炭分子筛CH4/N2吸附分离
林文胜,席芳,顾安忠
(上海交通大学制冷与低温工程研究所,上海200240)
Introduction
Methane contained in the coal bed methane(CBM)is a kind of clean fuel,but its emission to atmosphere would contribute to the greenhouse effect.The methane content in CBM is usually only 20%to 40%due to the mixing of air during the exploitation process[1].In spite of the large coal production,CBM is not well utilized in China.Besides its low methane content,CBM wells are often remote and the gas production of a single well is so small that delivering it through pipeline is economically unpractical.An effective way of utilizing CBM is to deliver it to local natural gas supply systems after liquefaction[2].For oxygen mixed in CBM,it must be removed firstly considering the safety issue.After that,the major and difficult task of utilization CBM is to separate methane from nitrogen economically.Although cryogenic distillation is a mature application,it usually needs higher energy consumption for the undesired liquefaction of nitrogen.An alternative way of separating the CH4/N2mixture is pressure swing adsorption (PSA)process[3-5].
In recent years,researchers have found that it is favorable to adopt the mechanism of kinetic selectivity to produce enriched methane with certain pressure.Principally,a kinetic separation is achieved by virtue of the difference in diffusion rates which is higher for the smaller nitrogen molecular diameter compared to methane.Although simple in principle,it is a relatively difficult separation compared to an equilibrium separation because of the low values of the diffusion time constants and the adverse equilibrium selectivity towards methane and nitrogen.Ackleyetal[6]enriched methane from 50%to 80%by vacuum pressure swing adsorption (VPSA).However,it is hard to enrich methane up to 90%through kinetic PSA while the methane content is lower than 50%in the feed gas until now[7].The selection of appropriate adsorbent is crucial to the successful separation of methane and nitrogen.
In our recent studies[8-9],single bed kinetic PSA separation experiments were carried out under a wide range of adsorption pressures (p),feed gas velocities (v)and different methane contents in feed gas with a selected carbon molecular sieve(CMS).The breakthrough curves showed that at the initial stage of adsorption there was only methane at the outlet of adsorption bed,indicating that methane could be enriched up to 90%with a single PSA unit.
In this paper,the same two simulated methane-nitrogen mixtures (30%and 50%methane,respectively)were separated using a two-bed PSA unit with the same CMS adsorbent.The experimental studies under various conditions of adsorption pressures,feed gas velocities and cycle times were investigated.
1 Experimental
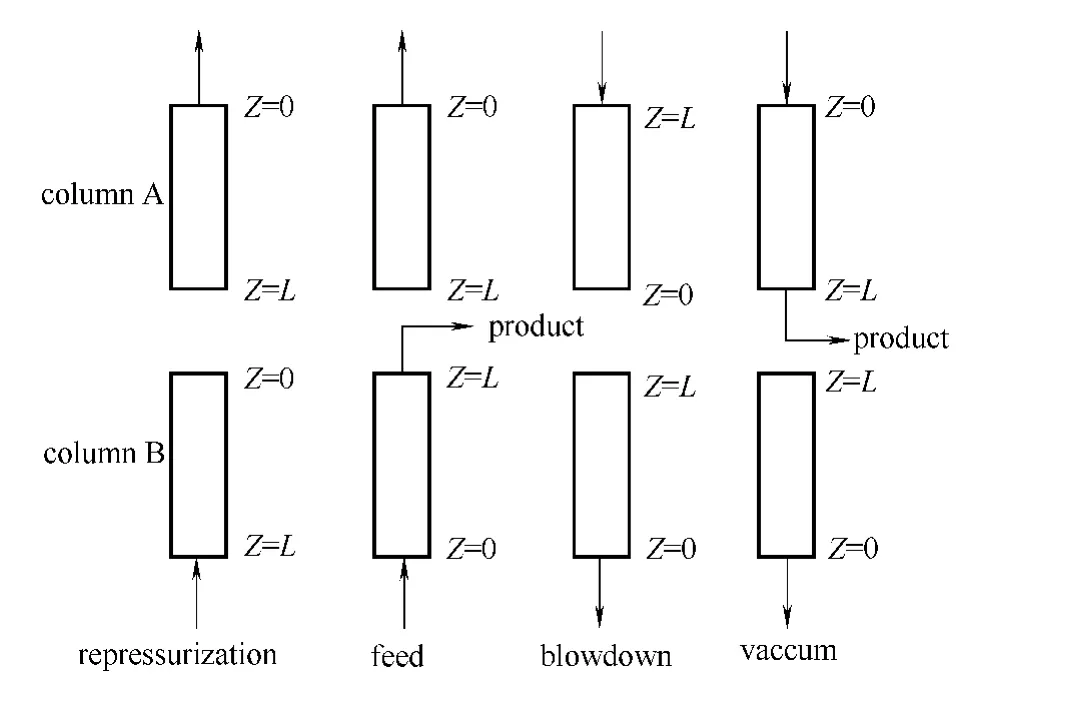
Fig.1 Schematic of steps involved in PSA cycle
A simple two-bed PSA process operated in a cyclic manner as shown in Fig.1comprises four basic steps:repressurization,feed,counter-current blowdown and counter-current vacuumization.High pressure feed flows through bed B where the faster diffusing nitrogen is preferentially adsorbed,leaving a stream richer in methane at the outlet of the bed after the regenerated bed repressurized with product.Vacuum regeneration is used to remove most of the nitrogen which has been adsorbed in order to avoid slow uptake of methane which would saturate the adsorbent and reduce its effectiveness for taking up nitrogen during the subsequent adsorption step.
