Novel potential for optimization of antitubercular therapy:Pulmonary delivery of rifampicin lipospheres
Chrn Singh,L.V.Seshu Kumr Koduri,Arti Singh, Srsij Suresh,1,*
Novel potential for optimization of antitubercular therapy:Pulmonary delivery of rifampicin lipospheres
Charan Singha,L.V.Seshu Kumar Koduria,Arti Singhb, Sarasija Suresha,1,*
aDepartment of Pharmaceutical Technology(Formulations),National Institute of Pharmaceutical Education and Research(NIPER),Sector 67,S.A.S.Nagar(Mohali),Punjab 160062,India
bPharmacology Division,University Institute of Pharmaceutical Sciences,Panjab University,Chandigarh
160014,India
ARTICLE INFO
Article history:
Received 14 April 2015
Received in revised form 31 July 2015
Accepted 2 August 2015
Available online 24 August 2015
Rifampicin
Phospholipid
Cyclodextrin
Vitamin C
Inhalation
Lipospheres
The aim of the present work is to develop rifampicin loaded phospholipid lipospheres containing sulfphobutyl ether β-cyclodextrin andVitamin C for inhalation to test their potential for deep lung delivery.The fi ndings of the solid state characterization revealed the amorphous nature of the lipospheres.These exhibited a better fl owability,an aerodynamic diameter in the range of 1.76 to 3.99 μm.Moreover,the fi ne particle fraction and emitted dose was found in the range of 68.84-83.73%and 80-93%,respectively.Moreover,lipospheres exhibited enhanced/equivalent ef fi cacy in vitro in H37Rv strain.Hence,the results show the potential of lipospheres for pulmonary delivery of rifampicin.
©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Tuberculosis(TB)is a chronic infectious disease caused by Mycobacterium tuberculosis(MTB).According to World Health Organization(WHO),1.5 million people died from TB,including 360,000 among people who were HIV-positive(WHO Global Tuberculosis Report 2014)[1].A major paradigm shift in the treatment ofTB occurred with the introduction of rifampicin(RMP), the last landmark drug introduced forTB treatment[2].RMP is the semi-synthetic hydrazine derivative of rifampicin B[3].In addition to bactericidal effect on MTB,it exhibits excellent late Original Research Papersterilizing action on semi-dormant organisms undergoing short bursts of metabolic activity.However,some of the challenges that this potent antibiotic faces include poor water solubility, low oral bioavailability,relatively short biological half-life(1.5-5 h)[3],induction of a prehepatic fi rst-pass metabolism, hepatotoxicity and adverse effects due to multiple doses[4]. Hence,conventional oral treatment of TB is not only longterm[5]but also associated with disadvantages of side effects and systemic toxic effects due to high doses[6].Vitamin C(Ascorbic acid)(AA)is a water soluble vitamin which is a known antioxidant[7,8]and free radical scavenger[9].AA has been reported to modulate RMP-induced hepatotoxicity in vivo[10]and of other drug molecules as well[11,12].Moreover,due to the presence of high iron concentration,reactive oxygen species production and DNA damage,AA has sterilizing action on MTB in vitro[13].Cyclodextrins(CDs)are macrocyclic oligosaccharides composed of(α-1,4)-linked α-L-glucopyranose units[14-16] with a hollow hydrophobic interior and a hydrophilic outer surface.Application of CD in pharmaceutical,food and cosmetic industry is extensive because of its inexpensive and nontoxic nature.In pharmaceutical industry,they have been employed to enhance the drug aqueous solubility and thereby enhance the oral bioavailability of the encapsulated drug[17-21]. However,natural CDs are associated with problems of limited solubility and toxicity related issues.To address this problem, alkyl moieties such as hydroxyalkyl or methyl on free hydroxyl groups of CD were introduced[15,22].Sulphobutyl ether β-cyclodextrin(SBE-β-CD)is a chemically modi fi ed β-CD,cyclic hydrophilic oligosaccharide.Also,the solubility in water for SBE-β-CD is signi fi cantly higher than the parent β-CD.Furthermore, SBE-β-CD does not exhibit the nephrotoxicity associated with β-cyclodextrin[8].Moreover,no cytotoxic effects of SBE-β-CD have been reported[23].
Pulmonary delivery via inhalation is a common technique of drug administration to patients with a variety of lung diseases[24,25].This is the favoured route of administration of drugs over both parenteral and oral route of drug administration.The principal advantages include reduced systemic side effects and higher dose levels of the applicable medication at the site of drug action.Unlike the oral route of drug administration,pulmonary inhalation is not subject to fi rst-pass metabolism.Administration of drugs to the lungs via the inhaled route offers rapid onset of action,high local concentration by direct delivery to the airways and hence high therapeutic ratio[26,27].However,the airway geometry of the lung poses a challenge for delivery into the alveoli.For effective delivery deep into the lung,the particle size should be between 1 and 5 μm[28],with mass median aerodynamic diameter (MMAD)and geometric standard deviation(GSD)of aerosolized particles.Particles above 5 μm are likely to be deposited in the upper respiratory tract while particles below 1 μm could be exhaled during expiration[29].
