Flame Retardancy and Thermal Property of Poly(ethylene terephthalate)/Modified Cyclotriphosphazene Composites
WANG Jiao-ning(汪娇宁),SU Xing-yong(苏兴勇),YA Lan(雅 兰),CHEN Wen-ting(陈雯婷),CHEN Fei(陈 飞),CHEN Ying(陈 樱),MAO Zhi-ping(毛志平)
College of Chemistry,Chemical Engineering&Biotechnology,Key Laboratory of Science&Technology of Eco-textile,Ministry of Education,Donghua University,Shanghai 201620,China
Flame Retardancy and Thermal Property of Poly(ethylene terephthalate)/Modified Cyclotriphosphazene Composites
WANG Jiao-ning(汪娇宁),SU Xing-yong(苏兴勇),YA Lan(雅 兰),CHEN Wen-ting(陈雯婷),CHEN Fei(陈 飞),CHEN Ying(陈 樱),MAO Zhi-ping(毛志平)*
College of Chemistry,Chemical Engineering&Biotechnology,Key Laboratory of Science&Technology of Eco-textile,Ministry of Education,Donghua University,Shanghai 201620,China
In this study,hexachlorocyclotriphosphazene(HCCP) modified by boric acid and 3-aminopropyltriethoxysilane(KH-550) in solvent diglyme(FR-HCCP)was used as the flame retardant for poly(ethylene terephthalate) (PET) composites.The flame retardancy and thermal property of pure PET and flame-retarded PET composites were mainly investigated.The flame retardancy was investigated by limited oxygen index(LOI)and UL-94 vertical burning test.The results showed that the composites could achieved an increased UL-94 V-0 rating and LOI value 30.2,when the content of FR-HCCP was just 1%. The pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS) study demonstrated that introducing FR-HCCP into PET would prevent the polymer pyrolysis during heating.TGA analysis showed that the addition of FR-HCCP could improve the char formation of the system.Roman spectra showed the order degree of residue was increasing by adding the additive.The morphology and the chemical structure of the charred residue were detected by SEM and FTIR,respectively.Results demonstrated that a good barrier was formed by the char of the composite,which protected the inside of the composite during burning.
boron-silicon;polyphosphazene;flame retardant;poly (ethylene terephthalate)(PET)
Introduction
Poly(ethylene terephthalate)(PET)is a very important polymer for producing high-performance engineering plastics,fibers,films,and bottles due to its good mechanical properties,resistance to fatigue, high rigidity, low cost, high transparency, high processability, and moderate recyclability[1].However,like otherorganic thermoplastic polymers,their uses are limited by their combustibility[2-3].PET almost totally converts to volatile fragments upon exposure to heat,providing fuel to flame.It is a challenging task to flame-retard these polymers.Although halogen-containing flame retardants which have been commonly used for PET in past decades show remarkable efficiency,their potential to corrode metal components and more pressingly,the toxicity of the hydrogen halide formed during combustion are the obvious disadvantages.These disadvantages restrict their use in some applications[4-5].
Hexachlorocyclotriphosphazene(HCCP)is a unique class of inorganic-organic material with excellent flame retardant property due to the alternating phosphorus and nitrogen atoms.Meanwhile itisactive to form differentderivativesby substituting the two chlorine groups attaching to each phosphorus atom[6].In view of the advantages of phosphazene efforts have been performed to study it in recent years[7].The derivatives contain flame retardant, special rubbers,biomedicine,liquid crystal[8-11],etc.
Currently,various efforts have focused on developing synergistic effects of specific element with flame retardant characteristics,such as B,Si,P,N,halogen(Cl,Br,and I),Sb and Bi.For instance,P-N,P-Si,Sb-Cl,Bi-Cl,and many other combinations have been incorporated into the structures of high performance flame retardants[12-18].
In this study,a novel flame retardant containing P,N,Si,and B was synthesized from HCCP by substituting the chlorine with KH-550 which modified by boric acid.The effect of synthetic substance on the flame retardancy and thermal property of PET composites was mainly investigated.The composites were prepared by melt blending pure PET with FR-HCCP,named as PET/FR-HCCP composites.Three levels of FRHCCP(1%,3%,and 5% of the total quantity) were considered for the blends.
1 Experimental
1.1 Materials
Boric acid,triethylamine(TEA),and diglyme were purchased from Shanghai Chemical Reagents Co.,China.HCCP,which was recrystallized twice from petroleum ether,was provided by Zibo Lanyin Chemical Co.,Ltd(Shandong province,China).The silane used in this research was KH-550 obtained from Shanghai Yuanye Biotechnology Ltd.,China.Pristine PET chip was supplied by Sinopec Yizheng Chemical Fiber Company Limited(Jiangsu province,China).