The experimental unit is shown in Fig.2.The two stainless steel columns were packed with a known amout of CMS (Table 1),which was produced by Changxing Shanli Chemical Material Technology Co.,Ltd.Activation of CMS was done at 423Kovernight under high vacuum conditon.Each column was connected to three pneumatic valves:one for the inlet of feed gas,one for the outlet of product in feed step and the inlet of product gas in repressurization step,and the other for the outlet of absorbed nitrogen in blowdown and vacuumization steps.The pneumatic valve,which was used to protect the experimental apparatus from possible explosion,was driven by pressurized air.The circulation of the pressurized air was controlled by two-way solenoid valve.Sequential switching of solenoid valves was controlled by relays.An electronic mass flow controller (MFC)(±0.3%)was used to controll the feed gas flow.The product and feed stream are analysed by withdrawing gas samples through a manual sampling probe and submitting them to a GC900A chromatograph.The experimental unit was evacuated for 12hours and purged with pure helium before each experiment.The pressure of the unit was controled by a back pressure valve.
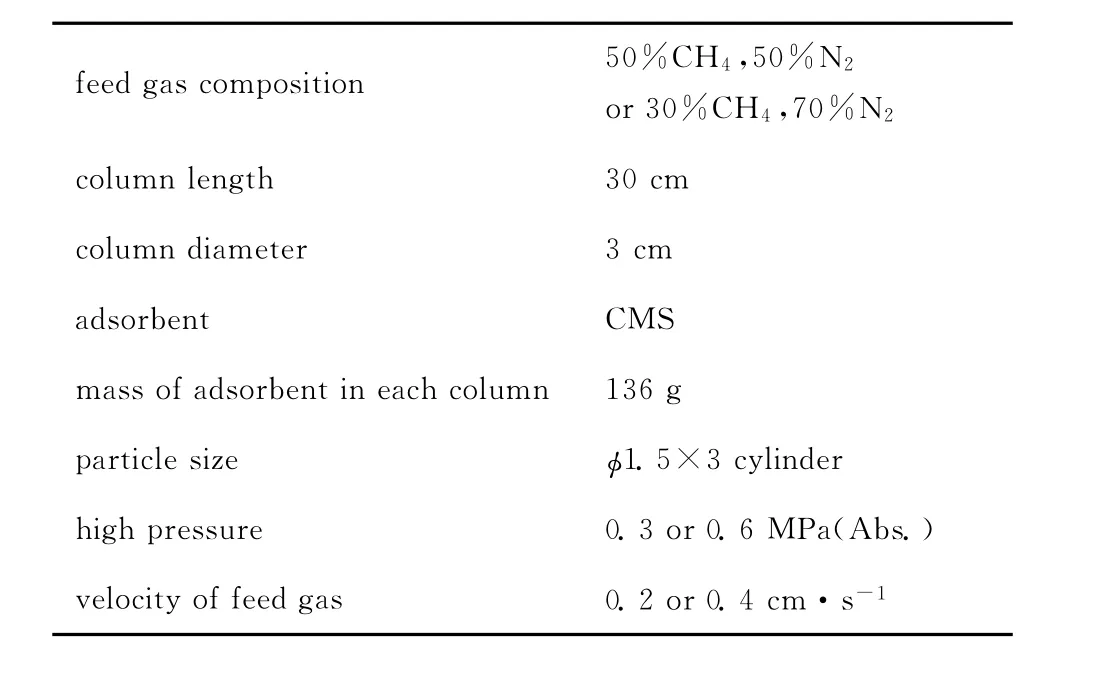
Table 1 Experimental conditions of two-bed PSA unit
2 Results and discussions
It has been proved that velocity of feed gas,adsorption pressure,and cycle time can affect the performance of a PSA unit[10].Our recent study[9]showed that the greater the velocity of feed gas was,the lower the methane purity was in product while the velocity was between 0.2—1.0cm·s-1.In this two-bed PSA research,velocities of 0.2 and 0.4cm·s-1were chosen according to the single-bed experiments.The adsorption pressures studied were 0.2and 0.5MPa.
