Th22细胞因子协同促进小鼠输卵管上皮细胞抗沙眼衣原体感染体外研究
徐玲娟,赵秀敏,朱丹阳,许 文
Th22细胞因子协同促进小鼠输卵管上皮细胞抗沙眼衣原体感染体外研究
徐玲娟1,赵秀敏2,朱丹阳2,许 文3
目的 体外研究Th22细胞因子(IL-22、TNF-α)在小鼠输卵管上皮细胞抵抗沙眼衣原体感染过程中的作用。方法 体外培养Th22细胞,ELISA检测Th22上清中细胞因子含量及其对体外培养的小鼠输卵管上皮细胞(MOECs)表达Th1趋化因子、抗菌肽的影响;Transwell实验检测MOECs上清对Th1细胞的趋化能力;分别用免疫荧光法、PCR及7-乙氧基试卤灵(7-ethoxyresrufin, 7-ER)法检测Th22上清对输卵管上皮细胞抑制Chlamydiatrichomatis(Ct)能力、促进胰岛再生源蛋白3g(regenerating islet-derived protein-3g,Reg3g)Reg3g表达及细胞活性的影响。结果 Th22细胞以分泌IL-22、TNF-α为主。Th22上清可诱导MOECs表达Th1细胞趋化因子CXCL9/10/11及抗菌肽mBD-2表达,活化MOECs对Th1细胞具有趋化作用并抑制Ct生长。同时,Th22上清可促进MOECsReg3g表达及增强其活性。结论 Th22细胞因子能增强MOECs介导的天然免疫功能,促进其损伤修复,在Ct及其它性传播疾病中可能发挥重要作用。
细胞因子;IL-22;TNF-α;小鼠输卵管上皮细胞;Th22细胞
Th22细胞是一类新发现的辅助性T细胞,以膜表面表达CCR10及分泌IL-22、TNF-α为主要特征,在监督和协调引起炎症的免疫细胞过程中发挥作用[1-2]。研究表明,IL-22可单独或协同IL-17或TNF-α诱导上皮细胞产生抗微生物肽及多种趋化因子,在皮肤黏膜损伤修复及抗微生物感染中发挥重要作用[3-4]。以IL-22还可做为分子佐剂,可有效增强DNA疫苗诱导的Th1免疫反应。在慢性以及过敏性炎症疾病,如牛皮癣、过敏性湿疹等疾病中; Th22细胞功能的丧失将使慢性炎症疾病恶化。因此,Th22细胞可能是治疗慢性炎症疾病的潜在靶标。在Ct、阴道毛滴虫等性传播病原体感染的患者阴道分泌物中IL-22明显升高[5],因此,我们推测Th22细胞及其细胞因子在生殖道黏膜免疫中亦发挥重要作用。本实验应用体外培养小鼠输卵管上皮细胞模型, 分析两种关键Th22细胞因子IL-22、TNF-α对小鼠输卵管上皮细胞免疫功能的影响, 为时一步探讨Th22细胞及其细胞因子在生殖道黏膜免疫中的作用。
1 材料与方法
1.1 鼠 雌性6~8周龄的BALB/c小鼠购自中科院上海实验动物中心。