1.2 Synthesis of the flame retardant FR-HCCP
Boric acid and diglyme were introduced into a 250 mL three-necked bottleequipped with amechanicalstirrer,a dropping funnel,and a thermometer.The mixture was stirred at 90℃ until all of the boric acid dissolved.KH-550 was then added(a Si/B molar ratio of 0.5 was employed)from a dropping funnel.The reaction mixture was heated at 95℃ in an oil bath with stirring for 12 h,after which it was allowed to cool to 65℃.HCCP and TEA,as the binding acid agent,were introduced into the above mentioned mixture and stirred at 65℃for 8 h under nitrogen atmosphere.The resulting solution was then filtered and washed using water in order to remove the residual reactants and TEA hydrochloride,then dried overnight at 80℃ in a vacuum oven.The product hexa(aminopropyltriborosiloxane) cyclotriphosphazene obtained was a faint yellow solid,labeled as FR-HCCP.The reaction was shown in Scheme 1.
FTIR(KBr disk):the peaks at 1096 and 1050 cm-1are attributed to Si—O bond,the peaks at 1471 and 762 cm-1are assigned to B—O bond,the peaks at 955 and 695 cm-1are belonged to Si—O—B bond,the peak at 912 cm-1is assigned to P—N bond,and the peaks at 1390 and 1223 cm-1are assigned to
Solid state13C CP/MAS NMR:the peaks at δ 42.8,δ 22.0 and δ 10.9 are assigned to C—N,C—C,and C—Si bonds of propyl from KH-550,respectively.
Solid state31P direct-polarization/MAS NMR:the peak at δ 3.21 is assigned to phosphorus from HCCP.
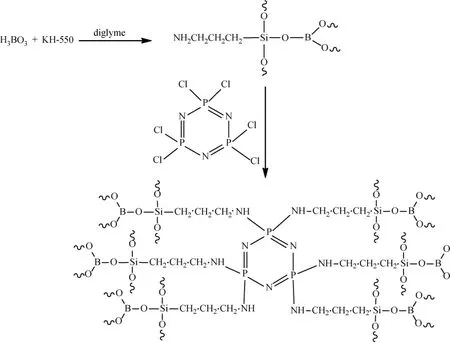
Scheme 1 Hexa(aminopropyltriborosiloxane)cyclotriphosphazene(FR-HCCP)
1.3 Preparation of PET composites
PET chips and FR-HCCP powders were dried overnight in vacuum at 120℃ before use.The composites were prepared by mixing PET chips and different amounts of FR-HCCP(1%,3%,and 5%of the total quantity)on a Torque Rheometer(HAAKE RC90,HAAKE Company,Germany)at a temperature of 280℃for 8 min with a rotation speed of 80 r/min-1.The blended materials were then pressed on a miniature injection molding machine(SZ-5-Q,Dehong Rubber and Plastic Machinery Co.,Ltd.)at 275℃ to produce sheets of various dimensions,recorded as material PET/FR-HCCP which was used in all tests.
1.4 Characterization
FTIR spectra of the samples(KBr)were recorded on a Varian 640 Fourier transform spectrometer(Varian Technology Co.,Ltd.)at room temperature(25℃).The samples were scanned 32 times over the spectral range of 4000-400 cm-1with a nominal resolution of 4 cm-1.
Thermo-gravimetric analysis(TGA)was performed on a TG 209F1thermobalance(NETZSCH-Gerätebau GmbH,Germany)in nitrogen atmosphere flow.The weight of the samples was kept within 3-5 mg.Samples were heated at ambient temperature of 900℃ with a heating rate of 5℃ /min.
Pyrolysis-gas chromatographyemass spectrometry(Py-GC/ MS)analysis was carried out by a system combined with a FRONTIER PY-2020iD Pyrolyser(Japan)and a SHIMADZU GC-MS QP2010(Japan).The pyrolysis temperature was 600℃and the GC column was an HP DB-5MS capillary column(30 m length,0.25 mm diameter,and 0.25 mm film thickness).The GC temperature program started at 40℃ holding for 3 min,and then heated up to 300℃ within 15 min and maintained for 15 min.
The flame retardancy of all samples was determined by limited oxygen index(LOI)and UL-94 vertical burning test.LOI data of all samples were obtained at room temperature on an ATS 1004050 FAA oxygen indexinstrumentproduced by ATSFAAR Industries(Italy),according to ISO4589-1984 standards.Vertical burning rates of all samples were conducted on an XMC-2 instrument produced by Xinma Analysis Instrument Factory(ShandongProvince,China) accordingto UL-94 standards.
Scanning electron microscopy(SEM)analysis was carried out using a T-1000 scanning microscope(HITACHI,Japan).All the sample surfaces studied were sprayed with gold to avoid charging under the electron beam.
Raman spectrum was test using in Via-Reflex Micro-Raman Spectroscopy system(Renishaw,UK).
Element Analysis of P,Si,and B were measured on Prodigy inductively coupled plasma (ICP) emission spectrometer(Leeman,China).