Therefore,the aim of this study was to evaluate RMP-loaded dry powder formulation containing sulphobutylether β-cyclodextrin(SDRPL-CD),Vitamin C(SDRPL-AA)and combination of both SBECD and AA(SDRPL-Comb).RMP has previously shown interesting potential for treating pulmonary TB infection via inhalation[4,30,31]and was chosen in this report as a model molecule.The reports are not available for inhaled phospholipid based lipospheres of RMP containing sulphobutylether β-cyclodextrin andVitamin C along with the in vitro antimycobacterial ef fi cacy and aerosol performance studies.
2. Materials and methods
2.1. Materials
RMP was obtained as a kind gift by Sandoz,Mumbai.The phospholipid(Lipoid S-75)sample was kindly provided by Lipoid Ludwigshafen,Germany.HPLC grade methanol was purchased from Fisher Scienti fi c,UK.Dichloromethane and other chemicals were obtained from Loba Chemie,Mumbai,India. Vitamin C was purchased from HiMedia,Mumbai.SBECD was obtained as a kind gift sample from Cydex Pharmaceuticals USA.All other chemicals were of analytical grade.
2.2. Methods
2.2.1. Preparation of spray dry powders
The spray drying open cycle system process was performed using the BUCHI Mini Spray Dryer B-191 attached with high-performance cyclone(BUCHI Labortechnik G,Flawil,Switzerland).Co-spray dried particles(SDRPLs)were obtained by spray drying organic solvent(dichloromethane)and aqueous solutions RMP,PL and SBECD and AA,respectively.The feed solution(3.5%w/w)was passed through a stainless steel 0.7 mm diameter atomizing nozzle via a peristaltic pump at a fl ow rate of 4 ml/min(pump rate 12%)employing atmospheric gas for drying at a fl ow rate between 30 and 40 kg/h.A set inlet temperature of 80°C±2°C(primary drying step)resulted in outlet of 55°C±3°C(secondary drying step)with an aspirator rate of 90%.The resultant dry powder particles were blown through a high-performance cyclone separator and collected in the sample container.Dry powder formulation was stored in glass vials sealed with para fi lm at room temperature.
2.2.2. Fourier transform infra red spectrometry
Fourier transform infra red spectrometry(FTIR)spectra of samples were recorded with a FTIR spectrometer(Nicolet, Impact 410,USA)employed with a denudated triglycine sulphate detector.The spectra were scanned in the region 450-4000 cm-1derived from 11 single average scans.Potassium bromide pellet method was employed for the analysis.The data were collected and analyzed using Omnic 5.1a software(Thermo Nicolet,USA).These are similar conditions to those previously reported[5].
2.2.3. Thermal analysis
Thermograms were obtained on a Mettler Stare System(Mettler Toledo,DSC 821e,Switzerland)using similar conditions previously reported in the literature[5].Approximately 2-8 mg of powder was carefully weighed into hermetic anodized aluminium DSC pans.An empty,hermetically sealed,anodized aluminium pan was used as reference.Samples were heated at a rate of 20°C/min over a temperature range of 25°C to 300°C.Nitrogen gas at purge rate of 100 ml/min was used as the purging gas.
2.2.4. Crystallinity determination
The crystalline nature and non-crystallinity of samples were examined using X-ray powder diffraction(XRPD)(Bruker axs System,D8,Germany)with a slit detector Cu Kα radiation(35 kV, 20 mA)source.The scan range was 3 to 40°2θ with a scan rate of 10°/min at room temperature in a continuous scan mode. The samples were placed on a horizontal quartz glass sample holder plate.
2.2.5. Hot stage microscopy under cross polarizer
Hot stage microscopy(HSM)studies were performed under Leica DMPL polarized microscope(Leica MicrosystemsWetzlar GmbH, Wetzlar Germany)equipped with Linkam LTS 350 hot stage. Photomicrographs were captured using JVS colour video camera and analyzed using Linksys32 software.The powder samples were placed on glass slides with cover glass and heated at the rate of 10°C/min from 25°C to 250°C.
