ZnO/V2O5纳米管阵列的合成及其光电化学性能研究
刘兆清,黄志翠,张秋丽,曾环娜,李 楠,苏育志
(广州大学化学化工学院,广东广州 510006)
ZnO/V2O5纳米管阵列的合成及其光电化学性能研究
刘兆清,黄志翠,张秋丽,曾环娜,李 楠,苏育志
(广州大学化学化工学院,广东广州 510006)
通过简单直接的两步电化学沉积法成功制备了核壳结构的ZnO/V2O5纳米管阵列.通过采用XRD,SEM,TEM和XPS等表征手段对这些制备的ZnO/V2O5核壳纳米管结构的物相和微结构进行分析.光电化学测试结果表明:ZnO/V2O5核壳纳米管阵列相比于单一的ZnO纳米棒阵列具有明显增强的光电化学性能,使其有望在光解水领域得到广泛的应用.
ZnO/V2O5;纳米管阵列;光电化学性能
Converting solar energy to chemical energy has been a focus of great attention in photoelectrochemical(PEC)application for solving the current global energy crisis and environment problems.PEC water splitting has been regarded as a proven and effective method for hydrogen production by utilizing solar en-ergy[1].Since the PEC water splitting on rutile TiO2anode electrode has been firstly reported by FUJISHIMA,et al[2]in 1972,varieties ofmetal oxide semiconductors have been extensively used and reported in PEC water splitting application[3-4].
Among the abundantmultifunctional one-dimen-sional(1D)metallic oxides,ZnO NRAs has been extensively investigated in photocatalysis[5],solar cells[6]and supercapacitors[7]due to its excellent carrier mobility with large exciton binding energy of 60 meV,facile synthesis,nontoxicity,easy immobilization and unique chemical and physical properties. Particularly,it has been widely served as a photoanode in water splitting attributed to the appropriate band gap that cross the redox potential of water photoelectrolysis[8-9].However,the further research and application of ZnO NRAs are seriously restricted by its intrinsic defects,such as wide band gap(Eg=3.2 eV),poor visible light absorption ability,and high recombination of photogenerated electron-hole pairs and so on,which inevitably leading to the negligible visible light photocatalytic performance. Therefore,many researchers have put their efforts to promote the photoelectrochemical performance of ZnO NRAs.Incorporating twometal oxide semiconductors into an integrated structure is regarded as a promising strategy because the resulted product exhibits increased physicochemical properties compared to onefold semiconductor.Recently,ZnO NRAs-based heterostructure,such as ZnO/Er2O3[10],ZnO/CdTe[11],ZnO/Ag2O[12],ZnO/NiO[13],ZnO/CuO[14]and ZnO/CuS[15],with enhanced photoelectrochemical performance have already been successfully constructed.
Among the versatile candidates,vanadium peotoxide(V2O5)has acquired special attention due to its several attractive advantages such as low band gap(Eg=2.2 eV),ease of synthesis,low cost,abundance as well as the layered structure and superior optical properties,which enables it to be used as optical switches[16],catalysts[17],solar cells[18]and so on.Furthermore,ZnO/V2O5heterostructure could absorb the ultraviolet and visible light from the entire solar spectrum,promote the separation efficiency and also suppress the recombination of photogenerated electron-hole pairs,thus resulting in the enhanced photocatalytic property.In other word,it can serve as a promising,effective and practical photoanode material in the application of PEC water splitting.
Herein,highly dense and ordered ZnO/V2O5core/shell NTAs has been successfully synthesized through a simple and directed two-step electrochemical deposition method.The as-prepared ZnO/V2O5heterostructure exhibits enhanced PEC water splitting performance compared to pure ZnO,which can be mainly ascribed to the formation of heterojunction that not only promotes the separation efficiency but also restrains the recombination rate of photogenerated electron-hole pairs.Such heterostructured composite can be used as a potential catalyst in PECwater splitting for hydrogen production.
1 Experiment
1.1 Synthesis of ZnO/V2O5NTAs electrode
All chemical reagents used were analytical grade which were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China)and were used directly withoutany purification.An aqueous solution contains 0.02 M Zn(NO3)2,0.01 M NH4Ac and 0.01 M hexamethylenetetramine(HMT)served as the electrolyte,ZnO NRAswas grown on the fluorine tin oxide(FTO)substrate in a traditional two-electrode system with the current density of-2.0 mA cm-2at 90℃for 50 min.ZnO/V2O5NTAs were prepared by a second electrochemical deposition process.Typically,the FTO substrate covered with ZnO NRAs was immersed into a solution of 0.005 M NH4VO3,0.01 M Na2EDTA and 0.02 M NH4Ac with current density of-1.0 mA cm-2at 90℃for 15 min.