2 Results and Discussion
2.1 Thermal behavior

Fig.1 TGA curves of FR-HCCP,PET and the composites in nitrogen atmosphere
TGA was provided as a simple method to judge the thermal stability of the composites[29].Figure 1 showed the TGA curves of FR-HCCP,purePET andPET/FR-HCCP compositeswithdifferent contents of FR-HCCP(1%,3%,and 5%of the total quantity)under nitrogen atmosphere.Corresponding thermal factors were listed in Table 1 including the initial degradation temperature(IDT),the temperature of the maximum mass loss rate(Tmax),and the residue of the materials at 600℃ and 900℃.

Table 1 Thermal factor for PET and PET/FR-HCCP composites in nitrogen
For FR-HCCP(curve a),the thermal degradation process was mainly separated into three steps.The first step of mass loss was before 100℃ in the thermograms which probably was the exhaustion of small amount of water in system,and quality loss was about 5%.Following the system was in a relatively steady state of thermal degradation,about from 100℃ to 300℃ only small organic molecules such as amimo propyl in KH-550 degraded.The third step of mass loss was from 300℃ to 600℃ in the thermograms.In this process,the mass loss was relatively serious because the thermal degradation of small organic molecules further happened and the hydroxyl degraded happened in the step.From Table 1 it could be clearly seen that the residue of FR-HCCP was about 68.7%in 900℃.It may be a good flame retardant for the good char forming performance.
By comparing the thermal degradation process of pure PET and PET composites,the difference of IDT was very small,roughly from 360℃ to 460℃.The thermal behavior of the composites indicated that the addition of FR-HCCP did not change the initial thermal degradation process of PET[30-33],but the carbon residue was increased with the increasing of the flame retardant additives,as shown in Table 1.Pure PET left no residual char above 900℃ while PET/FR-HCCP composites left about 7.62%-9.04%increasing with the content of FR-HCCP.The phenomenon illustrated that the addition of FR-HCCP could promote the carbon formation of this system.Therefore,the addition of the FR-HCCP was effective to protect the material at the high temperature and reduce the decomposition of the polymer.When the amount of FR-HCCP in PET was only 1%,the residue received by measurement could reach 7.80%at 900℃ in nitrogen atmosphere compared with almost 0 of pure PET.
2.2 Flame retardant performance of PET/FRHCCP composites
Table 2 gave LOI and UL-94 data ofPET/FR-HCCP composites and pure PET,respectively.The LOI value and the result of UL-94 test are two parameters to evaluate the flammability of flame retardant materials.The dimensions for LOI samples were 125 mm ×6.5 mm ×3.2 mm,and 125 mm ×12.5 mm ×3.2 mm for UL-94 testing samples.It could be seen that the addition of FR-HCCP was beneficial to improve the flame retardancy of PET.Comparing PET/FR-HCCP composites with pure PET,the sample could reach the UL-94 V-0 rating and the LOI value increased from 24.0 to 30.2 when the content of FR-HCCP was just 1%.Furthermore,the composites exhibited a scarcely-dripping behavior during flame ignition.This phenomenon demonstrated that FRHCCP could improve the thermal stability of polymers and slow the burning speed.The worthy point of attention of this research was the flame combustion time of our sample containing additives,which was 3 s.

Table 2 Flammability properties of PET/FR-HCCP composites
The excellent flame retardancy of the material originated from Si,B,P,and N elements in FR-HCCP.When organic flame retardant containing Si and B elements burnt out,the synergistic flame retardant effect of P and N element in the phosphorus could notonly make materialsdehydrate and carbonize and form the carbonized layer which can insulate heat off the air,but also generate incombustible ammonia which can dilute the concentration of combustible gas produced during the combustion process,thus decreased the heat release of the composite.Meanwhile the melting flame retardant containing Si and B moved to the surface of the substrate through the gap of the material,thus a compact and stable coking protective cover was formed.Compared with conventional carbon layer,the organic Si and B carbon layer which was formed by the structure more compact and stable.At the same time boric acid salt was formed in the combustion process,melted in high temperature to convert to a stable boron glass material,and covered on the polymer surface.The protective covering played a role of separating heat,cutting off oxygen,and preventing melting drop down,thereby achieving the effect of improving the flame retardancy of the material.Together,we believe that the flame retardant plays the dual role of gas phase flame retardant mechanism and condenses phase flame retardant mechanism in polymer matrix,and they improve the thermal stability of the materials together[34].
2.3 Py-GC-MS analysis
FR-HCCP was pyrolysed at 600℃ by Py-GC/MS to get a more precise insight into the products of thermal decomposition.Table 3 showed the main pyrolysis products of FR-HCCP the Py-GC/MS analysis.The first and major pyrolysis products of FR-HCCP were CHx.It was deduced that these volatile fragments mainly arose from decomposition of silane coupling agent.It should be noticed that compounds containing P,B,and Si atoms were not detected in the pyrolysis indicating that the cyclotriphosphazenes and Si-B structural unit were still remained in the residue.