2.2.6. Morphology evaluation
Scanning electron microscopy(SEM)of the samples was evaluated using SEM,equipped with a Hitachi S-3400 microscope (Hitachi Ltd,Tokyo,Japan).Samples were placed on double sided adhesive carbon tabs(Ted Pella,Inc.,Redding,CA,USA),which were adhered to aluminium stubs(Ted Pella,Inc.),and were coated with gold thin fi lm using a HummerVI sputtering system from Hitachi.The coating process was operated at 25 mA discharge current for 20 s.
2.2.7. Drug content and entrapment ef fi ciency
RMP content in the spray dried powders was determined using LCMS(Thermo Scienti fi c,LTQ XL,Germany).For this analysis,methanol and ammonium acetate buffer(10 mM,pH 3.4) system was used in the ratio of 70:30.The column used for chromatographic separation was Phenomenex C18column (250 mm×4.6 mm,5 μm)and fl ow rate was 1 ml/min at 4°C column temperature.The detection wavelength was 333 nm and injection volume was 20 μl.
Entrapment ef fi ciency(%EE)was calculated using the equation shown below:
2.2.8. Bulk and tap density
For this,a known mass of powder was poured into a graduated cylinder,which is then tapped for de fi ned number of times. For calculation of tap and bulk densities,we noted the initial volume and fi nal volume(after tapping).Owing to the sample size constraint,a 10 ml graduated cylinder was fi lled with 1000 mg of dry powder for testing and 1250 taps were applied for this measurement.The static powder fl ow was determined by Carr’s compressibility index(CI).This was determined by the following equation:
2.2.9. Powder fl owability
For assessment of powder fl owability,measurement of angle of repose is the most frequently adopted method.Powder was poured through a funnel to form a cone-shaped pile with an angle,α,to the horizontal.The value of α was calculated by measuring the height and radius of the pile formed.Powder fl owability is inversely proportional to the angle of repose i.e. a large angle of repose is indicative of poor fl ow properties while a small angle of repose indicates a free fl owing powder[32]. Apart from angle of repose properties,Hausner ratio was also determined.
2.2.10.Speci fi c surface area
To determine the surface area,approximately 100 mg of each formulation were dried at 50°C for 30 min under vacuum and dead volume of sample cell was measured at room temperature.Nitrogen adsorption and desorption measurements were performed using sample cells and empty reference cell immersed in liquid nitrogen[33].Each measurement was repeated twice to obtain the average surface area calculated by BET equation(Brunauer-Emmett-Teller)method.
2.2.11.In vitro antimycobacterial activity by BACTEC method The antimycobacterial activity of SDRPL-CD,SDRPL-AA and SDRPL-Comb formulation and RMP was tested on MTB H37Rv strain(Tuberculosis Research Centre,Chennai,India).Brie fl y, the bacteria were cultured in Middlebrook 7H9 liquid medium (HiMedia,India)supplemented with 10%albumin,dextrose,and catalase(ADC;HiMedia,India)to mid-log phase and then frozen in aliquots at-70°C until needed.We checked the purity of culture by Ziehl-Neelsen staining(HiMedia,India).We dissolved the samples in dimethyl sulphoxide(DMSO)and fi lter sterilized through 0.2 μm DMSO safe fi lters(PALL Life Sciences)before testing.The BACTEC 460 TB system(Becton Dickinson,USA)was employed to determine a growth index (GI)of the MTB.GI is the quantitative measure of14CO2liberated by metabolism of14C-labelled substrate in a medium and expressed in numbers on a scale from 0 to 999.Brie fl y,0.1 ml of samples were transferred to 12B BACTEC vials,in duplicate for each sample/drug concentration,unless mentioned otherwise,and incubated at 37°C in 5%CO2atmosphere.GI was calculated daily under aerobic condition until in 1:100 controls;a value greater than 30 was obtained.In order to determine the per cent inhibition,undiluted control reading was used.Appropriate positive and negative controls were also included in the calculation.The growth inhibition was expressed as a ratio of GI of drug to the respective control vial.
The per cent growth inhibition was calculated for each drug concentration[34,35].
2.2.12.In vitro pulmonary deposition studies
Aerosol performance of SDRPL-CD,SDRPL-AA and SDRPLComb formulation was assessed using an eight stage,nonviable Anderson Cascade Impactor with a preseparator(Graseby-Anderson,Atlanta,USA)operating at an air fl ow of 28.3 l/m[36]. To overcome the particle bounce and re-entrainment phenomenon,the impaction plates were coated with 1.5%w/v of HPMC (4000 cps)gel in water.Five hydroxypropyl methylcellulose hard capsules(Size 3;Quali-V®;Qualicaps®Inc,Whitsett,NC,USA) were loaded with 20 mg of powder per capsule followed by actuation time of 10 s.The drug content deposited in individual impaction plates was rinsed with methanol and subjected to HPLC analysis.From the drug deposition data the emitted dose (ED), fi ne particle fraction(FPF),mean median aerodynamic diameter(MMAD)and geometric standard deviation(GSD)were calculated[37]as follows:
2.2.13.Statistical analysis
The values are reported as the mean±SD.The minimum level of signi fi cance was set at P<0.05.All statistical analyses were performed using the GraphPad Prism 5.0 software(Graph pad software[CA,USA]).