1.2 Characterization
X-ray diffraction(XRD)measurement was performed on a PANalytical PW3040/60 diffractometer with monochromatized Cu Kαradiation(λ=0.154 18 nm).The field emission scanning electron microscopy(FE-SEM,JEOL JSM-7001F)and transmission electron microscopy(TEM,JEM2010-HR)were employed to examine the surface morphologyand crystalmicrostructure.The chemical composition and surface electronic stateswere determined by X-ray photoelectron spectroscopy(XPS,ESCALab250).
1.3 Photoelectrochem icalmeasurements
The photoelectrochemical performance was investigated with a CHI 760D model electrochemical workstation(Shanghai Chenhua)in a quartz cell. The as-prepared ZnO/V2O5NTAs grown on FTO substrate was used as the working electrode(working area was fixed to 2.25 cm2),a saturated Ag/AgCl electrode and platinum plate were served as the reference electrode and counter electrode,respectively.A 0.5 M Na2SO4+0.1 M Na2S aqueous solution was used as the electrolyte,and a 300 W Xe lamp with visible light(λ>420 nm)was served as the light source.
2 Results and discussion
XRD patterns have been employed to identify the phase and composition of the as-prepared products(Fig.1).It can be clearly seen that all the diffraction peaks in the black and red patterns can be well-assigned to the hexagonal wurtzite phase of ZnO(JCPDS card no.36-1451)with lattice parameters a=3.25Å,b=3.25Åand c=5.21Åand the tetragonal phase of V2O5(JCPDS card no.45-1074)with lattice parameters a=14.26Å,b=14.26Å and c=12.58Åexcept the diffraction peaks for SnO2which come from the FTO substrate.No impurity phase could be found from the patterns,indicating the as-prepared products are highly pure.Furthermore,the intense and sharp peaks demonstrate that the products are high degree of crystallinity.Fig.2 presents the EDS spectrum of ZnO/V2O5NTAs,the existence of V,Zn and O elements signals further confirms the high purity and suggests the ZnO/V2O5core/shell structurewas obtained through the two step electrodeposition.
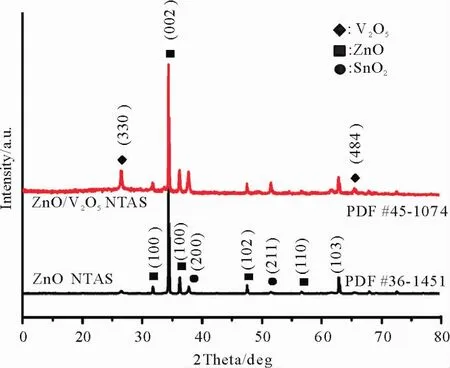
Fig.1 XRD patterns of ZnO NRAs and ZnO/V2O5NTAs
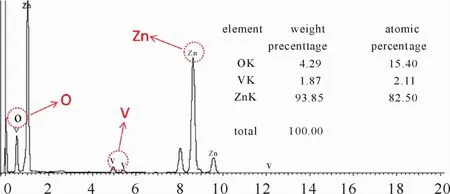
Fig.2 EDS spectrum of ZnO/V2O5NTAs
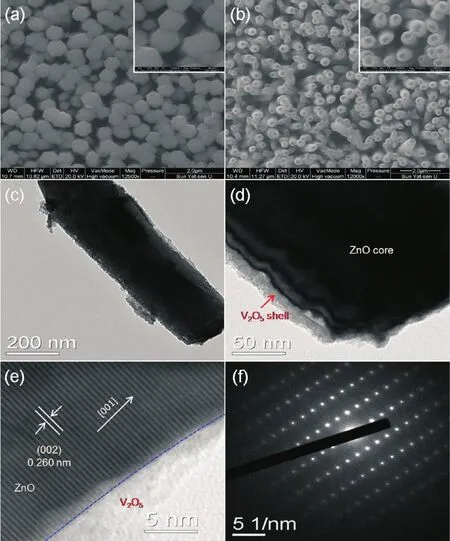
Fig.3 SEM images of(a)ZnO NRAs and(b)ZnO/V2O5NTAs;(c~e)TEM and HRTEM images of ZnO/V2O5NTAs;(f)SAED pattern of ZnO
Fig.3 displays the typical SEM and TEM images of pure ZnONRAs and ZnO/V2O5NTAs.Obviously,compared to the pure ZnO nanorods(Fig.3a),thehexagon disappeared and nanotubes formed after the electrodeposition of V2O5(Fig.3b),their diameters are in the range of 200~300 nm.Fig.3c and 3d show the TEM images of the as-prepared ZnO/V2O5NTAs,it can be clearly seen that the core-shell structure formed after the electrodeposition of V2O5. Moreover,the enlarged HRTEM image and selected area electron diffraction(SAED)pattern of ZnO are presented in Fig.3e and f.The lattice spacing of ZnO estimated from the HRTEM image was found to be about 0.260 nm,which matches well to the(002)plane of ZnO in the wurtzite structural phase.The HRTEM image and SAED pattern reveal that the ZnO is of single crystalline,while the V2O5has a poor crystalline.