Table 3 Main pyrolysis products of FR-HCCP
Table 4 listed the pyrolysis products of PET and PET/5% FR-HCCP composites respectively.Data in the table indicated that both component and relative concentration of pyrolysisproducts for PET and PET blends were different.Zhang et al.reported that the mechanism of PET degradation was a free radical process[35].The initial and main fracture step of degradation was the formation of carboxylic acid and vinyl ester which could undergo further degradation to form anhydride and acetaldehyde;with the furtherdegradation the vinylester produced small molecules such as ketones,ethylene,and carboxylic acids.While carboxylic acids produced by molecular chain rupture generated terephthalic acid and vinyl esters.Further degradation of terephthalic acid formed benzene,benzoic acid,and CO2.The major products in the pyrolysis for neat PET were CO2,benzene,benzoic acid,and its ester compounds.But many researchers also considered that the mechanism of PET degradation was a random fracture mechanism. Early researchers such as Buxbaum[36]established the random fracture mechanism of PET degradation by comparing with the ester model compounds.They considered that in the progress of thermo-degradation,the first oxygen atom in carboxyl of polyester's main chain attacked the β-hydrogen atom of ester bond,then after a transition state of hexatomic ring,carbonyl acid and vinyl acid were generated from the random fracture of ester bond.As the rising of temperature these main products were further degraded and produced some small molecular weight volatile products such as carbon dioxide and carbon monoxide.Bednas et al.[37]thought the ester cracking reaction of carbonyl acid and vinyl acid generated from primary pyrolysis further happened,thus we obtained the carbonyl acid and vinyl acid of shorter molecular chain and some small molecules products with carboxyl and vinyl.Carboxyl products further cracked into CO2and CO for vinyl products.In summary,random fracture mechanism indicated that in the case of heating the main chain of the polymer were ruptured without rules,and generated many fragments of different molecular weight.And then,at high temperature,small molecular weight volatile products were produced.In fact,the degradation progress of PET is a very complicated process,and it is not confined to a single mechanism.But the degradation of polymers are the joint action of many mechanisms.
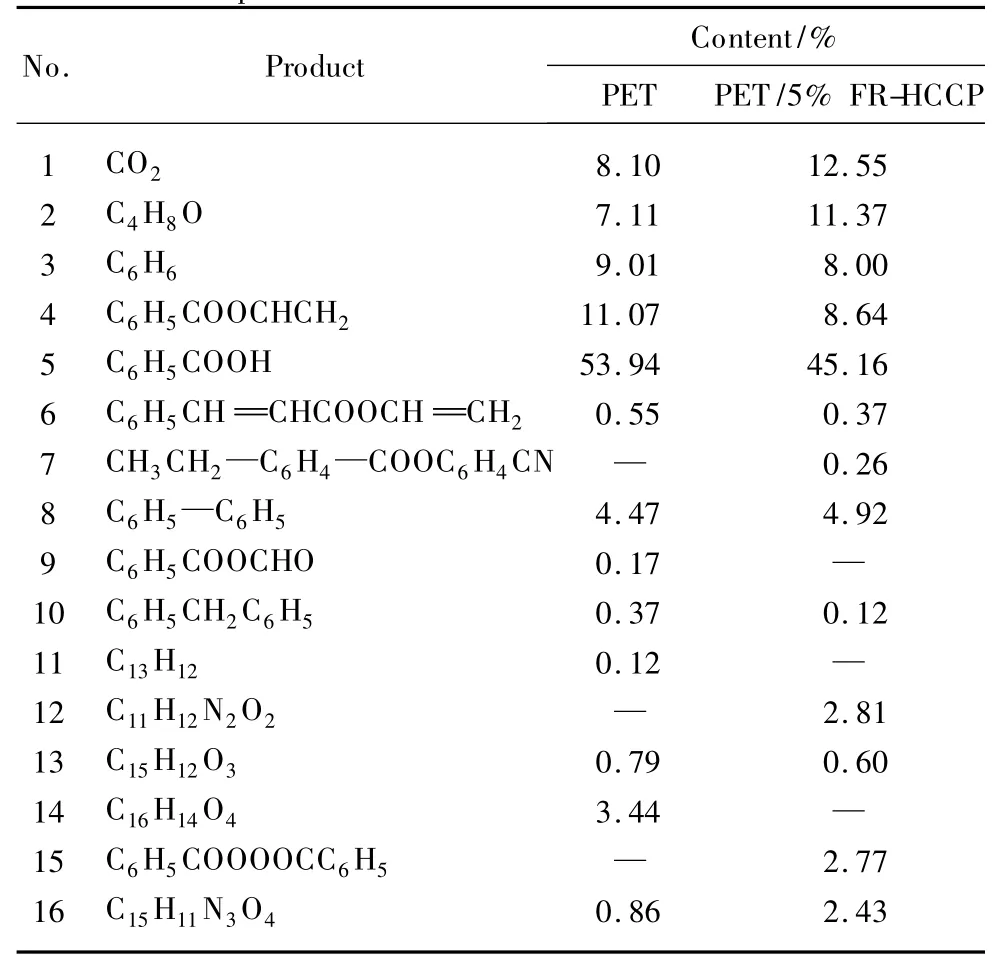
Table 4 Main pyrolysis products of PET and PET/5%FR-HCCP composites
Under the same condition,the concentration of CO2accounted for 12.55%in the pyrolysis products of PET/5% FR-HCCP,compared with 8.10% of PET.CO2was the decarboxylation productof benzoic acid from pyrolysis compounds,and in fact the content of main breakdown products benzoic acid was really reduced compared PET blends with neat PET.As known,CO2was non-flammable and in favour of retardancy for fire spread.That was to say,an interaction between PET and FR-HCCP happened and generated more incombustible CO2.
