Effects of curcumin on sodium currents of dorsal root ganglion neurons in type 2 diabetic neuropathic pain rats
Bo MENG, Lu-lu SHEN, Xiao-ting SHI, Yong-sheng GONG, Xiao-fang FAN, Jun LI, Hong CAO
1. Department of Anesthesiology, the 2nd Affiliated Hospital of Wenzhou Medical University, Wenzhou 325027, China;
2. Teaching Center of Medical Functional Experiment, Wenzhou Medical University, Wenzhou 325035, China
Effects of curcumin on sodium currents of dorsal root ganglion neurons in type 2 diabetic neuropathic pain rats
Bo MENG1, Lu-lu SHEN1, Xiao-ting SHI1, Yong-sheng GONG2, Xiao-fang FAN2, Jun LI1, Hong CAO1
1. Department of Anesthesiology, the 2nd Affiliated Hospital of Wenzhou Medical University, Wenzhou 325027, China;
2. Teaching Center of Medical Functional Experiment, Wenzhou Medical University, Wenzhou 325035, China
doi 10.13459/j.cnki.cjap.2015.06.009
Along with the development of economy and society, type 2 diabec mellitus (T2DM) has become one of the most common diseases at the global level. As one of the complications of T2DM, diabetic neuropathic pain (DNP) stubbornly and chronically af f ects the health and life of human beings. In the pain field, dorsal root ganglion (DRG) is generally considered as the first stage of the sensory pathway where the hyperexcitability of injured neurons is associated with dif f erent kinds of peripheral neuropathic pains. The abnormal electrophysiology is mainly due to the changed properes of voltage-gated sodium channels (VGSCs) and the increased sodium currents (INa). Curcumin is an acve ingredient extracted from turmeric and has been demonstrated to ameliorate T2DM and its various complicaons including DNP ef f ecvely. The present study demonstrates that the INaof small-sized DRG neurons are signif i cantly increased with the abnormal electrophysiological characteriscs of VGSCs in type 2 diabec neuropathic pain rats. And these abnormalies can be ameliorated efficaciously by a period of treatment with curcumin.
type 2 diabetic mellitus; diabetic neuropathic pain; dorsal root ganglion; sodium currents; curcumin
Introduction
A multicountry, joint research project showed that the age-standardized prevalence of adult diabetes was 9.8% in men and 9.2% in women in 2008, up from 8.3% and 7.5%, respectively, in 1980, and, the number of diabetic patients increased from 153 million in 1980 to 347 million in 2008 [1]. Type 2 diabetic mellitus (T2DM) is the predominant type accounting for at least 90% diabetics. As a serious and chronic metabolic disease, T2DM could predispose someone to multiple-organ/system dysfunction. Diabetic neuropathic pain (DNP), a kind of peripheral neuropathic pain, could af f ect the health and life of human beings chronically and stubbornly [2]. Dorsal root ganglion(DRG) is normally considered as the first stage of the sensory pathway, whose injured neurons display increased frequency of action potential generation and increased spontaneous activities, both of which are mainly due to the changed electrophysiological properties of voltage-gated sodium channels (VGSCs) and the increased sodium currents (INa) [3]. VGSCs are widely distributed in excitable cells and play a crucial role in control of membrane excitability and action potential. Based on the dif f erential sensitivity to tetrodotoxin (TTX), VGSCs can be classif i ed into TTX-sensitive (TTX-S) and TTX-resistant (TTX-R) isoforms.e majority of nociceptive DRG neurons are in small size mainly accepting Aδ-f i bers and C-f ibers and expressing TTX-R isoforms among which two TTX-R isoforms, Nav1.8 and Nav1.9, are tightly related to neuropathic pain [4]. Moreover, it had been reported that DNP was associated with the abnormal expression of VGSCs and the increased INain diabetic rats [5,6]. However, in the above DNP studies, the diabetic rats were induced only by streptozocin (STZ) injection which would not exactly ref f ect the pathophysiology of T2DM [4,5,6]. In the present study, wewould establish T2DM rat model to study DNP and its pathogenesis.