1.2 主要试剂 HeLa 229 cells及CtE型株购自ATCC。抗沙眼衣原体LPS多克隆抗体购自Abcam。抗鼠IL-22、TNF-α抗体及鼠IgG1同型对照抗体(BD 公司,美国)。抗鼠CD3、CD28, IFN-γ、IL-4 单抗及重组鼠TNF-α, IL-6, and IL-2;CXCL-9, -10, 及-11 ELISA kits 均购自 R&D公司。mBD-2试剂盒购自Phoenix Pharmaceuticals公司。FITC标记抗鼠CD4单抗、PE标记抗鼠CXCR3单抗购自Pharmingen。7-乙氧基试卤灵(7-ethoxyresrufin , 7 -ER )购自Sigma公司。
1.3 Th22细胞诱导及小鼠输卵管上皮细胞的原代培养 磁珠分离BALB/c小鼠脾CCR10+CD4+T细胞,以1 μg/mL抗CD3、2 μg/mL抗CD28、5 μg/mL IFN-γ、5 μg/mL IL-4 单抗及细胞因子50 ng/IL-6、20 ng/mL TNF-α100 U/mL IL-2 (Th22 条件)刺激[3],诱导Th22细胞极化,流式分析表型(BD FACS Calibur)。于第7 d,以抗CD3、抗CD28抗体刺激,收集上清,-80 ℃冻存。
MOECs的原代培养参见文献[6]。1×105MOECs/孔,接种接种于24孔板中,加入培养基2 mL, 细胞贴壁生长至85% 时,准备Th22上清干预。干预前基础培养基饥饿培养细胞过夜, 次日上午于各培养皿中分别加入Th22上清,分别于24、48、72 h 后分离上清,-80 ℃冻存。
1.4 ELISA检测细胞因子 依说明书操作,检测Th22上清中IL-22, TNF-α, IL-17, IFN-γ、IL-4 含量及 MOECs上清中mBD-2 and CXCL-9, -10及-11含量。
1.5 Transwell迁移实验 磁珠分离BALB/c 小鼠脾细胞中CD4+T 细胞,抗CD3 (1 μg/mL), 抗CD28 (2 μg/mL), 5 μg/mL 抗鼠IL-4及25 ng/mL IL-12 (Th1 条件) 刺激48 h后,离心、移除上清,补新鲜完全RPMI-1640,继续培养48 h,以诱导CXCR3表达。2×105CD4+T细胞加于Transwell上室,下室加MOECs培养上清。37 ℃,5% CO2培养4 h 后置于 4 ℃ 20 min以分离膜上细胞,流式分析迁移的CD4+T数及其CXCR3表达。
迁移阻断实验:抗CXCR3 抗体(5 μg/ml)或同型对照抗体预先与激活的淋巴细胞在37 ℃孵育30 min, 然后用所收集的细胞上清液做迁移实验。实验重复3 次。
1.6 免疫荧光检测活化MOECs抑制Ct增殖能力 3×104MOECs/孔置于96孔板中,1.2×103CtEB与100 μL MOECs培养上清(见上述)于37 ℃孵育45 min后加入上述孔中,室温250×g离心40 min,弃上清,补200 μL完全1640(含10%胎牛血清)。37 ℃,5% CO2继续培养40 h,10% 甲醇固定10 min, 加入兔抗Ct特异性脂多糖抗体200 μL, 37 ℃ 1 h, PBS洗涤后加入羊抗兔IgG-FITC 200 μL 30 min, PBS洗涤后甘油封片,荧光显微镜下(40×)计数检测包涵体。