It was deduced that FR-HCCP decomposed first in the early stages of degradation,generating gaseous pyrolysis products and forming charred residue which contained P and N elements.The phosphorus could promote the formation of the char which inhibited the release of the volatile products to form a porous foamed mass.This structure could effectively inhibit further gases diffusing and protect the polymer from heat and flame[35].At the same time the coking protective cover formed by Si and B played an important role in hindering diffusion of volatile degradation products on PET degradation and contributed to the enhancement of thermal stability of the composites[38-39].
2.4 SEM analysis of the char residues
The SEM photos of carbon residue could show intuitively the morphology of polymer after burning.Figure 2 showed that the charred surface layer of pure PET was very thin and the holes were all large,with diameters in the range 150-500 μm.Compared with that of pure PET,the char layer structure of materialPET/FR-HCCP wasnotsimilar, itwas more consistent,and the char crust was very thick(Fig.3).Holes were of diameter 15 μm or less.The thick char crust not only reduced the rates of formation of volatile gases,but also provided a barrier to heat and flame penetration into the inner layers of the material,thereby resisting the start and spread of fire.In general,FR-HCCP in a PET matrix played a moreactive role in retarding condensed phase flames and in this way inhibited flame propagation[27].
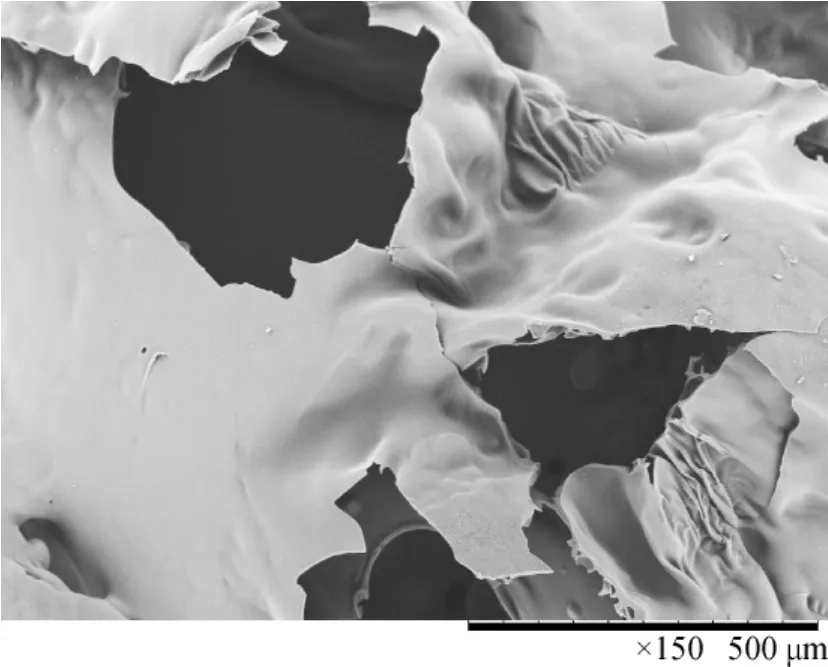
Fig.2 SEM image of pure PET char crusts

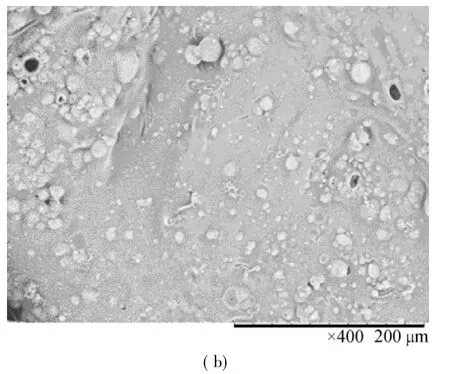
Fig.3 SEM images of PET/3%FR-HCCP char crusts inner(a)and outer(b)
2.5 FTIR investigation of the residue
FTIR spectrum of the residual char of FR-HCCP were given in Fig.4.The strong absorption peaks of 1096 and 1201 cm-1were attributed to Si— =O and P N,respectively.It indicated that the cyclotriphosphazene and silicon were still remained in the residue;it consisted with the results of Py-GCMS analysis.