Curcumin is one of the most important phytochemicals from turmeric which derives from the rhizome of the plantCurcuma longahas been used medically in India for centuries. So far, a lot of researches and studies have focused considerable interest on curcumin due to its efficacy in treating numerous disorders including diabetes mellitus and its complications [7,8,9]. The purpose of the current research was to reveal curcumin’s therapeutic mechanisms by investigating its effects on pain threshold and INaof DRG neurons in type 2 diabetic neuropathic pain rats.
Materials and methods
Animals and reagents
All experiments for this study were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University and abided by the guidelines of the International Association for the Study of Pain. Eighty-f i ve male Sprague Dawley (SD) rats (160-180 g) were provided by the animal center of Wenzhou Medical University. The rats were housed four to a cage at a temperature of 21-24 °C and maintained a 12-hour light/dark cycle withad libitumaccess to food and water.
The STZ was dispersed into a 1% stock solution with a citric acid salt buf f er at pH 4.3. Curcumin was dispersed into a 25 mg/ml stock solution with 1% corn oil and stored at 4 °C away from light. Collagenase (type I) and trypsin (type I) were dissolved in dulbecco’s minimum essential medium (DMEM, without phenol red), and dispersed into 0.1% and 0.3% enzyme solutions, respectively, and subsequently stored at -20 °C. All the above reagents were purchased from Sigma Chemical Co. St. Louis, MO, USA.
Induction of type 2 diabetic neuropathic pain
Based on the method reported by Srinivasan et al. [10], T2DM rat model was induced as follows modifi ed in some respects by our laboratory [11]. Eightyfive SD rats were randomized into group N (n=20) and group M (n=65) aer two weeks of adaptive feeding. Meanwhile, the basic values of blood glucose, serum insulin and pain threshold were measured. Aer that group M rats were fed on a high fat-fructose diet (HFD) for eight weeks while group N rats were fed with normal diet . After all of the above ten weeks feeding, the blood samples were collected from the tail vein of the two groups rats for measurement of serum insulin by ELISA. And, insulin sensitivity index (ISI) was calculated using the formula of ISI=1/ (blood glucose × serum insulin). For statistical analysis, the natural logarithm of ISI (ln(ISI)) should be performed since ISI is a non-normal distribution [12]. If the difference of ln(ISI) was significant between group N and group M (P<0.05), a single low dose of 35 mg/kg STZ would be injected intraperitoneally; if not, the rats would be kept on the feeding regime for one week. Aer three days of STZ injection, if the blood glucose levels were 16.7 mmol/l or higher, the T2DM rat model was induced successfully. Aer two weeks, if the pain threshold of the T2DM rats was downgraded below 85% basic value, these rats would be considered as successfully developing into type 2 diabetic neuropathic pain.
Groups
Pain threshold measurement
Mechanical withdrawal threshold (MWT)
MWT was measured using the Model 2390 Electronic von Frey Anesthesiometer (IITC/Life Science Instruments, Chicago, IL, USA). Rats were placed individually into wire mesh-bottom cages and acclimated for 20 minutes before testing. A 0.5-mm diameter von Frey probe was manually applied to the plantar surface of the bilateral hind feet with the force increasing. When feet retraction responses, such as licking and/or shaking the paw were observed, the minimum pressure was recorded as MWT.
Thermal withdrawal latency (TWL)
The Model 33 Analgesia Meter (IITC/Life Science Instruments, Chicago, IL, USA) was used to measure TWL. Rats were placed in a plexiglas chamber located on an elevated glass floor that was maintained cleanand dry during the testing.e rats were given 10 min to habituate. The parameter settings were as follows: the heat emission intensity of the laser head was 20% at idle state and 60% when heating the plantar surface of the bilateral hind paw; the cut-of f time was 25 s, and the triggering temperature was 30 °C.
Acute isolation of DRG neurons
At the above three time points, aer pain threshold measurement, the rats were deeply anesthetized with 4% chloral hydrate and L4-L6 DRGs of both sides were acutely isolated from spinal column.e DRGs were provisionally placed in an ice cold artif i cial cerebrospinal fluid (ACSF) incubated with 95% air + 5% CO2. Aer pruning the conjoint nerve fi bers and connective tissue, the DRGs were cut into pieces and moved into 0.1% collagenase digesting for 50 minutes, and then 0.3% trypsin digesting for 10 minutes, during which 95% air + 5% CO2was kept infused and the temperature was maintained at 37 °C [13]. Aer the total 60 minutes digesting, the DRG fragments were removed and rinsed three times with ACSF to stop the enzymatic digestion, then dispersed into cell suspension with special glass pipettes. Before electrophysiological recording, 3-5 drops of the cell suspension were dropped on an acid-cleaned glass coverslip standing for 20 minutes to adherence.