1.7 PCR检测Reg3β表达Ct感染MOECs 24 h、48 h后,RT-PCR检测再生蛋白(regenerating protein 3β,Reg3β) mRNA表达,引物序列为 5′-CGCATTAGTTGCCCCAAGG-3′ and 5′-TCCAGGCCTCTTTTGGCAG-3′. 提取Th22上清处理的MOECs总RNA,合成 cDNA。
1.8 细胞活性检测Ct感染MOECs 24 h、48 h后,依说明书操作,荧光分光光度计检测试卤灵吸光度。
2 结 果
2.1 Th22细胞诱导 Th22 细胞是新发现的CD4+T 细胞,以膜表达CCR10及分泌IL-22为特征。因此,在本研究中采用抗CD3、CD28、IFN-γ、IL-4单抗及细胞因子IL-6、TNF-α 、IL-2组合刺激BALB/c小鼠来源的CCR10+CD4+T细胞诱导Th22细胞极化。7 d后,以抗CD3、CD28刺激活化后,流式分析表明所得CD4+T 细胞表达Th22标志CCR10 及IL-22 及 TNF-α(图1). ELISA分析上清进一步表明,所得T细胞高表达Th22特征细胞因子IL-22、TNF-α而低表达Th1、Th2、Th17细胞因子IFN-γ、IL-4及IL-17(表1)。
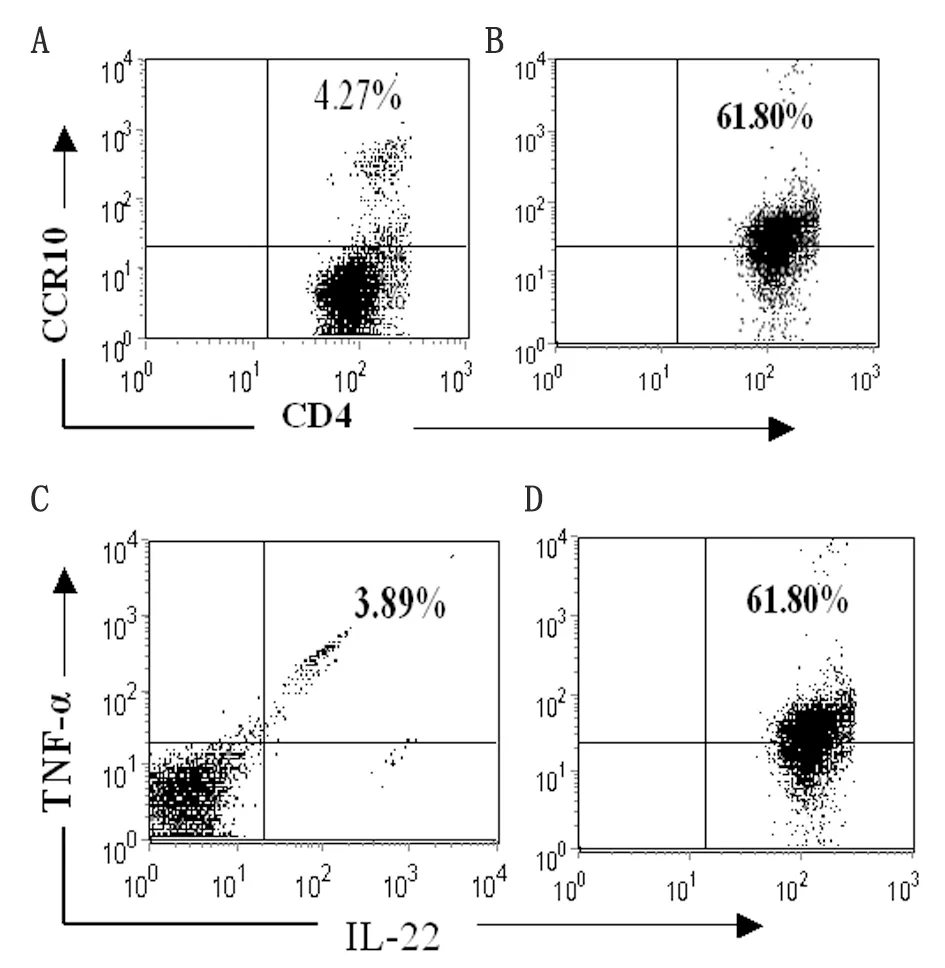
By flow cytometry, freshly isolated CCR10+CD4+T cells from the spleens of normal mice (left panel) and polarized Th22-type cells (right panel) were stained with anti-CCR10, anti-CD4, anti-IL-22, and anti-TNF-α Abs after permeabilization. The percentage of cells that were double-positive for CCR10 and CD4 (A and B) or IL-22 and TNF-α are shown (C and D).
图1 Th22细胞诱导
Fig.1 Polarization of CD4+T cells into IL-22- and TNF-α-producing cells
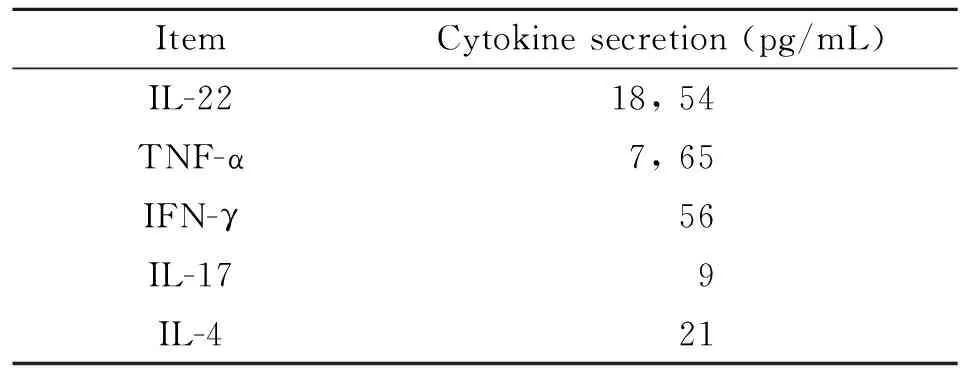
表1 Th22细胞因子检测
Note: We set up Th22-type cells from spleens of BALB/c mice. Supernatants were measured 48 hrs after anti-CD3/CD28 stimulation.