In order to gain the chemical position and surface electronic state,the as-prepared products were subjected to surface analysis by X-ray photoelectron spectroscopy.Fig.4a shows the typical survey XPS spectrum of the products,the elements carbon(C),zinc(Zn),vanadium(V)and oxygen(O)are detected.The peak position at284.60 eV in C 1s spectrum(Fig.4b)is ascribed to the C-C bonding[19]. Fig.4c exhibits the high-resolution XPS spectrum of Zn 2p,two symmetric peaks at 1 045.51 and 1 022.25 eV are assigned to Zn 2p1/2and Zn 2p3/2,indicating the existence of Zn2+in the ZnO/V2O5heterostructure[20].Fig.4d displays the spectra of V 2p-O 1s,the V 2p core-level spectrum with V 2p1/2and V 2p3/2peaks at525.10 and 517.15 eV is consistentwith V5+state in V2O5[21].As for O 1s spectrum,the peaks labeled“a”at533.03 eV and“b”at530.05 eV can be attributed to the absorbed oxygen molecules and O2-in the crystal lattice[22].All the XPS results further confirm the coexistence of ZnO and V2O5in the ZnO/V2O5heterostructure.
UV-vis diffuse reflectance spectra of the as-prepared samples are shown in Fig.5.The pure ZnO NRAs shows almost no absorption in the visible light range due to itswide band gap(ca.3.16 eV),with an absorption edge of about390 nm.While the ZnO/ V2O5NTAs exhibits strong absorption in both ultraviolet and visible light range,in which an apparent red shiftoccurs compared to pure ZnO NRAs.The results imply that the visible light response of the composite is improved by introducing V2O5to the ZnO,and a lower band gap is beneficial for the electronic transitions,which subsequently leads to the enhanced photoelectrochemical and photocatalytic performances.
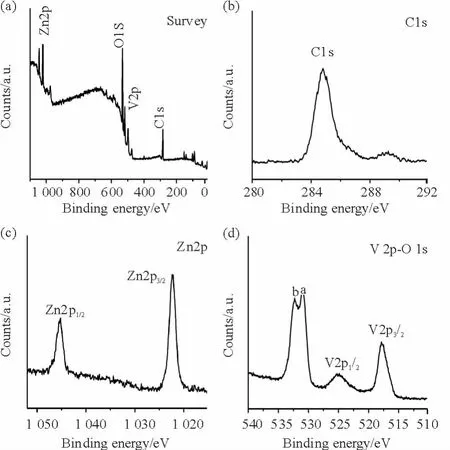
Fig.4 XPS spectra of ZnO/V2O5NTAs:(a)survey,(b)C 1s,(c)Zn 2p and(d)V 2p-O 1s
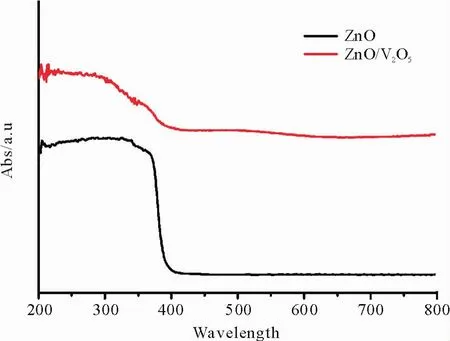
Fig.5 UV-vis diffuse reflectance spectrum of ZnO NRAs and ZnO/V2O5NTAs
To evaluate the PEC water splitting performance of the as-prepared ZnO/V2O5NTAs,electrochemical measurements were conducted in 0.5 M Na2SO4+0.1 M Na2S solution.Fig.6a presents the currentdensity versus potential(J-V)curves for the pure ZnO NRAs and ZnO/V2O5NTAs under visible light irradiation in a potential range of-0.8~1.2 V vs.Ag/AgCl.Obviously,the ZnO/V2O5NTAs exhibits greatly enhanced current density compared to the pure ZnO NRAs under visible light illumination,a maximum photocurrent density of 9.61 mA·cm-2at 1.2 V vs.Ag/AgCl for ZnO/V2O5NTAs was obtained,which was around 3.75 times than that of pure ZnO NRAs(2.56 mA·cm-2).Fig.5b shows the corresponding photocurrent response to on-off cycling,from which it can be seen that the photocurrent density generates promptly and increases sharply under irradiation,then decreases to zero point instantly as soon as the illumination is stopped.The transient photocurrent density of ZnO/V2O5NTAs(75.96 uA·cm2)is 2.77 times higher than that of pure ZnO NRAs(27.46 uA·cm-2),demonstrating the effective separation of photogenerated electro-hole pairs in the ZnO/V2O5heterostructures.The above results indicate that the as-prepared ZnO/V2O5NTAs could serve as a promising candidate for PEC water splitting application.