Whole cell patch clamp recording
Solutions preparation
ACSF (in mmol/L): 124 NaCl, 5 KCl, 10 HEPES, 2 MgSO4, 3 CaCl2, 23 NaHCO3, 3 NaH2PO4, 10 Glucose; pH was adjusted to 7.2-7.4 by NaOH [13].
Bath solution (in mmol/L): 80 NaCl, 50 Choline-Cl, 30 TEA-Cl, 2 CaCl2, 0.2 CdCl2, 10 HEPES, 10 Glucose; pH was adjusted to 7.4 by NaOH [6].
Pipette solution (in mmol/L): 70 CsCl, 34 NaCl, 30 tetraethylammonium-Cl (TEA-Cl), 10 EGTA, 1 CaCl2, 2 Mg-ATP, 0.05 GTP, 10 HEPES, 10 Glucose; pH was adjusted to 7.2 by CsOH [5].
Electrodes preparation and cell sealing
Patch electrodes were pulled from borosilicate glass pipettes (BF 150-86-10, Sutter Instruments, Novato, CA, USA) with horizontal micropipette puller (Model P-97, Sutter Instruments, Novato, CA, USA).e micropipette shank should be short, and the radius should be large to reduce the electrode resistance. Pipette solutions were fi ltered into electrodes for 1/3 total length.e electrode resistance was kept in the range of 2-4 MΩ while the micropipette touched the bath solution surface.
Small-sized neurons (diameter ≤ 25 μm) with smooth and intact cell membranes were chosen for INarecording. The pipette was moved slightly to bath solution at 45° by directional thrusters (MP-285, Sutter Instruments, Novato, CA, USA), and the junction potential was corrected while the pipette contacted the liquid surface. Keep the pipette moving to the selected neuron and the seal current would decrease suddenly while it touched the cell membrane. Negative pressure was then exerted into the pipette to form gigaohm seal (> 1 GΩ), subsequently, the pipette capacitance was compensated. The cell membrane was broken through by a little negative pressure or combined with ZAP function, finally the whole cell patch clamp was established aer the membrane capacitance was compensated.e series resistance should be also compensated for recording INa(Prediction ≥ 80%).
Sodium currents recording
Statistical analysis
Results
Induction of insulin resistance
Insulin resistance, as a key pathological feature of T2DM, could be well reflected by ISI value. After eight weeks of HFD feeding, compared with group N, the blood glucose levels of group M increased (P<0.05), which, however, was not high enough to meet the diagnostic criteria of T2DM (≥ 16.7 mmol/l). Additionally, combined with the increased plasma insulin levels of group M (P<0.05), the dif f erence of ln(ISI) between group M and group N was also signif i cant (P<0.05, Tab.1), which indicated that insulin resistance was successfully induced in group M rats. Subsequently, group M rats were intraperitoneally injected with 35 mg/kg STZ to induce T2DM.
Ef f ects of curcumin on pain threshold
After two weeks of STZ injection, about 85% group M rats were successfully induced into type 2 diabetic neuropathic pain. Mechanical allodynia and thermal hyperalgesia were separately determined by measuring MWT and TWL. At Day 3 of curcumin treatment, the pain of group DCur rats was not effectively relieved, and there were no significant differences in both of MWT and TWL compared with group DNP. However, after 7 days’ treatment, the pain of group DCur rats showed a significant remission compared with group DNP and group DSC, and this effectiveness was still obvious at Day 14 of curcumin treatment (Fig.1).
The basic electrophysiological properties of DRG neurons
Whole cell capacitance (Cm) was recorded from 322 DRG neurons, the mean value of which was 14.7 ± 2.9 pF, which further suggested that the chosen DRG neurons were small-sized. Additionally, there was no signif i cant dif f erence in Cm among dif f erent groups pointing that the sizes of chosen DRG neurons of each group had a high homogenicity.