2.2 Th22上清诱导MOECs产生Th1趋化因子CXCL9/10/11及抗菌肽mBD-2 为检测Th22上清能否刺激MOECs天然免疫功能,MOECs经Th22上清作用24、48及72 h后,收集培养上清,ELISA检测CXCL9、CXCL10、CXCL11及mBD-2浓度。如图2所示,Th22上清能够诱导小鼠输卵管上皮细胞产生CXCL9、CXCL10、CXCL11及mBD-2, anti-IL-22/TNF-α抗体显著抑制Th22上清的刺激作用,排除了其它细胞因子干扰的可能。
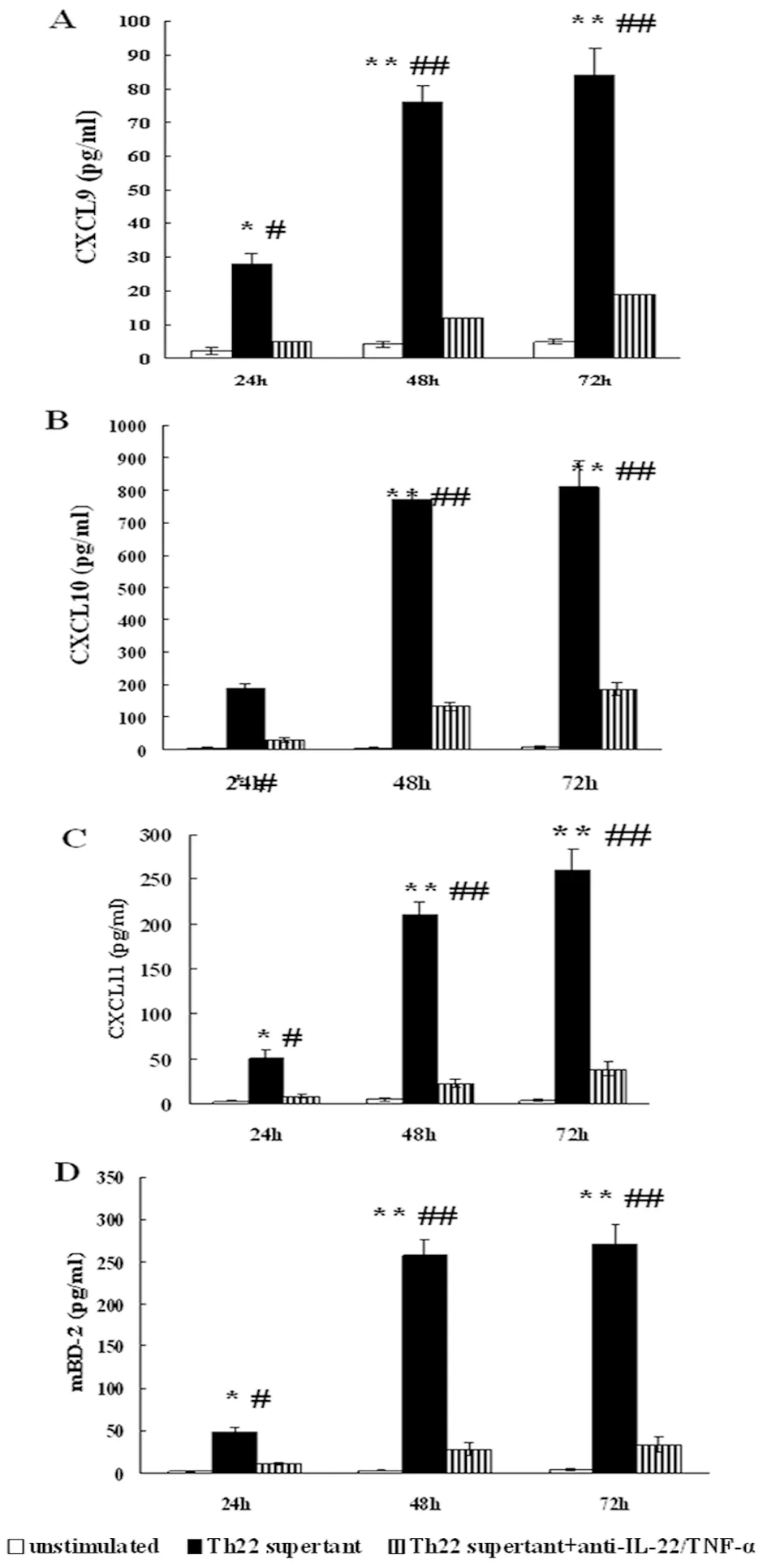
MOECs were stimulated with medium alone, IL-22, TNF-α, IL-22+TNF-α, or Th22 cell supernatant for 24, 48, or 72 hrs. For neutralization experiments, Th22 cell supernatants were pretreated with anti-IL-22+TNF-α mAbs for 30 min and then were used to stimulate MOECs. The levels of (A) CXCL-9, (B) CXCL-10, (C) CXCL-11, and (D) mBD-2 over a time-course in cell-free supernatants were quantified using ELISA kits. *P<0.05, **P<0.01, compared with the untreated control group; #P<0.05, ##P<0.01, compared with corresponding Th22 supernatant untreated by anti-IL-22/TNF-α mAbs.
图2 ELISA检测Th22上清刺激MOECs表达Th1趋化因子CXCL9/10/11及抗菌肽mBD-2表达