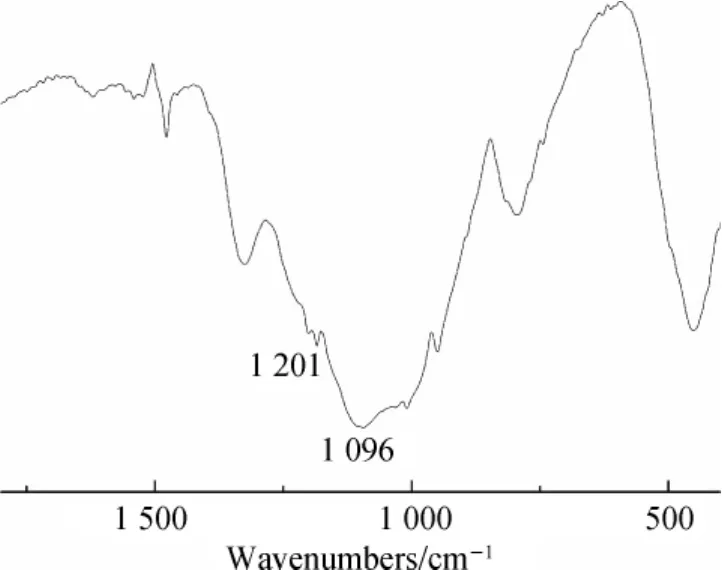
Fig.4 FTIR spectrum of the char of FR-HCCP
Figure 5 showed the FTIR spectra of charred residue of PET and PET/FR-HCCP composites.Compared with the char of pure PET,similar peaks of the composites residue were discovered expect for the bands at 1 099 and 798 cm-1corresponding to Si—O and Si—C products,respectively.These results proved the presence of silicon and boron in the PET residue.The result was consistent with the results of Py- GC-MS analysis;no Si and B was found in pyrolysis products.According to the spectra data,it was clearly concluded that the SiO2existed in the residue of the blends.

Fig.5 FTIR spectra of the chars of PET and PET/ FR-HCCP composites
2.6 Raman spectrum of the residue
Carbon layer structure order of the residue can be test by Roman spectrum.Figure 6 showed the Roman specctra of PET and PET composites with different content of additive.D peak about at 1350 cm-1represented the disordered degree of carbon layer and F peak about at 1600 cm-1represented the ordered degree of carbon layer.From Fig.6 we found that comparing with the pure PET,D peak relatively weakened meanwhile G peak enhanced,from which we learned that carbon layer order increased.At the same time,we also found that the order changed very little between differentcontentofadditive material.

Fig.6 Roman spectra of the chars of PET and PET/FRHCCP composites
2.7 Element analysis of the residue
In order to get the content of P,Si,and B elements in carbon residue more precisely,we performed the element analysis test by ICP.From the data in Table there were hardly any P,Si,and B elements in the residue of PET.But the contents of P,Si and B elements were increased along with the increasing of the additive.The result indicated that some of P,N,Si,and B material stayed in the carbon residue.The results were consistent with the Py-GC-MS.

Table 5 Element analysis data of PET and PET/FR-HCCP
3 Conclusions
In this paper,FR-HCCP as a kind of flame retardant was added to the PET by melt blending.The results of TGA showed that FR-HCCP improved the char formed acts of PET and enhanced the thermal stability of the polymer in nitrogen atmosphere.LOI values and UL-94 test results indicated that the addition of FR-HCCP could improve the flame retardancy of PET.The LOI value of the blend was 31.7 when the amount of FR-HCCP was only 5%.The Py-GC/MS study showed that the introduction of FR-HCCP into PET would inhibit the pyrolysis of composites during heating or burning.Roman spectra showed the order degree of residue was increasing by adding the additive.These results displayed that the residue with acontinuous outer surface and porous internal structure provided a good barrier to improve the thermal behaviours and flammability properties of the composites during burning.
[1]Bikiaris D.Can Nanoparticles Really Enhance Thermal Stability of Polymers?PartⅡ:An Overview on Thermal Decomposition of Polycondensation Polymers[J].Thermochim Acta,2011,523 (1/2):25-45.
[2]Wang Y Z,Chen X T,Tang X D,et al.A New Approach for theSimultaneousImprovementofFireRetardancy,Tensile Strength and Melt Dripping of Poly(ethylene terephthalate)[J].Journal of Materials Chemistry Communication,2003,13(6): 1248-1249.