From the above 322 DRG neurons, 281 neurons were successfully recorded resting membrane potential (RMP). Aer 3 days’ treatment with curcumin, the difference of RMP between normal control rats and type 2 diabetic neuropathic pain rats was significant (P<0.05) while there was no significant dif f erence between group DNP and group DCur. But after 7 days of treatment, the RMP of group DCur (-56.9±6.6 mV) was higher than that of group DNP (-49.8±5.3 mV,P<0.05). And, this therapeutic ef f ect was continued to Day 14 when the RMP of group DCur was -57.8±5.1 mV while group DNP -47.9±6.7 mV (P<0.05).
Ef f ects of curcumin on sodium currents
Be universally known, inward sodium currents were the key factor for action potential production whose abnormality was the basic electrophysiological mechanism of neuropathic pain. In the present study, INawere successfully recorded from 229 DRG neurons.e typical INatraces of each group between 5 ms and 35 ms of the total 40 ms wave width were shown in Figure 2. The peak current density was used as the statistical indicator for analyzing the amplitude of INawhich was the calculation of the peak current (pA) divided by the corresponding Cm (pF). The peak current was the one elicited by depolarizing neurons to -10 mV, around where the amplitude of INaof most of the neurons reached a maximum.
At Day 3 of treatment, the peak current density of the type 2 diabetic neuropathic pain rats was signif i cantly higher than that of normal rats (P<0.05), the values of which in each group were as follows: group Con 69.0±7.0 pA/pF, group DNP 124.9±8.6 pA/pF, group DCur 118.8±8.0 pA/pF, and group DSC 122.0±8.4 pA/pF. At Day 7, there was a significant decrease in group DCur (98.4±9.0 pA/pF) while the other two neuropathic pain groups showed no obvious remission for group DNP 129.4±4.7 pA/ pF and group DSC 125.5±6.7 pA/pF between which there was no significant difference (P=0.374). After 14 days’ treatment with curcumin, compared with group DNP (128.1±6.0 pA/pF), there was still an amelioration in group DCur (85.8±10.0 pA/pF,P<0.05), moreover, which was further lower than that at Day 7 (P<0.05, Fig.3).
In order to investigate the kinetics characteristics of VGSCs, current-voltage (I-V) curves were plotted in which the current was replaced with the current density for statistical comparison (Fig.4), and from which it could be found that the maximal peak current of each group was elicited around -10 mV, which was why we chose the peak current density at -10 mV for the above analysis. As shown in Figure 4, compared with control rats, the I-V curves of all the neuropathic pain rats shied down signif i cantly with the activation threshold of VGSCs slightly decreased. However, after a period of curcumin treatment, at Day 7 and Day 14, the mean I-V curve of group DCur showed an obvious upgrade compared with groups DNP and DSC, but was still below group Con.

Tab. 1 Comparison of blood glucose, insulin levels, and ln (ISI) .

Fig. 1 Effects of curcumin on pain threshold. At Day 7 and Day 14 of curcumin treatment, compared with group DNP, the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) of group DCur both significantly increased. Con: Normal rats without any treatment; DNP: Model rats without any treatment; DCur: Model rats with an intraperitoneal injection of curcumin 100 mg/kg/d for 14 days; DSC: Model rats with an intraperitoneal injuection of corn oil 4 ml/kg/d for 14 days. (*P< 0.05).
Discussion
Type 2 diabetic neuropathic pain rat model was established in the present study for investigating the pathogenesis of DNP and the ef f ects of curcumin on DNP on electrophysiology ground.e above results of the experiments showed that the INaof small-sized DRG neurons signif i cantly increased, with less negative RMP values of neurons and decreased activation threshold of VGSCs in type 2 diabetic neuropathic pain rats; furthermore, all these abnormalities could be ameliorated by an over 7-day curcumin treatment period resulting in the remission of hyperalgesia. During the whole experimental process, there was no signif i cant dif f erence between group DNP and group DSC in any observed index.