3. Result and discussion
3.1. FTIR
The FTIR spectra of RMP,PL,PM,SBECD,AA,SDRPL-CD,SDRPLAA and SDRPL-Comb formulations are shown in Fig.1(upper legend).The characteristic absorption peaks of RMP present at 1734 cm-1(νC=O acetyl stretching)and 1566 cm-1(νC=C stretching)in the physical mixture indicate the additive effect(Fig.1C). In the spectra of AA(Fig.1D)and SBECD(Fig.1E),the characteristic peaks were observed at 3412(νO-H bond)and 3221 cm-1(νC-H bond)and 3394(νO-H bond)and 1650 cm-1(bending vibration of νC=C),respectively.However,in the spectra of the SDRPL formulations(Fig.1F-H),the intensity of four characteristic absorption peaks of RMP at 1655 cm-1(νC=N asymmetric stretching),1566 cm-1(νC=C stretching),1252 cm-1(νC-O-C-ether group)and 1430 cm-1(νC-N stretching)was changed. These fi ndings suggested possible inter-molecular interactions between the components in the dry powder.
3.2. DSC
The thermograms of RMP,PL,SBECD,AA,PM,SDRPL-CD,SDRPLAA and SDRPL-Comb formulations are shown in Fig.1(lower legend).The AA(Fig.2A)and RMP(Fig.1B)thermogram shows an endothermic peak at 195°C and 187-193°C,which represents the melting point of AA and RMP samples,respectively. There are two endothermic peaks in the thermogram of PL (Fig.1C)at 153°C and 210°C.The thermogram of PM(Fig.1D) shows that the endothermic peak corresponding to the melting point of the AA is present but reduced.However,there was no melting endothermic peak present in the SDRPL-CD,SDRPLAA and SDRPL-Comb formulations(Fig.1F-H).Absence of these characteristic endothermic peaks is an indication of the amorphous nature of the formulations[38,39].
3.3. PXRD
PXRD is the direct method for determination of basic structure information of a material.Crystalline behaviour of free RMP,SDRPL-CD,SDRPL-AA and SDRPL-Comb formulations were examined by studying its PXRD.As shown in Fig.2,the PXRD diffractogram pattern of RMP(Fig.2A)and AA(Fig.2H)shows distinct sharp diffraction peaks(i.e.the presence of long range molecular order),indicating its crystalline nature.Fig.2B and D presents the diffractogram pattern of PL which displayed peaks at position 2°and 8°and halo pattern of SBECD,indicating its amorphous nature while in diffractogram pattern of PM(Fig.2C),there are still reduced diffraction peaks.This could be due to the presence of RMP and AA in the sample.However, no such diffraction peaks were observed in the diffractogram pattern of SDRPL formulations as displayed in Fig.2E-G,indicative of its non-crystallinity.Hence,based on the PXRD results it is evident that SDRPL formulations are amorphous in nature and are in agreement with DSC fi ndings.
3.4. HSM
Photomicrographs of RMP,PL,PM,SDRPL-CD,SDRPL-AA and SDRPL-Comb formulations are shown in Figs 3-8.The RMP micrographs exhibited the birefringency(blue arrow)pattern (Fig.3A-E).Gradually,it diminishes as the temperature is increased.This kind of pattern con fi rms the crystalline nature of the RMP.In Fig.4A-E,melting of the crystals(black arrow) of RMP was observed by deformation of the crystals.On the contrary,the micrographs of SDRPL-CD(Fig.5A-E),SDRPL-AA (Fig.6A-E)and SDRPL-Comb(Fig.7A-E)showed softening(black arrow)of the particles.This is probably due to the amorphous nature of the SDRPL formulations following spray drying process.Representative micrograph of SDRPL-CD is presented under the cross-polarizer light in Fig.8A-E wherein completely dark images that lack birefringency are present.The softening and lack of birefringency pattern in the SDRPL formulations is evident of their amorphous nature[5].Overall,HSM fi ndings con fi rmed the amorphous nature of the formulations.These results are in agreement with DSC and PXRD data.