Fig.2 MOECs producing Th1 cell-associated chemokines and mBD-2 after IL-22+TNF-α stimulation
2.3 活化MOECs具有趋化Th1细胞的作用 T细胞介导的细胞免疫在清除Ct等性传播病原中发挥关键作用。CXCL9、CXCL10、CXCL11可与效应T细胞表面CXCR3受体结合募集T细胞进入炎症部位。本研究中我们通过Transweell实验观察Th22上清活化的MOECs趋化CXCR3+T细胞的能力。结果显示活化的MOECs具有趋化活化T细胞的能力,与未刺激对照组比较差异有统计学意义。抗anti-IL-22及TNF-α抗体显著抑制Th22上清诱导的趋化作用。
2.4 Th22细胞因子具有促进MOECs损伤修复的作用 由于IL-22 在上皮细胞的损伤修复中扮演重要作用,在本研究中,我们以体外感染Ct的MOECs为模型,进一步检测了Th22上清对生殖道上皮细胞的保护作用。首先,我们检测了Th22上清活化的MOECs能否抑制Ct生长。结果表明,以Th22上清活化的MOECs具有抑制生长的能力,与未处理对照组比较具有显著差异。以anti-IL-22及TNF-α抗体封闭的Th22上清处理的MOECs抑制Ct生长的能力显著下降。 接着,我们检测了Th22
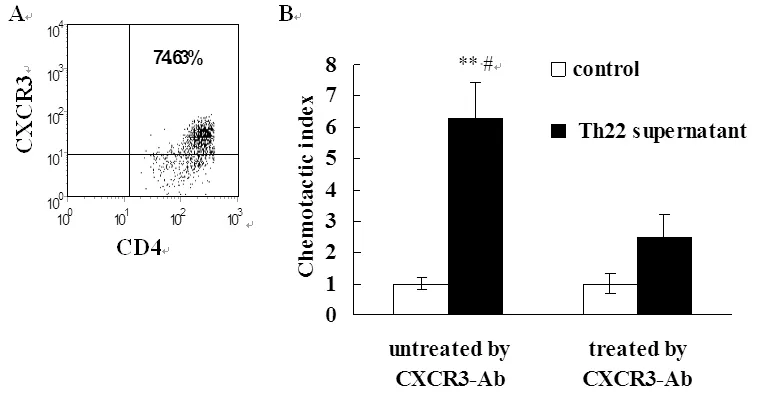
MOECs were stimulated by Th22 supernatant for 48 hrs. Then, supernatants were collected and analyzed by chemotaxis assay to assess the chemotactic activity of supernatants for Th1 cells. **P<0.01, compared with control; #P<0.05, compared with corresponding T cells that were not treated with CXCR3 antibody.