3 Conclusions
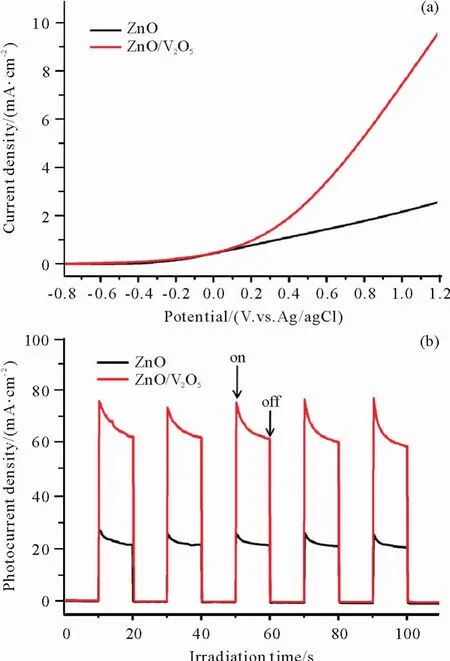
Fig.6 (a)Current density versus potential(J-V)curves for the pure ZnO NRAs and ZnO/V2O5NTAs under visible light irradiation;(b)Photocurrent response of the photoanodes prepared from pure ZnO NRAs and ZnO/V2O5NTAs to light on-off under visible light illumination
In summary,ZnO/V2O5core/shell NTAs has been successfully synthesized by a simple and directed two-step electrochemical deposition process.The as-prepared ZnO/V2O5NTAs exhibits enhanced PEC water splitting performance compared to pure ZnO NRAs,indicating the potential application as photoanode in future hydrogen production.
[1] YANG JH,WANG D,HAN H X,et al.Roles of cocatalysts in photocatalysis and photoelectrocatalysis[J].Acc Chem Res,2013,46(8):1900-1909.
[2] FUJISHIMA A,HONDA K.Electrochemical photolysis of water at a semiconductor electrode[J].Nature,1972,238(5358):37-38.
[3] KUDO A,MISEKIY.Heterogeneous photocatalystmaterials for water splitting[J].Chem Soc Rev,2009,38(1):253-278.
[4] WANGM Y,SUN L,LIN ZQ,et al.p-n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities[J].Energy Environ Sci,2013,6(4):1211-1220.
[5] CHANG H X,SUN ZH,HO K Y,etal.A highly sensitive ultraviolet sensor based on a facile in situ solution-grown ZnO nanorod/grapheme heterostructure[J].Nanoscale,2011,3(1):258-264.
[6] BID Q,BOSCHLOO G,SCHWARZMULLER S,et al.Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod arrays solid-state solar cells[J].Nanoscale,2013,5(23):11686-11691.
[7] HE Y B,LIG R,WANG Z L,etal.Single-crystal ZnO nanorod/amorphous and nanoporousmetal oxide shell composites:Controllable electrochemical synthesis and enhanced supercapacitor performances[J].Energy Environ Sci,2011,4(4):1288-1292.
[8] YU JX,HUANG B B,QIN X Y,etal.Hydrothermal synthesis and characterization of ZnO filmswith different nanostructures[J].Appl Sur Sci,2012,257(13):5563-5565.
[9] QIU JH,GUOM,FENG Y J,et al.Electrochemical deposition of branched hierarchical ZnO nanowire arrays and its photoelectrochemical properties[J].Electrochim Acta,2011,56(16):5776-5782.