3.5. SEM
Dry powder particles were successfully produced by using spray drying technology.The shape and surface morphology of all samples were visualized via SEM and displayed in Figs 9 and 10.The image shown in Fig.9A con fi rms the irregular size and morphology of RMP crystal,which is in agreement with PXRDanalysis that showed the crystalline nature of RMP.Fig.9B displayed the shrunk and irregular shape of PL.Moreover,in Fig.9C and D a spherical shape and cubic crystals are observed of SBECD andAA,respectively.The PM(Fig.10A)revealed presence of RMP crystal along with SBECD and AA.SDRPL-CD,SDRPL-AA and SDRPL-Comb(Fig.10B-D)presented the formation of fi ne particles,indicated by the spherical morphology within the size range of 1 μm to 5 μm,which shows the potential for delivery to the lungs[32,40].In conclusion,the particle size exhibited by all the powder formulations was in the respirable range.
3.6. Powder characterization
The tapped density of spray dried formulations was similar for all powders,and ranged between 0.26±0.003 gcm-3and 0.33±0.005 gcm-3(data not presented).The better aerosolization of the formulation is associated with the lower powder densities[41,42].Carr’s Index,Angle of Repose and Hausner ratio were used to provide a measure of the fl ow properties. In Carr’s Index,a value less than 25%indicates good fl owing powder,whereas value greater than 25%indicates cohesive fl owing powder[43].Carr’s Index values in the SDRPL-CD, SDRPL-AA and SDRPL-Comb formulations varied from 11.7% (excellent fl owability)to 13.5%(good fl owability).Moreover,Angle of Repose and Hausner ratio of the spray dried formulations were in the range of 31.8±4 to 35.1±3 and 1.03±0.02 to 1.21±0.05,respectively.Results of the powder characterization were not signi fi cant(P>0.05)with pure SDRPL formulation without CD and AA(data not presented).Findings of these parameters further proved the good fl owing behaviour of thepowders.Hence,it can be concluded that the developed SDRPL formulations were found to have potential for the pulmonary delivery of poor-aqueous soluble drugs.Powder characterization data are compiled in Table 1.
3.7.In vitropulmonary deposition studies
In vitro pulmonary deposition study results of SDRPL-CD,SDRPLAA and SDRPL-Comb formulations after 28.3 l/m using the eight stage Anderson Cascade Impactor are shown in Table 2 and Fig.11.To investigate in vitro aerosol powder performance,we used Size 3 HPMC capsules.Aerodynamic studies by Andersen Cascade Impactor were then conducted on these preparations that met the delivered dose speci fi cations(>75%) and showed a respirable fraction higher than 50%.Ef fi ciency of inhalation delivery system depends on particle size and particle size distribution which are considered as the critical factorsfor deposition of particles in the alveolar region.An aerodynamic particle size of 1-3 is required for optimal delivery to the lung.
The ef fi ciency of powder recovery was found to be>80%. This indicated that the maximum amount of the drug was delivered by the device and small amount of the drug was retained in the inhaler device.The per cent mass fraction of the aerosolized particles from DPIs on the different stages of cascade impactor is graphically presented in Fig.11.Each bar represents the powder of certain sizes deposited on the stages of the Anderson Cascade Impactor.Maximum FPF of 83.73±2% was observed with SDRPL-CD as compared to SDRPL-AA of68.84±4%and SDRPL-Comb of 69.01±3%(Table 2).No signi ficant difference was observed between SDRPL-AA and SDRPLComb.The per cent emitted dose was ranged from 80±4%of SDRPL-AA to 93±1.5%for SDRPL-CD(Table 2).For ef fi cient delivery of formulation to the lung,a combination of lower MMAD and higher FPF is required[44].Therefore,higher the FPF greater will be the deposition in the deeper lung resulting in enhanced ef fi cacy.SDRPL-CD was found to have a maximum FPF compared to SDRPL-AA and SDRPL-Comb(Table 2).This could be due to its less cohesive nature with lower surface area and vander Walls forces per unit mass of particles.In addition to this, these particles tend to separate easily and provide maximum fi nes.The mass median aerodynamic diameter(MMAD)and geometric standard deviation(GSD)of SDRPL-CD,SDRPL-AA and SDRPL-Comb were in the range of 1.76±0.23 to 3.99±0.13 and 2.11±0.09 to 3.28±0.1,respectively.Findings of the study also revealed that SDRPL-CD displayed the best aerosol powder performance among the various formulations in terms of ED, FPF and MMAD.Deposition of SDRPL-AA formulation in the cascade impactor was in the lower stages(stages 4 and 5)which is most relevant for deep lung penetration[45],while in case of SDRPL-CD and SDRPL-Comb,it was on stages 5 and 6 and stages 3 and 5 of impactor,respectively.Thus,these results demonstrated that the RMP loaded phospholipid lipospheres incorporated with SBECD and AA formulations have potential for inhalation delivery.

Table 1-Powder properties characterization.