图3 Th22上清活化的MOECs诱导Th1细胞迁移
Fig.3 Migration of Th1 cells induced by supernatants of MOECs treated with Th22 cell supernatant
上清对MOECs表达Reg3β的影响。Reg3β是促进上皮组织损伤修复的一个重要蛋白。结果表明,Th22上清具有促进Reg3β表达的作用。另外,我们的结果还证明了Th22上清具有促进MOECs活性的作用。以上结果表明,Th22细胞因子具有促进MOECs损伤修复的作用。
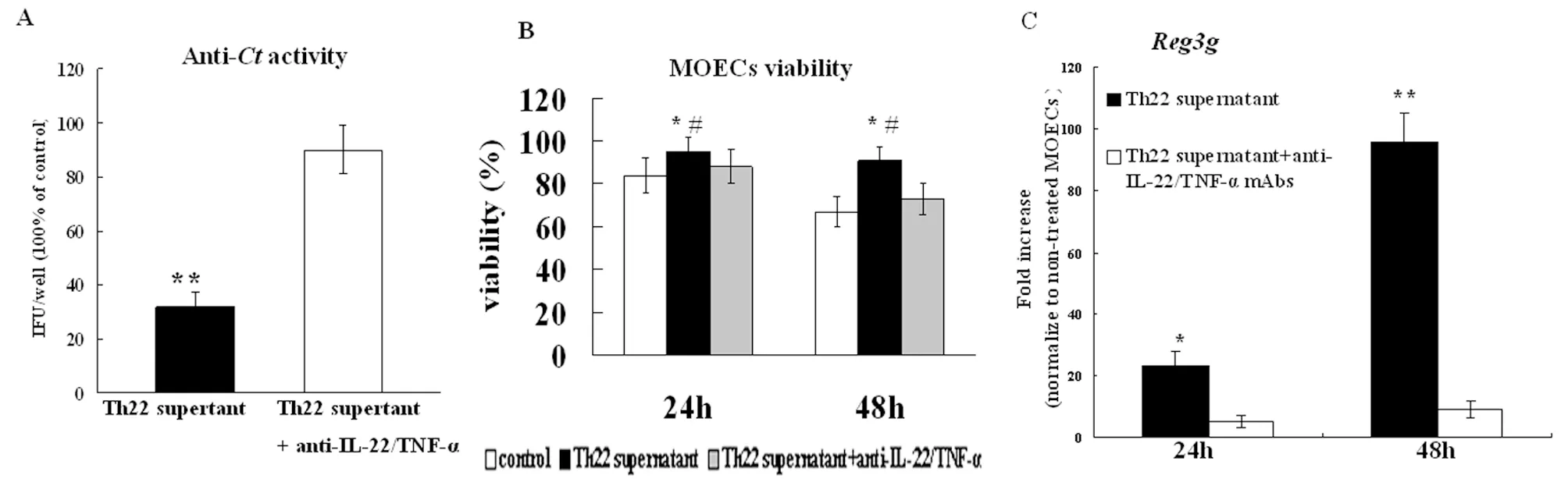
(A) MOEC supernatants from Th22 cell supernatant stimulation significantly inhibitedCtgrowth. Data are expressed relative values compared with the IFU/well resulting from infection with untreatedCt; (B) 24 and 48 h later, cell viability was quantified using Resorufin; (C)REG3βmRNA copy numbers were measured by quantitative RT-PCR.
Data are normalized to the expression ofβ-actin, and are expressed as the fold-increase over the average gene expression in untreated MOECs.
*P<0.05, **P<0.01, compared with the control group; #P<0.05, ##P<0.01, compared with the corresponding Th22 cell supernatant untreated by anti-IL-22 or TNF-α mAbs group.