[10]YE K H,WANG JY,LIN,et al.A facile way to synthesize Er2O3·ZnO core-shell nanorods for photoelectrochemicalwater splitting[J].Inorg Chem Commun,2014,45:116-119.
[11]LIU ZQ,XIE X H,XU Q Z,etal.Electrochemical synthesis of ZnO/CdTe core-shell nanotube arrays for enhanced photoelectrochemical properties[J].Electrochim Acta,2013,98:268-273.
[12]MA SS,XUE JJ,ZHOU Y M,etal.Photochemical synthesis of ZnO/Ag2O heterostructureswith enhanced ultravioletand visible photocatalytic activity[J].JMater Chem A,2014,2(20):7272-7280.
[13]DAIW,PAN X H,CHEN SS,etal.Honeycomb-like NiO/ZnO heterostructured nanorods:photochemical synthesis,characterization,and enhanced UV detection performance[J].JMater Chem C,2014,2(23):4606-4614.
[14]SIMON Q,BARRECA D,GASPAROTTO A,etal.Vertically oriented CuO/ZnO nanorod arrays:from plasma-assisted synthesis to photocatalytic H2production[J].JMater Chem,2012,22(23):11739-11747.
[15]BASU M,GARGA N,GANGULIA K.A type-IIsemiconductor(ZnO/CuSheterostructure)for visible light photocatalysis[J].JMater Chem A,2014,2(20):7517-7525.
[16]ZHU G M,QU Z B,ZHUANG G L,etal.CO oxidation by lattice oxygen on V2O5nanotubes[J].JPhys Chem C,2011,115(30):14806-14811.
[17]PENNER S,KLOTZER B,JENEWEIN B.Structural and redox properties of VOxand Pd/VOxthin film model catalysts studied by TEM and SAED[J].Phys Chem Chem Phys,2007,9(19):2428-2433.
[18]MENEZESW G,REISDM,BENEDETTITM,etal.V2O5nanoparticles obtained from a synthetic bariandite-like vanadium oxide:Synthesis,characterization and electrochemical behavior in an ionic liquid[J].JColloid Interf Sci,2009,337(2):586-593.
[19]QIN G H,WU X,ZHANG H J.Rational design of TiO2-V2O5-C nanostructure grafted by N-doped graphene with enhanced photocatalysis and lithium ion store performances[J].RSC Adv,2014,4(94):52438-52450.
[20]JIANG J,ZHANG X,SUN P B,et al.ZnO@BiOI heterostructures photoinduced charge-transfer property and enhanced visible-light photocatalytic activity[J].JPhys Chem C,2011,115(42):20555-20564.
[21]WANG Y,LIU L X,XU L,etal.Ag2O-TiO2-V2O5one-dimensional nanoheterostructures for superior solar light photocatalytic activity[J].Nanoscale,2014,6(12):6790-6797.
[22]ZOU CW,RAO Y F,AlyamaniA,etal.Heterogeneous lollipop-like V2O5-ZnO array a promising composite nanostructure for visible light photocatalysis[J].Langmuir,2010,26(14):11615-11620.
【责任编辑:周 全】
Directed synthesis of ZnO/V2O5nanotube arrays w ith enhanced photoelectrochem ical properties
LIU Zhao-qing,HUANG Zhi-cui,ZHANG Q iu-li,ZENG Huan-na,LINan,SU Yu-zhi
(School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China)
In this work,ZnO/V2O5core/shell nanotube arrays(NTAs)have been successfully synthesized via a simple and two-step electrochemical deposition process.The as-prepared ZnO/V2O5samples are characterized by power X-ray diffraction(XRD),scanning electron microscopy(SEM),transmission electron microscopy(TEM)and X-ray photoelectron spectroscopy(XPS).The as-prepared ZnO/V2O5NTAs exhibits enhanced photoelectrochemical(PEC)performance compared with pure ZnO nanorod arrays(NRAs),indicating the promising application in photoelectrochemicalwater splitting.
ZnO/V2O5;nanotube arrays;photoelectrochemical performance
O 649.4
A
date:2015-03-02; Revised date:2015-04-14
s:This work was supported by Natural Science Foundation of China(21306030),Guangdong Natural Science Foundation(s2013040015229),Scientific Research Project of Guangzhou Municipal Colleges and Universities(12014106184),the Fresh Talent Program of Guangzhou University(201302).
O 649.4
A
1671-4229(2015)03-0024-06
Biography:LIU Zhao-qing(1979-),male,associateprofessor,Ph.D.E-mail:lzqgzu@gzhu.edu.cn