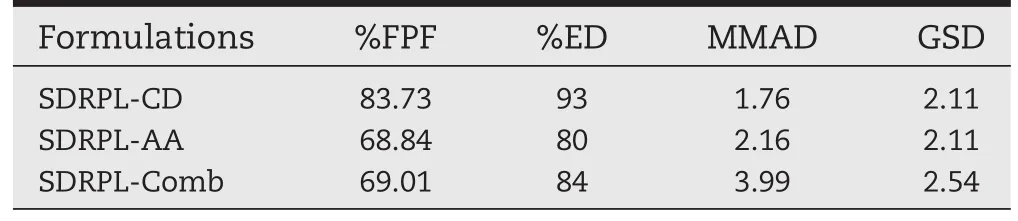
Table 2-In vitro aerosol performance data of SDRPL formulations.
3.8. In vitro antimycobacterial activity by BACTEC method
All the three SDRPL-CD,SDRPL-AA and SDRPL-Comb formulations were tested for antimycobacterial activity.All the formulations exhibited enhanced/equivalent ef fi cacy in vitro in MTB H37Rv strain.DMSO was employed as the solubilizing agent and its possible inhibitory effect on MTB growth was considered as well.The mechanism of action of RMP is to arrest DNA-directed RNA synthesis of MTB by interacting with the subunit of RNA polymerase[46].The MIC values of RMP and SDRPL formulations against MTB were found to be 0.05 and0.005,0.024,0.028 μg/ml,respectively.Thus,SDRPL-CD formulation exhibited signi fi cant(P<0.001)lowest MIC value among all the formulations.Also,in comparison to pure SDRPL lipospheres,it exhibited two folds(0.005 μg/ml vs 0.01 μg/ml) improved in vitro ef fi cacy.Probably,this may be due to the cyclodextrin-cholesterol complex formation and subsequently,removal of cholesterol from the membrane(Fig.12). It has been reported that MTB degrades the cholesterol to derivethe carbon and energy from this molecule for its growth[47-51]. As result of the cholesterol removal of membrane,fewer nutrients will be available for MTB and not able to survive in the cells.On the other hand,SDRPL-AA has also shown improved/ equivalent ef fi cacy in vitro but not as remarkable as SDRPLCD formulation.It is reported that sterilizing effect of the AA on the MTB culture is due to its pro-oxidant effect which results in increase in the ferrous ion concentration leading to ROS generation,alteration in lipid,imbalance in redox reaction and DNA damage(Fig.13).To our knowledge,this is the fi rst time that activity of a RMP loaded lipospheres formulation containing SBECD and AA has been reported.
4. Conclusion
In the present study,a novel DPI system of rifampicin for inhalation therapy was developed with the use of cyclodextrin and vitamin C.Micronized spray dry powders prepared with a spray drying technique showed suitable physicochemical and powder fl ow properties for the inhalable dry powder.SDRPLCD formulation presented excellent in vitro antimycobacterial ef fi cacy and aerosol performance.The in vitro deposition study using the cascade impactor also highlighted that spray dry powders consistently deposited in deep lung regions.Overall, the data from the study suggest an alternative option for inhalation delivery of an antitubercular drug directly into the lungs,which may enhance therapeutic ef fi cacy and greatly reduce adverse effects.The study establishes that rifampicin can be successfully loaded into phospholipid lipospheres for pulmonary delivery.Hence,it can be concluded that SDRPL formulations have potential for better management in the tuberculosis therapy.
REFERENCES
[1]Ramzi G,Didier R,Michel D.Dramatic reduction of culture time of Mycobacterium tuberculosis.Sci Rep 2014;4:4236-4240.
[2]Newer Anti-TB Drugs and Drug Delivery Systems.<http://apiindia.org/medicine_update_2013/chap86.pdf>; [accessed 23.03.15].
[3]Rao BS,Murthy KV.Studies on rifampicin release from ethylcellulose coated nonpareil beads.Int J Pharm 2002;231:97-106.
[4]Courtney AO,Lonji K,Hulda S,et al.Preparation of rifampicin/lactose microparticle composites by a supercritical antisolvent-drug excipient mixing technique for inhalation delivery.Powder Technol 2013;236:132-138.
[5]Charan S,Tara DB,Manjinder SG,et al.Novel rifampicinphospholipid complex for tubercular therapy:synthesis, physicochemical characterization and in-vivo evaluation.Int J Pharm 2014;460:220-227.
[6]Rajesh P,Sonali D.Preparation and characterization of controlled release poly-ε-caprolactone microparticles of isoniazid for drug delivery through pulmonary route.Powder Technol 2014;264:158-165.
[7]Yun-Zhong F,Shen Y,Guoyao W.Free radicals,antioxidants, and nutrition.Nutrition 2002;18:872-879.