图4 IL-22 and TNF-α 协同促进MOECs损伤修复
Fig.4 IL-22 and TNF-α co-operate to protect MOECs againstCt
3 讨 论
Th22细胞主要通过协调其它炎症细胞从而在慢性炎症疾病中发挥重要作用。IL-22、TNF-α是两个关键的Th22细胞因子。IL-22受体由IL-22R1与IL-10R2组成。 由于IL-22R1主要表达于皮肤黏膜细胞并决定细胞对IL-22的反应性,故上皮细胞等组织细胞为IL-22的主要靶细胞[7]。IL-22可单独或协同TNF-α还可诱导上皮细胞表达抗菌肽(如防御素、S100A7等)及Th1趋化因子CXCL9 /10/11的表达,具有抑制细菌生长及募集效应T细胞进入炎症部位的作用[4, 8]。IL-22还可促进上皮细胞损伤修复[4]。
包括我们前期研究在内的结果表明Ct等性传播病原体感染患者阴道分泌物中IL-22水平明显上升且输卵管上皮细胞可表达IL-22特异受体IL-22R1[9],因此我们推测Th22细胞及其细胞因子在生殖道黏膜免疫中亦发挥重要作用。由于mBD-2及CXCL9/10/11具有抗菌及趋化Th1细胞的作用,因此,在本研究中,我们建立了Th22细胞系并观察其培养上清对MOECs产生mBD-2及CXCL9/10/11的影响。结果表明,Th22细胞上清能有效诱导MOECs产生mBD-2及趋化因子CXCL9/10/11。中和IL-22、TNF-α可有效抑制抗菌肽及趋化因子的表达,因而排除了其它细胞因子干扰的可能。
女性生殖道是HIV、HPV、HSV-2及Ct等性传播病原体的入口[10]。T细胞介导的细胞免疫在清除这些性传播病原体的过程中发挥重要作用。然而由于特殊的解剖屏障,T细胞难于进入生殖道。而趋化因子CXCL9/1011可与效应T细胞及记忆T细胞上CXCR3受体结合促进其进入生殖道发挥效应作用[11]。有证据表明,沙眼衣原体感染小鼠输卵管组织中CXCL9、CXCL10 CXCL11表达明显升高,且与沙眼衣原体的清除有关[12]。本研究中,通过Transwell实验,我们亦证实了Th22活化的MOECs具有趋化CXCR3+T细胞的作用。另外,Th22活化的MOECs培养上清可显著抑制Ct的生长。这些结果表明,Th22细胞因子具有调节MOECs免疫功能的效应。
Reg3g蛋白家族属于C-类凝集素家族,具有抗菌、促进细胞增殖、抑制炎症和细胞凋亡的作用[13-14]。IL-22可活化STAT3信号途径,进而调节Reg3g蛋白的表达,促进上皮细胞损伤修复。我们前期的研究表明,IL-22可上调MOECs STAT3表达,因此在本研究中我们进一步观察了Th22细胞因子对MOECs表达Reg3g的影响。结果显示,IL-22、TNF-α可协同促进Reg3g的表达并增加MOECs的活性。这表明,Th22细胞因子具有促进MOECs损伤修复的作用。
综上所述,本研究认为,Th22细胞因子具有调节MOECs免疫功能并促进其损伤修复的作用,这为进一步研究Th22细胞及其细胞因子在生殖道黏膜免疫中的作用提供了实验依据。
[1]Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling[J]. J Clin Invest, 2009, 119(12): 3573-3585.
[2]Fujita H, Nograles KE, Kikuchi T, et al. Human Langerhans cells induce distinct IL-22-producing CD4+T cells lacking IL-17 production[J]. Proc Natl Acad Sci U S A, 2009, 106(51): 21795-21800.
[3]Sugita S, Kawazoe Y, Imai A, et al. Role of IL-22- and TNF-alpha-producing Th22 cells in uveitis patients with Behcet’s disease[J]. J Immunol, 2013, 190(11): 5799-5808.
[4]Eyerich S, Wagener J, Wenzel V, et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection withCandidaalbicans[J]. Eur J Immunol, 2011, 41(7): 1894-1901.
