09.001.
Leondiadis,L.,Vassiliadou,I.,Costopoulou,D.,Ferderigou,A.,2010. Levels of perfluorooctane sufonate(PFOS)and perfluorooctanoate(PFOA)in blood samplesfrom different groups of adults living in Greece.Chemosphere 8(1),1199-1206.http://dx.doi.org/10.1016/ j.chemosphere.2010.06.014.
Li,Z.H.,Zlabek,V.,Velisek,J.,Grabic,R.,M achova,J.,Kolarova,J.,Li,P.,Randak,T.,2011.Acute toxicity of carbamazepine to juvenile rainbow trout(Oncorhynchusmykiss):effects on antioxidant responses,hematological parameters and hepatic EROD.Ecotoxicol.Environ.Safe 74(3),319-327.http://dx.doi.org/10.1016/j.ecoenv.2010.09.008.
Liu,Y.,Wang,J.,Fang,X.,Zhang,H.,Dai,J.,2011.The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish(Danio rerio).Ecotoxicology 20(1),47-55.http://dx.doi.org/10.1007/s10646-010-0555-3.
Lotka,A.J.,1913.A naturalpopulation norm.J.Wash.Acad.Sci.3,241-248,289-293.
Marklund,S.,Marklund,G.,1974.Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase.Eur.J.Biochem.47(3),469-474.http://dx.doi.org/ 10.1111/j.1432-1033.1974.tb03714.x.
Mommaerts,V.,Hagenaars,A.,Meyer,J.,De Coen,W.,Swevers,L.,M osallanejad,H.,Smagghe,G.,2011.Impact of a perfluorinated organic compound PFOS on the terrestrial pollinator Bombus terrestris(Insecta,Hymenoptera).Ecotoxicology 20(2),447-456.http://dx.doi.org/10.1007/ s10646-011-0596-2.
M urakam i,M.,Adachi,N.,Saha,M.,M orita,C.,Takada,H.,2011.Levels,temporal trends,and tissue distribution of perfluorinated surfactants in freshwater fish from Asian countries.Arch.Environ.Contam.Toxicol. 61(4),631-641.http://dx.doi.org/10.1007/s00244-011-9660-4.
Olsen,G.W.,Church,T.R.,Larson,E.B.,Van Belle,G.,Lundberg,J.K.,Hansen,K.J.,Burris,J.M.,Mandel,J.H.,Zobel,L.R.,2004.Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle,Washington.Chemosphere 54(11),1599-1611.http://dx.doi.org/10.1016/j.chemosphere.2003.09.025.
Organization for Econom ic Cooperation and Development(OECD),2004. Testno.202:Daphnia sp.acute immobilisation test.In:OECDGuidelines for the Testing of Chemicals,Section 2:Effecton Biotic System.OECD Publishing,Paris.http://dx.doi.org/10.1787/9789264069947-en.
Organization for Econom ic Cooperation and Development(OECD),2012. Testno.211:Daphniamagna reproduction test.In:OECD Guidelines for the Testing Chemicals,Section 2:Effect on Biotic System.OECD Publishing,Paris.http://dx.doi.org/10.1787/9789264185203-en.
Pane,E.F.,M cGeer,J.C.,Wood,C.M.,2004.Effects of chronic waterborne nickel exposure on two successive generations of Daphnia magna.Environ.Toxicol.Chem.23(4),1051-1056.http://dx.doi.org/10.1897/03-208.
Pestana,J.L.T.,Loureiro,S.,Baird,D.J.,Soares,A.M.M.,2010.Pesticide exposure and inducible antipredator responses in the zooplankton grazer,Daphnia magna Straus.Chemosphere 78,41-48.http://dx.doi.org/ 10.1016/j.chemosphere.2009.10.066.
Plum lee,M.H.,Larabee,J.,Reinhard,M.,2008.Perfluorochemicals in water reuse.Chemosphere 72(10),1541-1547.http://dx.doi.org/10.1016/ j.chemosphere.2008.04.057.
Pow ley,C.R.,George,S.W.,Russell,M.H.,Hoke,R.A.,Buck,R.C.,2008. Polyfluorinated chemicals in a spatially and temporally integrated food web in the Western Arctic.Chemosphere 70(4),664-672.http:// dx.doi.org/10.1016/j.chemosphere.2007.06.067.
Qu,B.,Zhao,H.,Zhou,J.,2010.Toxic effects of perfluorooctane sulfonate(PFOS)on wheat(Triticum aestivum L.)plant.Chemosphere 79(5),555-560.http://dx.doi.org/10.1016/j.chemosphere.2010.02.012.
Renner,R.,2001.Grow ing concern over perfluorinated chem icals.Environ. Sci.Technol.35(7),154A-160A.http://dx.doi.org/10.1021/es012317k.
Rudel,H.,Muller,J.,Jurling,H.,Bartel-Steinbach,M.,Koschorreck,J.,2011. Survey of patterns,levels,and trends of perfluorinated compounds in aquatic organisms and bird eggs from representative German ecosystems. Environ.Sci.Pollut.Res.Int.18(9),1457-1470.http://dx.doi.org/ 10.1007/s11356-011-0501-9.
Shivakoti,B.R.,Tanaka,S.,Fujii,S.,Nguyen,P.H.L.,Nozoe,M.,Kunacheva,C.,Okamoto,R.,Seneviratne,S.T.M.L.D.,Tanaka,H.,2011. Perfluorinated compounds(PFCs)in Yodo River system,Japan.Water Sci. Technol.63(1),115-123.http://dx.doi.org/10.2166/Wst.2011.020.
Tatum-Gibbs,K.,Wambaugh,J.F.,Das,K.P.,Zehr,R.D.,Strynar,M.J.,Lindstrom,A.B.,Delinsky,A.,Lau,C.,2011.Comparative pharmacokinetics of perfluorononanoic acid in rat andmouse.Toxicology 281(1-3),48-55.http://dx.doi.org/10.1016/j.tox.2011.01.003.
U.S.Environmental Protection Agency(USEPA),2000.The Use of Cholinesterase Inhibition for Risk Assessment of Organophosphate and Carbamate Pesticides.USEPA,Washington,D.C.http://www.epa.gov/oppfead1/ trac/science/cholin.pdf[Retrieved February 22,2013].
U.S.Environmental Protection Agency(USEPA),2009.Final Third Drinking Water Contaminant Candidate List(CCL 3).USEPA,Washington,D.C.. http://www.epa.gov/ogw dw/ccl/pdfs/ccl3_docs/fs_cc3_final.pdf[Retrieved February 22,2013].
van Leeuwen,C.J.,Luttmer,W.J.,Griffieon,P.S.,1985.Theuseof cohortsand populations in chronic toxicity studiesw ith Daphnia magna:a cadmium example.Ecotoxicol.Environ.Saf.9(1),26-39.http://dx.doi.org/ 10.1016/0147-6513(85)90031-4.
Ventura,S.P.,Gonçalves,A.M.,Gonçalves,F.,Coutinho,J.A.,2010.Assessing the toxicity on[C3mim][Tf2N]to aquatic organisms of different trophic levels.Aquat.Toxicol.96(4),290-297.http://dx.doi.org/10.1016/ j.aquatox.2009.11.008.
Wei,Y.,Shi,X.,Zhang,H.,Wang,J.,Zhou,B.,Dai,J.,2009.Combined effectsofpolyfluorinated and perfluorinated compoundson primary cultured hepatocytes from rare m innow(Gobiocypris raru)using toxicogenom ic analysis.Aquat.Toxicol.95(1),27-36.http://dx.doi.org/10.1016/ j.aquatox.2009.07.020.
Xu,D.,Li,C.,Wen,Y.,Liu,W.,2013.Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate(PFOS).Environ.Pollut.174,121-127.http://dx.doi.org/10.1016/ j.envpol.2012.10.030.
Xuereb,B.,Lefe'vre,E.,Garric,J.,Geffard,O.,2009.Acetylcholinesterase activity in Gammarus fossarum(Crustacea Amphipoda):linking AChE inhibition and behaviouralalteration.Aquat.Toxicol.94,114-122.http:// dx.doi.org/10.1016/j.aquatox.2009.06.010.
Yi,X.,Kang,S.W.,Jung,J.,2010.Long-term evaluation of lethal and sublethal toxicity of industrial effluents using Daphnia magna and Moinamacrocopa.J.Hazard.Mater.178(1-3),982-987.http://dx.doi.org/ 10.1016/j.jhazmat.2010.02.034.
Zainuddin,K.,Zakaria,M.P.,A l-Odaini,N.A.,Bakhtiari,A.R.,Latif,P.A.,2012.Perfluorooctanoic acid(PFOA)and perfluorooctane sulfonate(PFOS)in surface water from the Langat River,Peninsular M alaysia.Environ.Forensics 13(1),82-92.http://dx.doi.org/10.1080/ 15275922.2011.643335.
Zhang,X.M.,Song,J.L.,Jin,Y.H.,2011.Study on reproductive toxicity of perfluorooctanesulfonate(PFOS)inmale quail.Asian J.Ecotoxicol.6(2),143-148(in Chinese).
Zhao,H.Z.,Lu,G.H.,Xia,J.,Jin,S.G.,2012.Toxicity of nanoscale CuO and ZnO to Daphnia magna.Chem.Res.Chin.Univ.28(2),209-213.
Zheng,X.M.,Feng,Z.,Liu,H.L.,2011.Toxicity of typical perfluorinated compounds to Daphniamagna and zebrafish(Brachydanio rerio)embryos. In:Persistent Organic Pollutants Forum 2010 and the Fifth Session National Academ ic Symposium of Persistent Organic Pollutants.Chinese Chem ical Society,Nanjing,pp.246-247(in Chinese).
Zhu,X.S.,Chang,Y.,Chen,Y.S.,2010.Toxicity and bioaccumulation of TiO2nanoparticle aggregates in Daphnia magna.Chemosphere 78(3),209-215.http://dx.doi.org/10.1016/j.chemosphere.2009.11.013.
Thiswork was supported by the National Natural Science Foundation of China(Grant No.51279061)and the Qing Lan Project of Jiangsu Province.
*Corresponding author.
E-mail address:ghlu@hhu.edu.cn(Guang-hua Lu).
Peer review under responsibility of HohaiUniversity.
http://dx.doi.org/10.1016/j.wse.2015.01.001
1674-2370/©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Water Science and Engineering2015年1期
Water Science and Engineering2015年1期
- Water Science and Engineering的其它文章
- M inimum wall pressure coefficient of orifice plate energy dissipater
- Equilibrium sediment transport in lower Yellow River during later sediment-retaining period of Xiaolangdi Reservoir
- Experimental study on slope sliding and debris flow evolution w ith and w ithout barrier
- Incipientmotion of sediment in presence of submerged flexible vegetation
- Experimental investigation of creep behavior of clastic rock in Xiangjiaba Hydropower Project
- Application of subsurfacewastewater infiltration system to on-site treatment of domestic sewage under high hydraulic loading rate