[8]Dabashish B,Manashi B,Sidney JS,et al.Free radicals and grape seed proanthocyanidin extract:importance in human health and disease prevention.Toxicology 2000;148:187-197.
[9]Devasagayam TP,Tilak JC,Boloor KK,et al.Free radicals and antioxidants in human health:current status and future prospects.J Assoc Physicians India 2004;52:794-804.
[10]Olufunsho A,Alade A,Vincent OO,et al.Modulatory activity of antioxidants against the toxicity of rifampicin in vivo.Rev Inst Med Trop Sao Paulo 2010;52:43-46.
[11]Faouzi D,Zine K,Mohammed D.Bene fi cial effects of vitamins(C+E)supplementation against nickel-induced hepatotoxicity in mice.Adv Biores 2013;4:67-76.
[12]Rabab RE,Hamouda FA,Abdel-Fatah A,et al.Protective role of vitamin C and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats.Am J Pharmacol Toxicol 2014;9:177-188.
[13]Catherine V,Travis H,Brian W,et al.Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction.Nat Commun 2013;4:1881-1891.
[14]Xiao CF,Li K,Huang R,et al.Investigation of inclusion complex of Epothilone A with cyclodextrins.Carbohydr Polym 2014;102:297-305.
[15]Jing W,Yanping C,Baoguo S,et al.Characterisation of inclusion complex of trans-ferulic acid and hydroxypropylβ-cyclodextrin.Food Chem 2011;124:1069-1075.
[16]Meng C,Yujiao W,Xiaomei X,et al.Inclusion complex of monochlorotriazine-beta-cyclodextrin and wormwood oil: preparation,characterization,and fi nishing on cotton fabric. J Text Inst 2015;106:31-38.
[17]Jian Z,Yong R,Chengliang Z,et al.Preparation and physicochemical characteristics of the complex of edaravone with hydroxypropyl-β-cyclodextrin.Carbohydr Polym 2011;83:1101-1105.
[18]Thambusamy S,Krishnan S,Krishnamoorthy S.Preparation and characterizations of solid/aqueous phases inclusion complex of 2,4-dinitroaniline with β-cyclodextrin. Carbohydr Polym 2014;107:72-84.
[19]Jozsef S.Cyclodextrin complexed generic drugs are generally not bio-equivalent with the reference products:therefore the increase in number of marketed drug/cyclodextrin formulations is so slow.J Inclusion Phenom Macrocyc Chem 2005;52:1-11.
[20]Louis DS,Leonard BJ,Carol AK.Voriconazole:a new triazole antifungal agent.Ann Pharmacother 2003;37:420-432.
[21]Astray G,Gonzalez-Barreiro C,Mejuto JC,et al.A review on the use of cyclodextrins in foods.Food Hydrocolloids 2009;23:1631-1640.
[22]Shaimaa MB-E,Seham AE,Mahmoud MG.Inclusion complexes of tadala fi l with natural and chemically modi fi ed β-cyclodextrins.I:preparation and in-vitro evaluation.Eur J Pharm Biopharm 2008;70:819-827.
[23]Fukuda M,Miller DA,Peppas NA,et al.In fl uence of sulfobutyl ether β-cyclodextrin(Captisol®)on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion.Int J Pharm 2008;350:188-196.
[24]Federico L,Antoine M,Jean CD,et al.Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD.Respir Med 2008;102:593-604.
[25]Harry H,Elsbeth W,Steven C,et al.Inhaled medication and inhalation devices for lung disease in patients with cystic fi brosis:a European consensus.J Cyst Fibros 2009;8:295-315.
[26]John SP,Simone F,Jeffry GW.The lungs as a portal of entry for systemic drug delivery.Proc Am Thorac Soc 2004;1:338-344.
[27]Timsinaa MP,Martin GP,Marriott C,et al.Drug delivery to the respiratory tract using dry powder inhalers.Int J Pharm 1994;101:1-13.
[28]Frijlink HW,De Boer AH.Dry powder inhalers for pulmonary drug delivery.Expert Opin Drug Deliv 2004;1:67-86.
[29]Remigius UA,Michael IU,Michoel A,et al.The lung as a route for systemic delivery of therapeutic proteins and peptides.Respir Res 2001;2:198-209.
[30]Son YJ,McConville JT.A new respirable form of rifampicin. Eur J Pharm Biopharm 2011;78:366-376.
[31]Patrick O,Anthony JH.Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization.Pharm Res 2000;17:955-961.
[32]Hitendra SM,Sadanand AG.Preparation,characterization and pulmonary pharmacokinetics of xyloglucan microspheres as dry powder inhalation.Carbohydr Polym 2014;102:529-536.