[5]Makinde HM, Zariffard R, Mirmonsef P, et al. IL-22 levels are associated withTrichomonasvaginalisinfection in the lower genital tract[J]. Am J Reprod Immunol, 2013, 70(1): 38-44.
[6]Li X,Xu W. The expression of murin β-defensin-2 in murine oviduct epithelial cells induced by IL-22[J]. Prog Obstet Gynecol,2014, 23(08): 627-629.
[7]Pham TA, Clare S, Goulding D, et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen[J]. Cell Host Microbe, 2014, 16(4): 504-516.
[8]Guilloteau K, Paris I, Pedretti N, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis[J]. J Immunol, 2010, 184(9): 5263-5270.
[9]Frazer LC, Scurlock AM, Zurenski MA, et al. IL-23 induces IL-22 and IL-17 production in response toChlamydiamuridarumgenital tract infection, but the absence of these cytokines does not influence disease pathogenesis[J]. Am J Reprod Immunol, 2013, 70(6): 472-484.
[10]Huo Z, Yu P, Cheng W. Preliminary study of primary and secondary infection in mice genital withChlamydiamuridarum[J]. Chin J Zoonoses, 2013, 29(10): 1000-1005.
[11]Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells[J]. Nature, 2012, 491(7424): 463-467.
[12]Maxion HK, Kelly KA. Chemokine expression patterns differ within anatomically distinct regions of the genital tract duringChlamydiatrachomatisinfection[J]. Infect Immun, 2002, 70(3): 1538-1546.
[13]Rendon JL, Li X, Akhtar S, et al. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury[J]. Shock, 2013, 39(1): 11-18.
[14]Gessner MA, Werner JL, Lilly LM, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense againstAspergillusfumigatus[J]. Infect Immun, 2012, 80(1): 410-417.
Potential protective role of the Th22 cytokines againstChlamydiatrichomatisinfection in genital tract
XU Ling-juan1,ZHAO Xiu-min2,ZHU Dan-yang2,XU Wen3
(1.DepartmentofLaboratoryMedicine,HospitalofTraditionalChineseMedicineofJinhuaCity,Zhejiang318300,China;2.DepartmentofObstetricsandGynecology,TaizhouFirstPeople’sHospital,Taizhou318020,China;3.DepartmentofMicrobiologyandImmunology,WengzhouMedicalUniversity,Wengzhou325035,China)
Th22 cells are a novel class of leukocytes characterized by the secretion of both IL-22 and TNF-α. Th22 cells have little or no direct impact on other immune cells, but exert selective effects on epithelia. It is not known, however, whether Th22 cells play a role in genital mucosal immunity. Here, we demonstrate that IL-22 and TNF-α synergistically induce several immunomodulatory molecules, such as the antimicrobial peptide mBD-2 (murine β-defensin 2) and the antimicrobial chemokines CXCL-9, -10, and -11 in primary murine oviduct epithelial cells (MOECs). The induction of innate immunity is relevant in aninvitroinfection model, in which MOECs stimulated with Th22 cell supernatants effectively inhibit the growth ofChlamydiatrichomatisand maintain the survival of the epithelia compared with untreated control. In summary, we demonstrate that the Th22 cell cytokines IL-22 and TNF-α play important roles in genital tract infection. The potential for Th22 cell cytokines to modulate innate immune mediators may lead to the development of new topical agents to treat and/or prevent immune-mediated STDs.
IL-22; defensin; chemokine; oviduct epithelial cell; Th22 cell
10.3969/cjz.j.issn.1002-2694.2015.08.012
许文,Email:wenxu.cn@gmail.com
1.浙江金华市中医院检验科,金华 321017; 2.浙江台州第一人民医院妇产科,台州 318300; 3.温州医科大学微生物学与免疫学教研室,温州 325035
Correponding author: Xu Wen, Email: wenxu.cn@gmail.com
R374
A
1002-2694(2015)08-0742-05
2014-10-30;
2015-03-26
浙江省自然科学基金((Y2 110670);温州市科技局项目(Y20110126)联合资助
Supported by the Zhejiang Provincial Natural Science Foundation (No. Y2 110670) and the Science and Technology Planning Project of Wenzhou Municipal (No. Y20110126)