According to an epidemiology study, the estimated worldwide prevalence of diabetic patients was 285 million and this value was predicted to rise to around 439 million by 2030 [14]. In most of the previous studies, the animal models of diabetes and its complications were induced only by STZ injection [4,5,6,8,9], which could not exactly simulate the clinical features of T2DM. In the present study, type 2 diabetic neuropathic pain rats were induced by the combination of 8-week HFD feeding and a single low dose of STZ injection, which separately modeled insulin resistance process and islet β-cell destruction process exactly reflecting the pathophysiology of T2DM.
As one kind of peripheral neuropathic pain, DNP manifests typical chronic neuralgia symptoms including hyperalgesia, autotomy, allodynia, and so on, which severely influenced diabetic patients’ life. A lot of studies had demonstrated that, in the injured primary afferent neurons, such as DRG neurons, a series of electrophysiological processes happened, for instance, membrane potential oscillations, ectopic fi ring, and so on, all of which were tightly related to the abnormal expression of VGSCs and the increased amplitude of INaleading to the lower action potential threshold [3,4]. Most nociceptive DRG neurons are small-sized mainly expressing TTX-R isoforms, compared with large-sized neurons mainly expressing TTX-S isoforms, which are more closely related to different kinds of neuropathic pain including DNP.e increased INaof DRG neurons had been reported by several diabetic and/or pain studies including one our own study [5,6,15]. In the present study, we specif i cally selected small-sized (diameter ≤ 25 μm) DRG neurons to investigate INa, which was further proved by the small value of Cm. Consequently, the results of the present study were similar to what we reported previously [15].
It has been demonstrated that curcumin could ameliorate T2DM and its complications [7,8,9]. Our laboratory has studied the pharmacological characteristics of curcumin for several years and has reported its analgesic effect in chronic constrictive injury rats [16,17]. Based on our previous findings, we divided the curcumin treatment period into three time points (Day 3, Day 7, and Day 14), and used intraperitoneal injection, anin vivoadministration, compared within vitroadministration, which could reduce the instability and toxicity of curcumin and guarantee the administration dose consistency for each group, however, by which the detailed therapeutic mechanisms of curcumin would be more dif-fi cult to be studied. Sharma et al. demonstrated that curcumin could attenuate thermal hyperalgesia of diabetic mice by its inhibitory action on nitric oxide and TNF-α release [18]. TNF-α, as an inflammatory factor, has been reported to regulate VGSCs by acutely modulating p38-MAPK of sensory neurons [19]. Moreover, a number of studies had proved curcumin’s effects on MAPK family proteins, such as p38-MAPK, JNK, ERK1/2 and CREB, which had also been demonstrated by our laboratory [16,20,21]. To sum up, curcumin might regulate VGSCs directly by a certain signaling pathway.
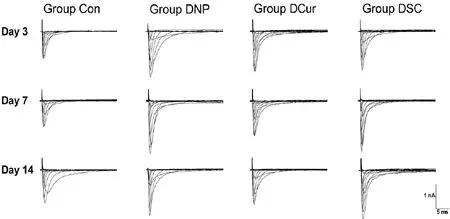
Fig. 2 Typical sodium currents traces of DRG neruns.