[3]Ban D M,Wang Y Z,Yang B,et al.A Novel Non-dripping Oligomeric Flame Retardant for Polyethylene Terephthalate[J].European Polymer Journal,2004,40(8):1909-1913.
[4]Cheema H A,Shafei A E,Hauser P J.Conferring Flame Retardancy on Cotton Using Novel Halogen-Free Flame Retardant Bifunctional Monomers: Synthesis, Characterizations and Applications[J].Carbohydrate Polymers,2013,92(1):885-893.
[5]Gao F, Tong L F, Fang Z P. Effectof a Novel Phosphorousenitrogen Containing Intumescent Flame Retardant on the Fire Retardancy and the Thermal Behaviour of Poly(butylene terephthalate)[J].Polymer Degradation and Stability,2006,91 (6):1295-1299.
[6]Zhang X,Zhong Y,Mao Z P.The Flame Retardancy and Thermal Stability Properties of Poly(ethylene terephthalate)/ Hexakis(4-nitrophenoxy)Cyclotriphosphazene Systems[J].Polymer Degradation and Stability,2012,97(8):1504-1510.
[7]Hoang D Q,Kim J.Synthesis and Performance of Cyclic Phosphorus-Containing Flame Retardants [J]. Polymer Degradation and Stability,2008,93(12):2042-2047.
[8]Xu J Z,He Z M,Wu W H,et al.Study of Thermal Properties of Flame RetardantEpoxy Resin Treated with Hexakis[p-(hydroxymethyl)phenoxy]Cyclotriphosphazene[J].Journal of Thermal Analysis and Calorimetry,2013,114(3):1341-1350.
[9]BoseS, Mukherjee M, Das C K, etal. Effectof Polyphosphazene Elastomer on the Compatibility and Properties of PES/TLCP Composites[J].Polymer Composites,2010,31 (3):543-552.
[10]Çiftçi G Y,Eçik E T,Yldrm T,etal.Synthesisand Characterization of New Cyclotriphosphazene Compounds[J].Tetrahedron,2013,69(5):1454-1461.
[11]Jiménez J,Laguna A,Molter A M,et al.Supermolecular Liquid Crystals with a Six-Armed Cyclotriphosphazene Core: from Columnar to Cubic Phases[J].Chemistry—A European Journal,2011,17(3):1029-1039.
[12]Lu H D,Wilkie C A.Study on Intumescent Fame Retarded Polystyrene Composites with Improved Flame Retardancy[J].Polymer Degradation and Stability,2010,95(12):2388-2395.
[13]Dogˇan M,Yilmaz A,Bayramli E.Synergistic Effect of Boron Containing Substances on Flame Retardancy and Thermal Stability ofIntumescent Polypropylene Composites[J]. Polymer Degradation and Stability,2010,95(12):2584-2588.
[14]Gérard C,Fontaine G,Bourbigot S.Synergistic and Antagonistic Effects in Flame Retardancy of an Intumescent Epoxy Resin[J].Polymers Advanced Technologies,2011,22(7):1085-1090.
[15]Song T,Li Z S,Liu J G,et al.Novel Phosphorus-Silicon Synergistic Flame Retardants:Synthesis and Characterization[J].Chinese Chemical Letters,2012,23(7):793-796.
[16]Yu Y M,Fu S Y,Song P A,et al.Functionalized Lignin by Grafting Phosphorus-Nitrogen Improves the Thermal Stability and Flame Retardancy of Polypropylene[J].Polymer Degradation and Stability,2012,97(4):541-546.
[17]Wang JN,MaoZ P.ModifiedMontmorilloniteand Its Application as a Flame Retardant for Polyester[J].Journal of Applied Polymer Science,doi:10.1002/app.39625.
[18]Gaan S,Sun G,Hutches K,et al.Effect of Nitrogen Additives on Flame Retardant Action of Tributyl Phosphate: Phosphorusenitrogen Synergism[J].Polymer Degradation and Stability,2008,93(1):99-108.
[19]Haaland D M,Brinker C J.In situ FT-IR Studies of Oxide and Oxynitride Sol-Gel-Derived Thin Films[J].Meterials Research Society Symposium Proceedings,1984,32(1):267-269.
[20]Villegas M A,Fernandez J M.Characterization of B2O3-SiO2Glasses Prepared via Sol-Gel[J].Journal of Materials Science,1988,23(7):2464-2478.
[21]Kamitsos E I,Karakassides M A,Chryssikos G D.Vibrational Spectra of Magnesium-Sodium-Borate Glasses.2.Raman and Mid-infrared Investigation of the Network Structure[J].The Journal of Physical Chemistry,1987,91(5):1073-1079.
[22]Parsons J L,Milberg M G.Vibrational Spectra of Vitreous B2O3.xH2O[J].Journal of the American Ceramic Society,1960,43 (6):326-330.