[33]Shashank PP,Sameer RM,Arvind KB.Generation of 1:1 carbamazepine:nicotinamide cocrystals by spray drying.Eur J Pharm Sci 2014;62:251-257.
[34]Huang TS,Tu HZ,Lee SS,et al.Antimicrobial susceptibility testing of Mycobacterium tuberculosis to fi rst-line drugs: comparisons of the MGIT 960 and BACTEC 460 systems.Ann Clin Lab Sci 2002;32:142-147.
[35]Jagannath C,Reddy MV,Kailasam S,et al.Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis.In vitro,intracellular,and in vivo studies.Am J Respir Crit Care Med 1995;151:1083-1086.
[36]Mahaveer C,Bijay P,Ambikanandan M.Nano-liposomal dry powder inhaler of tacrolimus:preparation,characterization, and pulmonary pharmacokinetics.Int J Pharm 2007;2:675-688.
[37]Jinghua D,Frederick GV,Xiaojian LD,et al.Design, characterization,and aerosolization of organic solution advanced spray-dried moxi fl oxacin and o fl oxacin dipalmitoylphosphatidylcholine(DPPC)microparticulate/ nanoparticulate powders for pulmonary inhalation aerosol delivery.Int J Nanomedicine 2013;8:3489-3505.
[38]Patil JS,Sarasija S.Physicochemical characterization,in vitro release and permeation studies of respirable rifampicincyclodextrin inclusion complexes.Indian J Pharm Sci 2009;71:638-643.
[39]Yu L.Amorphous pharmaceutical solids:preparation, characterization and stabilization.Adv Drug Deliv Rev 2001;48:27-42.
[40]Xiao W,Weifen Z,Don HJ,et al.Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery.Int J Nanomedicine 2013;8:1269-1283.
[41]Naumana RR,Peter CS.The in fl uence of formulation components on the aerosolisation properties of spray-dried powders.J Control Release 2005;110:130-140.
[42]Labiris NR,Dolovich MB.Pulmonary drug delivery.Part II:the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications.Br J Clin Pharmacol 2003;56:600-612.
[43]Tristan PL,Jane LB,Eddie F,et al.Chitosan-based spray-dried respirable powders for sustained delivery of terbutaline sulfate.Eur J Pharm Biopharm 2008;68:224-234.
[44]Arpana P,Abhay K,Milind P,et al.Montelukast-loaded nanostructured lipid carriers:part II pulmonary drug delivery and in vitro-in vivo aerosol performance.Eur J Pharm Biopharm 2014;88:169-177.
[45]Biswadip S,Biswajit M,Gurudutta P.Poly-lactide-coglycolide nanoparticles containing voriconazole for pulmonary delivery:in vitro and in vivo study. Nanomedicine 2013;9:94-104.
[46]Yu P,Jie L,Yufeng W,et al.Study of the rifampin monoresistance mechanism in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013;57:893-900.
[47]Isam IS,Nejat D.Ef fi cacies of cyclodextrin-complexed and liposome-encapsulated clarithromycin against Mycobacterium avium complex infection in human macrophages.Int J Pharm 2003;250:403-414.
[48]Maurine DM,Jennifer CC,Ankit KP,et al.Role of cholesterol in Mycobacterium tuberculosis infection.Indian J Exp Biol 2009;47:407-411.
[49]Anna B,Jacob P,Anna RG,et al.Mycobacterium tuberculosis is able to accumulate and utilize cholesterol.J Bacteriol 2009;191:6584-6591.
[50]Munoz S,Rivas-Santiago B,Jacob E.Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains.Scand J Immunol 2009;70:256-263.
[51]Cesar AL,Alex HV,Siewert JM.Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes.Sci Rep 2013;3:2071.doi:10.1038/ srep02071.
*Corresponding author.Department of PharmaceuticalTechnology(Formulations),National Institute of Pharmaceutical Education and Research(NIPER),Sector 67,S.A.S.Nagar(Mohali),Punjab 160062,India.
E-mail address:sarasija_s@hotmail.com(S.Suresh).
1Presently at Faculty of Pharmacy,MSRUAS,Bangalore-560057,India.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.003
1818-0876/©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2015年6期
Asian Journal of Pharmacentical Sciences2015年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- Rethinking bioequivalence and equivalence requirements of orally inhaled drug products
- Inhaled nicotine replacement therapy
- Inhalation of nanoparticle-based drug for lung cancer treatment:Advantages and challenges
- Practical,regulatory and clinical considerations for development of inhalation drug products
- Mathematical approach for understandingdeagglomeration behaviour of drug powder in formulations with coarse carrier
- The effects of surface morphology on the aerosol performance of spray-dried particles within HFA 134a based metered dose formulations