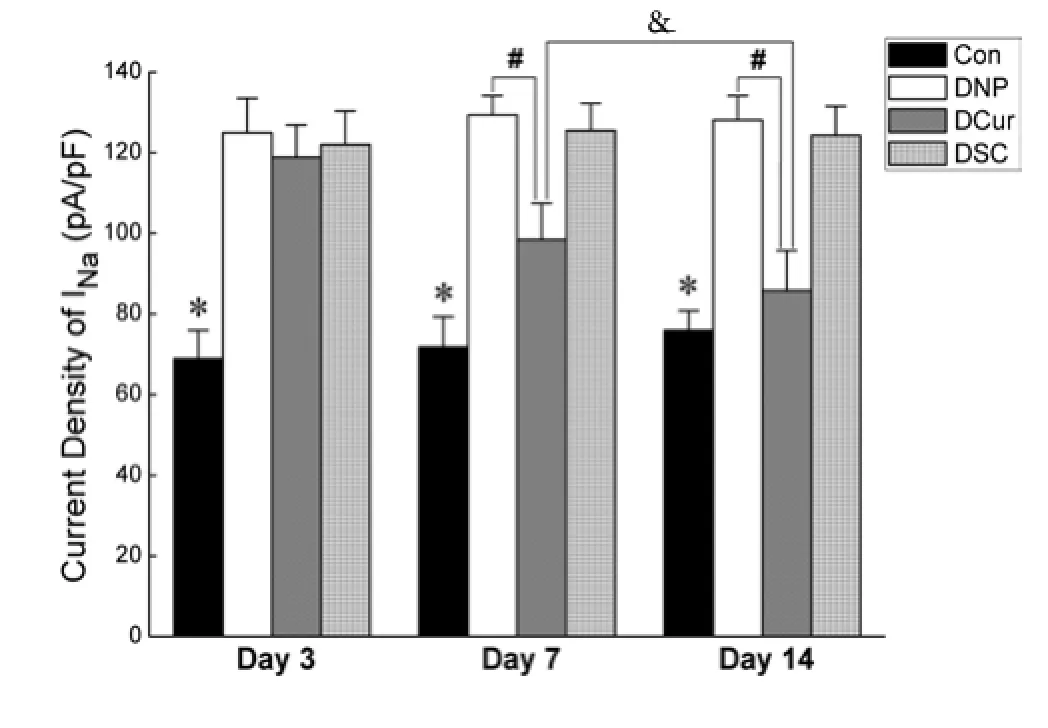
Fig. 3 Comparison of the peak current density.Compared with group Con, the peak current density of the other three diabetic neuropathic pain groups increased significantly (*P<0.05). After curcumin treatment, at Day 7 and Day 14, there was a noticeable decrease in group DCur (#P<0.05). Moreover, compared with these two time-points, the current density of group DCur at Day 14 was further decreased (&P<0.05).
It is well known that oxidative stress and inflammatory reaction play critical roles in the pathogenesis of T2DM, which could be effectively prevented or ameliorated by curucmin’s strong anti-oxidant and anti-inflammatory activities [7]. Moreover, it has been reported that curcumin could efficiently regulate the blood glucose and plasma insulin levels of diabetic animals [7,22,23], and these ef f ects might in turn af f ect neuropathic pain including modulating VGSCs and INaof DRG neurons.erefore, inasmuch as the multitargeted therapeutic features of curcumin, it might regulate VGSCs by an indirect way.
Conclusion
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81073125) and Natural Science Foundation of Zhejiang Province (Y2090252).e authors do not declare any conf f icts of interest relevant to this manuscript.
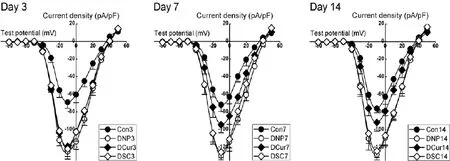
Fig. 4 Current-voltage (I-V) curves of VGSC. Compared with control rats, I-V curves of the diabetic neuropathic pain rats shifted down significantly. At Day 7 and Day 14, compared with groups DNP and DSC, the I-V curves of group DCur shifted up.
1. Danaei G, Finucane MM, Lu Y,et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants[J]. Lancet, 2011, 378(9785): 31-40.
2. Schreiber AK, Nones CF, Reis RC,et al. Diabetic neuropathic pain: Physiopathology and treatment[J]. World J Diabetes, 2015, 6(3): 432-444.
3. Nickel FT, Seifert F, Lanz S,et al. Mechanisms of neuropathic pain[J]. Eur Neuropsychopharmacol, 2012, 22(2): 81-91.
4. Chattopadhyay M, Zhou Z, Hao S,et al. Reduction of voltage gated sodium channel protein in DRG by vector mediated miRNA reduces pain in rats with painful diabetic neuropathy[J]. Mol Pain, 2012, 8: 17.
5. Hong S, Morrow TJ, Paulson PE,et al. Early painful diabetic neuropathy is associated with dif f erential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat[J]. J Biol Chem, 2004, 279(28): 29341-29350.
6. Hong S, Wiley JW. Altered expression and function of sodium channels in large DRG neurons and myelinated A-f i bers in early diabetic neuropathy in the rat[J]. Biochem Biophys Res Commun, 2006, 339(2): 652-660.