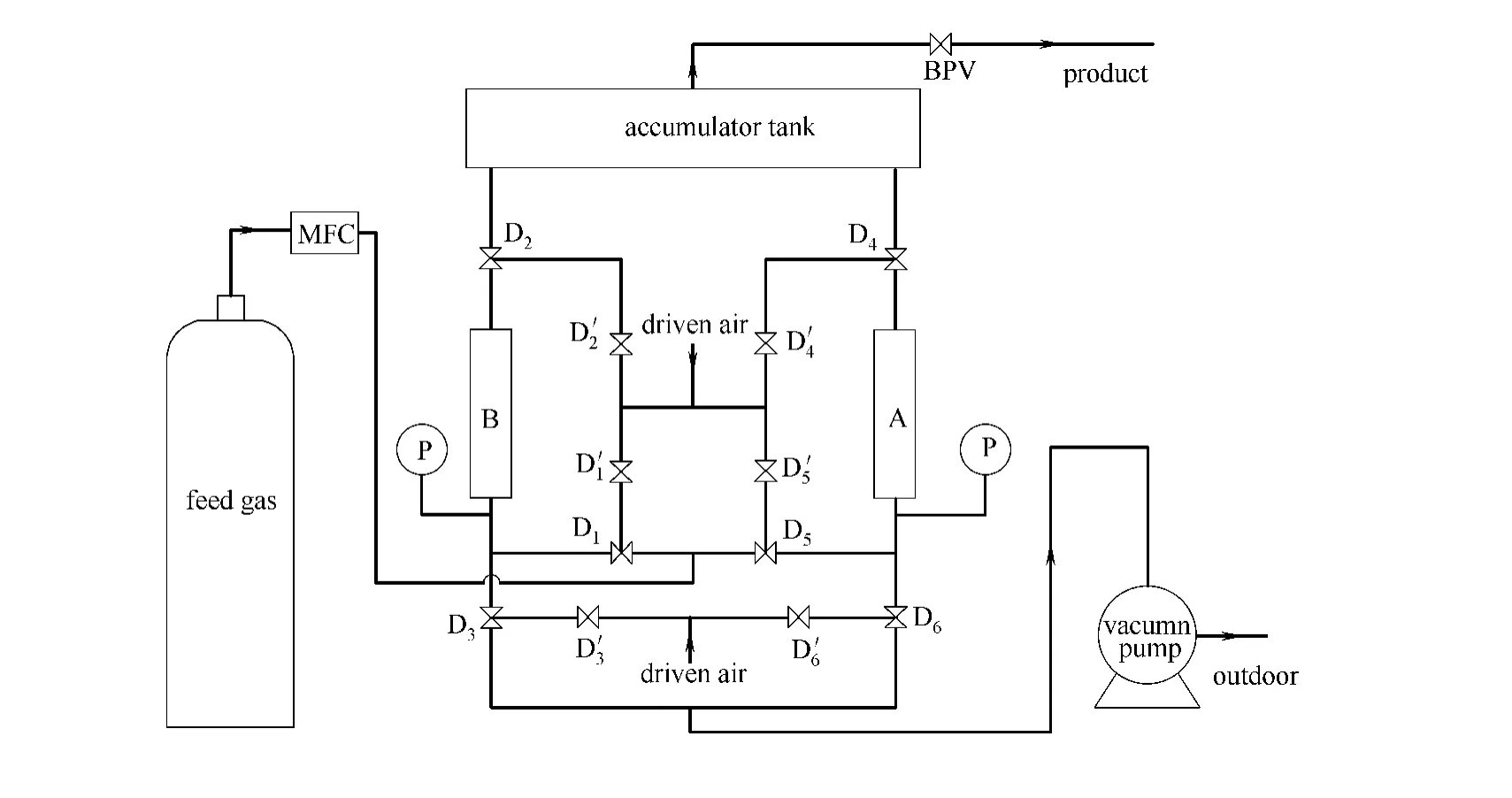
Fig.2 Schematic of two-bed PSA experimental unitD1,D2,D3,D4,D5,D6—solenoid valve;D′1,D′2,D′3,D′4,D′5,D′6—pneumatic valve;MFC—mass flow controller;A,B—column;BPV—back pressure valve;P—pressure gauge
The unit was purged and pressurized by helium at the beginning of experiments,so there are two kinds of curves in the methane concentration plots as showed in Fig.3.The solid ones were the methane concentration in methane and nitrogen binary mixture (C2)in buffer tank,manually eliminated helium.This profile represented the separation effect of the CMS directly.The hollow ones were the methane concentration in methane,nitrogen and helium ternary mixture(C3),which were the true methane concentration of the product in buffer tank,representing the productivity of adsorbent.In these figures,twas the adsorption time of a single bed.All the concentration points correspond to those measured 2sprior to the end of each cycle.
The results show that the methane concentration is enriched to a certain extent under each condition.Whilevis 0.2cm·s-1,C2is up to 90%under all experimental conditions,and the highest one is 95.45%.But it is different whilevis 0.4cm·s-1.C2is up to 90%only when the methane concentration in feed gas (C1)is 50%,and the highest one is 94.87% .Table 2is the summary of experiments.
It has to be noted that the reason of the slow increase ofC3is the much greater volume of buffer tank compared with the volume of column,in order to make sure that the pressure decline of the system after repressurization is small enough.It is interesting thatC2is 100%at the beginning of the adsorption.In fact,the nitrogen extent is too small to be analyzed though GC900A.In the enlarged profiles from the chromatograph,there is a chromatographic peak of nitrogen.
It can be seen from the results that cycle time has a great influence on methane purity.Methane purity in the product is higher while the cycle time is shorter no matter what other experiment conditions are.The influences of adsorption pressure and feed gas velocity are the same as that in the single-bed study:the increases of both parameters have negative effects on the methane purity.As can be seen from the profiles,while the methane concentration in feed gas is 30%,the accumulation of methane in buffer tank is slower than that when methane concentration is 50%.
3 Conclusions
Experimental PSA studies were performed with two methane-nitrogen feeds (50%and 30%methane in nitrogen)using a two-bed unit packed with a selected CMS.A four-step PSA cycle was used.The exit methane concentration ranged from 95.45%to 88.04%for the 50%feed and from 94.89%to 79.53%for the 30%feed.
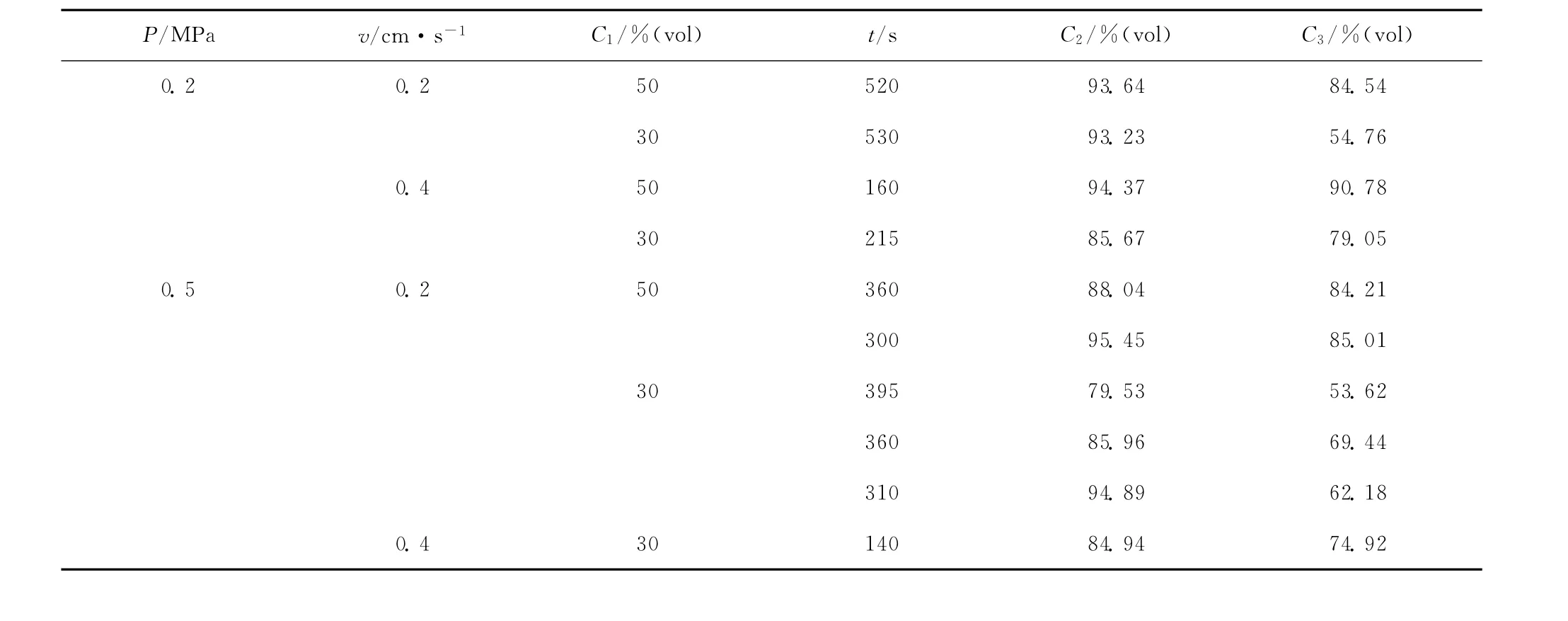
Table 2 Summary of two-bed PSA experiments with CMS (T=300K)
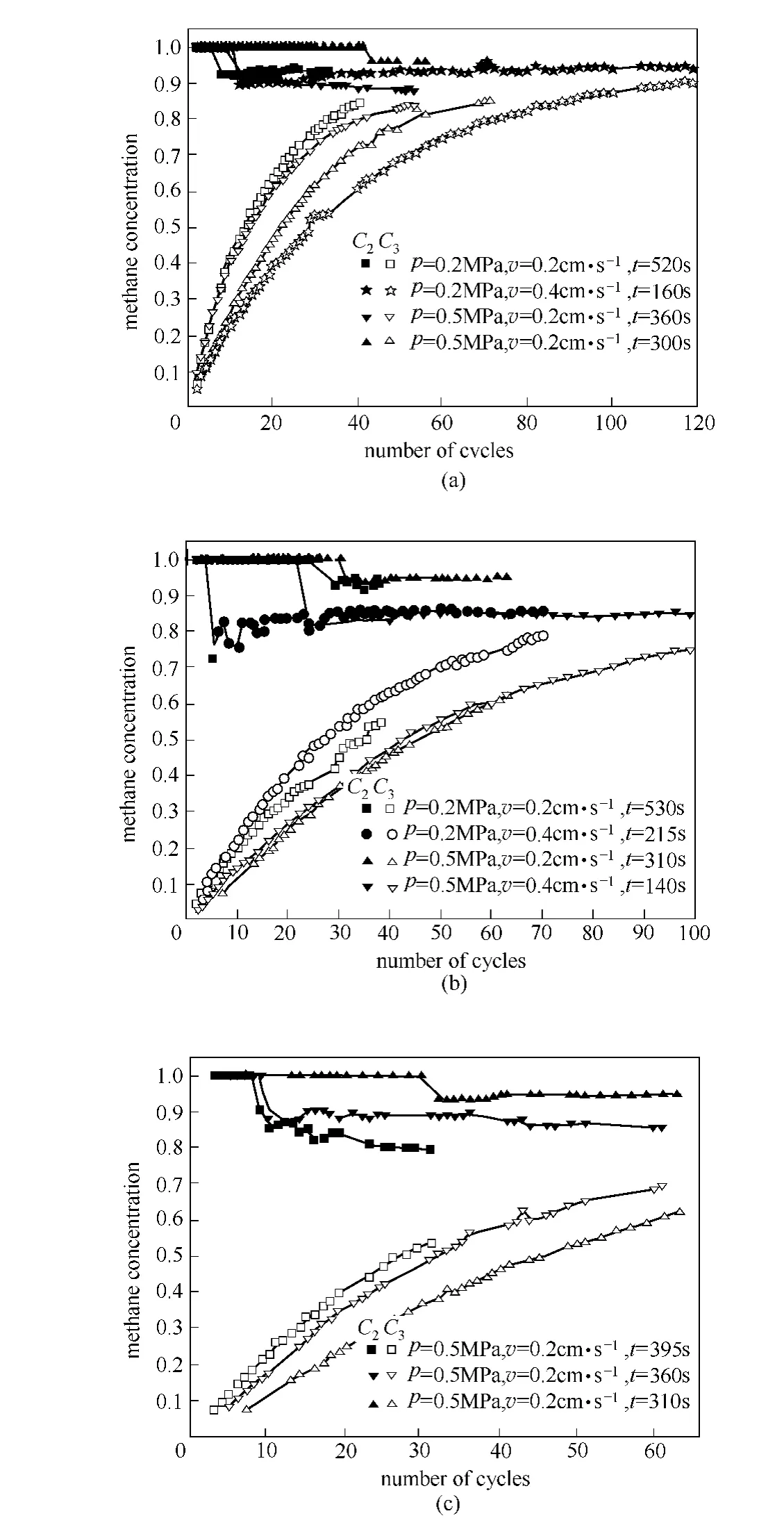
Fig.3 Methane concentration profiles while methane concentration was 50% (a)and 30% (b,c)in feed gas
The cycle time,feed gas velocity and adsorption pressure could affect the methane purity,and the methane concentration in feed gas significantly influences the accumulation speed of methane in buffer tank.It is easier to achieve methane purity above 90%at low pressure and low velocity.And cycle time has a negative effect on methane purity.
The study shows that it is possible to enrich methane concentration from a relatively low level to a commercially acceptable one,implying potential application in unconventional natural gas utilization practices.
[1] Nie Lihong(聂李红),Xu Shaoping(徐绍平),Su Yanmin(苏艳敏),Liu Shuqin (刘淑琴).Progress of recovery of low concentration coal bed methane [J].ChemicalIndustryand EngineeringProgress(化 工 进 展 ),2008,27 (10):1505-1511.
[2] Lin W S,Gu M,Gu A Z,Lu X S,Cao W S.Analysis of coal bed methane enrichment and liquefaction processes in China//Proceedings of the 15th International Conference &Exhibition on Liquefied Natural Gas[C].Barcelona,Spain:GTI/IGU/IIR,2007.
[3] Yu M,Primera-Pedrozo J N,Marcano-González M E,Hernández-Maldonado A J.Selective adsorption of N2over CH4in flexible Sr2+-and Ba2+-UPRM-5 (TEA)titanium silicates:effect of activation temperature [J].Chemical EngineeringJournal,2014,252:311-319.
[4] Sun Q,Wang M,Li Z,Li P,Wang W H,Tan X J,Du A J.Nitrogen removal from natural gas using solid boron:a first-principles computational study [J].Fuel,2013,109:575-581.
[5] Delgado J A,Uguina M A,Sotelo J L,Águeda V I,Gómez P.Numerical simulation of a three-bed PSA cycle for the methane/nitrogen separation with silicalite [J] .SeparationandPurificationTechnology,2011,77 (1):7-17.
[6] Ackley M W,Yang R T.Kinetic separation by pressure swing adsorption:method of characteristics model [J].AIChEJournal,1990,36 (8):1229-1238.
[7] Guo Pu (郭 璞),Li Ming (李 明).Research progress of separation of CH4/N2in coal-bed methane [J].Chemical IndustryandEngineeringProgress(化工进展),2008,27(7):963-967.
[8] Xi Fang(席芳),Lin Wensheng (林文胜),Gu Anzhong(顾 安 忠 ).Separation of methane/nitrogen mixture by pressure swing adsorption on carbon molecular sieve [J].JournaloftheChinaCoalSociety(煤炭学报),2011,36(6):1032-1035.
[9] Xi Fang(席芳),Lin Wensheng (林文胜),Gu Anzhong(顾 安 忠 ),Liu Wei(刘 薇 ),Qi Yanke (齐 研 科 ).Adsorption separation of coal-bed methane on activated carbon and carbon molecular sieve [J].CIESCJournal(化工学报),2010,61 (S2):54-57.
[10] Fatehi A.Separation of methane nitrogen mixtures by pressure swing adsorption using a carbon molecular sieve [J].GasSeparation&Purification,1995,31 (9):199-204.