[23]Jabra R,Phalippou J,Zarzycki J.Synthesis and Characterization of Glasses from SiO2-B2O3System Obtained by Hot-Pressing of Gels[J].Revue de Chimie minerale,1979,16(4):245-249.
[24]Kasgoz A,Misono T,Abe Y.Preparation and Properties of Polyborosiloxanes as Precursors for Borosilicate Formation of SiO2-B2O3Gel Fibers and Oxides by the Sol-Gel Method Using Tetraacetoxysilane and Boron Tri-n-butoxide[J].Journal of Polymer Science Part A:Polymer Chemistry,1994,32(6): 1049-1056.
[25]Tenney A S,Wong J.Vibrational Spectra of Vapor-Deposited Binary Borosilicate Glasses[J].TheJournal of Chemical Physics,1972,56(11):5516-5523.
[26]Tohge N,Matsuda A,Minami T.Coating Films of 20 B2O3· 80 SiO2by the Sol-Gel Method[J].Journal of the American Ceramic Society,1987,70(1):C13-5.
[27]Taft E A.Infrared Absorption of Chemical Vapor Deposited Borosilicate Glass Films[J].Journal of the Electrochemical Society,1971,118(12):1985-1988.
[28]Bois L,Haridon P L,Laurenta Y,et al.Characterization of a Boro-silicon Oxynitride Prepared by Thermal Nitridation of a Polyborosiloxane[J].Journal of Alloya and Compounds,1996,232(1/2):244-253.
[29]Satu E K,Andrew R,Marko P,et al.Determination of the Hydroxyl Group Content in Silica by Thermogravimetry and a Comparison with1H MAS NMR Results[J].Thermochim Acta,2001,379(1/2):201-212.
[30]Qin H L,Su Q S,Zhang S M,et al.Thermal Stability and Flammability of Polyamide 66/Montmorillonite Nanocomposites[J].Polymer,2003,44(24):7533-7538.
[31]Davis R D,GilmanJW,VanderHartD L.Processing Degradation of Polyamide 6/Montmorillonite Clay Nanocomposites and ClayOrganicModifier[J].Polymer Degradation and Stability,2003,79(1):111-121.
[32]Xie W,Gao Z M,Pan W P,et al.Thermal Degradation Chemistry of Alkyl Quaternary Ammonium Montmorillonite[J].Chemistry of Materials,2001,13(9):2979-2990.
[33]Qin H L,Zhang Z G,Feng M,et al.The Influence of Interlayer Cations on the Photo-Oxidative Degradation of Polyethylene/ Montmorillonite Composites[J].Journal of Polymer Science Part B:Polymer Physics,2004,42(16):3006-3012.
[34]Zhou W J,Chen K,Yang H,et al.Preparation and Flame Retardancy of Polyborosiloxane[J].Rare Metal Materials and Engineering,2010,39(S2):211-214.
[35]Zhang J J,Ji Q,Zhang P,et al.Thermal Stability and Flame-Retardancy Mechanism of Poly(ethyleneterephthalate)/Boehmite Nanocomposites[J].Polymer Degradation and Stability,2010,95(7):1211-1218.
[36]Buxbaum L H.Degradation of Poly(ethylene terephthalate)[J].Angewandte Chemie International Edition in English,1968,7 (3):182-190.
[37]Bednas M E,Day M,Ho K,et al.Combustion and Pyrolysis of Poly(ethylene terephthalate).Ⅰ.The Role of Flame Retardants on Products of Pyrolysis[J].Journal of Applied Polymer Science,1981,26(1):277-289.
[38]Qin H L,Zhang S M,Zhao C G,et al.Flame Retardant Mechanism of Polymer/Clay Nanocomposites Based on Polypropylene[J].Polymer,2005,46(9):8386-8395.
[39]Xu X F,Ding F F,Qian Z Z,et al.Degradation of Poly (ethylene terephthalate)/Clay Nanocomposites during Melt Extrusion:Effect of Clay Catalysis and Chain Extension[J].Polymer Degradation and Stability,2009,94(1):113-123.
TQ322.4+2
A
1672-5220(2015)03-0384-06
date:2014-03-17
*Correspondence should be addressed to MAO Zhi-ping,E-mail:donghuatougao@126.com
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Effect of Sludge Retention Time on the Fate of Proteins and Polysaccharides in AAO Process
- Compressive Strength Estimation for the Fiber-Reinforced Polymer(FRP)-Confined Concrete Columns with Different Shapes Using Artificial Neural Networks
- Force-Based Quadrilateral Plate Bending Element for Plate Using Large Increment Method
- Effects of 1,7-Bromine Substitution at Bay Area on Self-assembly Behavior and Photo Physical Properties of Perylene Diimide
- Theoretical and Experimental Analyses of Poisson Ratios for Plain-Woven Fabrics
- Dynamic Engaging Characteristics of Wet Clutch in Automatic Transmission