7. Meng B, Li J, Cao H. Antioxidant and antiinf f ammatory activities of curcumin on diabetes mellitus and its complications[J]. Curr Pharm Des, 2013, 19(11): 2101-2113.
8. Peeyush Kumar T, Antony S, Soman S,et al. Role of curcumin in the prevention of cholinergic mediated cortical dysfunctions in streptozotocin-induced diabetic rats[J]. Mol Cell Endocrinol, 2011, 331(1): 1-10.
9. Soetikno V, Watanabe K, Sari FR,et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-alpha and PKC-beta1 activity in streptozotocin-induced type I diabetic rats[J]. Mol Nutr Food Res, 2011, 55(11): 1655-1665.
10. Srinivasan K, Viswanad B, Asrat L,et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening[J]. Pharmacol Res, 2005, 52(4): 313-320.
11. Dang JK, Wu Y, Cao H,et al. Establishment of a rat model of type II diabetic neuropathic pain[J]. Pain Med, 2014, 15(4): 637-646.
12. Muniyappa R, Lee S, Chen H,et al. Current approaches for assessing insulin sensitivity and resistancein vivo: advantages, limitations, and appropriate usage[J]. Am J Physiol Endocrinol Metab, 2008, 294(1): E15-26.
13. Tu WZ, Cheng RD, Cheng B,et al. Analgesic effect of electroacupuncture on chronic neuropathic pain mediated by P2X3 receptors in rat dorsal root ganglion neurons[J]. Neurochem Int, 2012, 60(4): 379-386.
14. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and2030[J]. Diabetes Res Clin Pract, 2010, 87(1): 4-14.
15. Meng B, Shen LL, Shi XT,et al. Effects of curcumin on TTX-R sodium currents of dorsal root ganglion neurons in type 2 diabetic rats with diabetic neuropathic pain[J]. Neurosci Lett, 2015, 605: 59-64.
16. Li X, Liu RH, Cao H,et al. Effects of curcumin on behavior and p-ERK, p-CREB, c-fos expression in dorsal root ganglion in chronic constrictive injury rats[J]. Chin J Appl Physiol, 2009, 25(3): 418-422.
17. Zheng J, Zheng C, Cao H,et al. Curcumin down-regulates CX3CR1 expression in spinal cord dorsal horn and DRG in neuropathic pain rats[J]. China J Chin Mater Med, 2011, 36(18): 2552-2556.
18. Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha[J]. Phytother Res, 2007, 21(3): 278-283.
19. Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha[J]. J Neurosci, 2006, 26(1): 246-255.
20. Shi X, Zheng Z, Li J,et al. Curcumin inhibits Abeta-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways[J]. Neurosci Lett, 2015, 594: 105-110.
21. Jeong CW, Yoo KY, Lee SH,et al. Curcumin protects against regional myocardial ischemia/ reperfusion injury through activation of RISK/ GSK-3beta and inhibition of p38 MAPK and JNK[J]. J Cardiovasc Pharmacol Ther, 2012, 17(4): 387-394.
22. Seo KI, Choi MS, Jung UJ,et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice[J]. Mol Nutr Food Res, 2008, 52(9): 995-1004.
23. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R,et al. Curcumin extract for prevention of type 2 diabetes[J]. Diabetes Care, 2012, 35(11): 2121-2127.
Hong CAO, MD, PhD, Professor, Department of Anesthesiology, the 2nd Affiliated Hospital of Wenzhou Medical University, 109 Xuyuanxi Road, Wenzhou 325027, China. Tel: 86-577-86699767; Fax: 86-577-86699767; E-mail: caohongwz@163.com
Received 2015-11-12; accepted 2015-11-20
- 中国应用生理学杂志的其它文章
- Wnt/β-catenin pathway might underlie the MET in transdifferentiation from MSC to MSC-derived neuron
- Impacts of exposure to 900 MHz mobile phone radiation on liver function in rats
- Ultrastructural study on route of gut bacterial translocation in a rat after spinal cord injury
- Establishment and evaluation of a mouse model of bronchial asthma with Yin deficiency syndrome
- Leptin receptor of the hind brain nuclei is involved in the conditioned taste preference of rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats

